Abstract
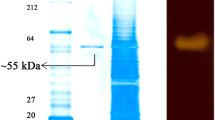
A halophilic α-amylase (EAMY) gene from Escherichia coli JM109 was overexpressed in E. coli XL10-Gold and the recombinant protein was purified and characterized. The activity of the EAMY depended on the presence of both Na+ and Cl−, and had maximum activity in 2 M NaCl at 55 °C and pH 7.0. When 2 % (w/v) soluble starch was used as substrate, the specific activity was about 1,090 U mg−1 protein. This is the first report on identifying a halophilic α-amylase with high specific activity from non-halophilic bacteria.



Similar content being viewed by others
References
Amoozegar MA, Malekzadeh F, Malik KA (2003) Production of amylase by newly isolated moderate halophile, Halobacillus sp. strain MA-2. J Microbiol Meth 52(3):353–359
Bernfeld P (1955) Amylase, α and β. Methods Enzymol 1:149–158
Coronado M, Vargas C, Hofemeister J, Ventosa A, Nieto JJ (2000) Production and biochemical characterization of an α-amylase from the moderate halophile Halomonas meridiana. FEMS Microbiol Lett 183:67–71
Fukushima T, Mizuki T, Echigo A, Inoue A, Usami R (2005) Organic solvent tolerance of halophilic alpha-amylase from a Haloarchaeon, Haloarcula sp. strain S-1. Extremophiles 9:85–89
Gupta R, Gigras P, Mohapatra H, Goswami VK, Chauhan B (2003) Microbial α-amylases:a biotechnological perspective. Proc Biochem 38:1599–1616
Hashim SO, Delgado O, Hatti-Kaul R, Mulaa FJ, Mattiasson B (2004) Starch hydrolysing Bacillus halodurans isolates from a Kenyan soda lake. Biotechnol Lett 26:823–828
Hutcheon GW, Vasisht N, Bolhuis A (2005) Characterisation of a highly stable α-amylase from the halophilic archaeon Haloarcula hispanica. Extremophiles 9:487–495
Li X, Wang HL, Li T, Yu HY (2012) Purification and characterization of an organic solvent-tolerant alkaline cellulase from a halophilic isolate of Thalassobacillus. Biotechnol Lett 34:1531–1536
Mevarech M, Frolow F, Gloss LM (2000) Halophilic enzymes: proteins with a grain of salt. Biophys Chem 86:155–164
Mijts BN, Patel BK (2002) Cloning, sequencing and expression of an alpha-amylase gene, amyA, from the thermophilic halophile Halothermothrix orenii and purification and biochemical characterization of the recombinant enzyme. Microbiology 148:2343–2349
Rui Y, Hiroko T, Matsujiro I, Tsutomu A, Masao T (2011) Salt-dependent thermo-reversible α-amylase: cloning and characterization of halophilic α-amylase from moderately halophilic bacterium, Kocuria varians. Appl Microbiol Biotechnol 89:673–684
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant no. 31160311), Science and Technology Development Project of Guangxi (Contract no. 11107008-3) and the Natural Science Foundation of Guangxi (Contract no. 2012GXNSFAA053051).
Author information
Authors and Affiliations
Corresponding author
Additional information
Yutuo Wei and Xiaobo Wang contributed equally to this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wei, Y., Wang, X., Liang, J. et al. Identification of a halophilic α-amylase gene from Escherichia coli JM109 and characterization of the recombinant enzyme. Biotechnol Lett 35, 1061–1065 (2013). https://doi.org/10.1007/s10529-013-1175-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-013-1175-9