Abstract
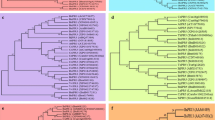
Two biotic stress resistance related genes from the full-length cDNA library of Brassica rapa cv. Osome were identified from EST analysis and determined to be pathogenesis-related (PR) 12 Brassica defensin-like family protein (BrDLFP) and PR-10 Brassica Betv1 allergen family protein (BrBetv1AFP) after sequence analysis and homology study with other stress resistance related same family genes. In the expression analysis, both genes expressed in different organs and during all developmental growth stages in healthy plants. Expression of BrDLFP significantly increased and BrBetv1AFP gradually decreased after infection with Pectobacterium carotovorum subsp. carotovorum in Chinese cabbage. Expression of these two genes significantly changed after cold, salt, drought and ABA stress treatments. These two PR genes may therefore be involved in the plant resistance against biotic and abiotic stresses.



Similar content being viewed by others
References
Ahmed NU, Park JI, Seo MS, Kumar TS, Lee IH, Park BS, Nou IS (2011) Identification and expression analysis of chitinase genes related to biotic stress resistance in Brassica. Mol Biol Rep. doi:10.1007/s11033-011-1139-x
Barratt DHP, Clark JA (1991) Proteins arising during the late stages of embryogenesis in Pisum sativum L. Planta 184:14–23
Breiteneder H, Pettenburger K, Bito A, Valenta R, Kraft D, Rumpold H, Scheiner O, Breitenbach M (1989) The gene coding for the major birch pollen allergen Betv1, is highly homologous to a pea disease resistance response gene. EMBO J 8:1935–1938
Broekaert WF, Cammue BPA, De Bolle MFC, Thevissen K, De Samblanx GW, Osborn RW (1997) Antimicrobial peptides from plants. Crit Rev Plant Sci 16:297–323
Cardoza V, Stewart CN Jr (2004) Brassica biotechnology: progress in cellular and molecular biology in vitro cell. Dev Biol Plants 40:542–551
Chiang CC, Hadwiger LA (1991) The Fusarium solani-induced expression of a pea gene family encoding high cysteine content proteins. Mol Plant Microbe Interact 4:324–331
Crowell DN, John ME, Russell D, Amasino RM (1992) Characterization of a stress-induced, developmentally regulated gene family from soybean. Plant Mol Biol 18:459–466
Epple P, Apel K, Bohlmann H (1997) ESTs reveal a multigene family for plant defensins in Arabidopsis thaliana. FEBS Lett 400:168–172
Garcia-Olmedo F, Molina A, Alamillo JM, Rodriguez-Palenzuela P (1998) Plant defense peptides. Biopolymers 47:479–491
Gu Q, Kawarta EF, Morse M-J, Wu H-M, Cheung AY (1992) A flower-specific cDNA encoding a novel thionin in tobacco. Mol Gen Genet 234:89–96
Haas BJ, Volfovsky N, Town CD, Troukhan M, Alexandrov N, Feldmann KA, Flavell RB, White O, Salzberg SL (2002) Full-length messenger RNA sequences greatly improve genome annotation. Genome Biol 3(6):0029.1–0029.12 (research)
Hamel F, Bellemare G (1995) Characterization of a class I chitinase gene and of wound-inducible, root and flower-specific chitinase expression in Brassica napus. Biochim Biophys Acta 1263(3):212–220
Hashimoto M, Kisseleva L, Sawa S, Furukawa T, Komatsu S, Koshiba T (2004) A novel rice PR-10 protein, RSOsPR10, specifically induced in roots by biotic and abiotic stresses, possibly via the jasmonic acid signaling pathway. Plant Cell Physiol 45(5):550–559
Korkina LG (2007) Phenylpropanoids as naturally occurring antioxidants: from plant defense to human health. Cell Mol Biol 53:15–25
Kragh KM, Nielsen JE, Nielsen KK, Dreboldt S, Mikkelsen JD (1995) Characterization and localization of new antifungal cysteine rich proteins from Beta vulgaris. Mol Plant Microbe Interact 8:424–434
Lee SC, Hwang IS, Choi HW, Hwang BK (2008) Involvement of the pepper antimicrobial protein CaAMP1 gene in broad spectrum disease resistance. Plant Physiol 148:1004–1020
Lo SC, Hipskind JD, Nicholson RL (1999) cDNA cloning of a sorghum pathogenesis-related protein (PR-10) and differential expression of defense-related genes following inoculation with Cochliobolus heterostrophus or Colletotrichum sublineolum. Mol Plant Microbe Interact 12:479–489
Matton DP, Brisson N (1989) Cloning, expression, and sequence conservation of pathogenesis-related gene transcripts of potato. Mol Plant Microbe Interact 2:325–331
McGee JD, Hamer JE, Hodges TK (2001) Characterization of a PR-10 pathogenesis-related gene family induced in rice during infection with Magnaporthe grisea. Mol Plant Microbe Interact 14:877–886
Midoh N, Iwata M (1996) Cloning and characterization of a probenazole-inducible gene for an intracellular pathogenesis-related protein in rice. Plant Cell Physiol 37:9–18
Molarda CR, Brugiere N, Moille M, Carrayol E, Limami AM (2004) Molecular characterization of a gene encoding a vegetative storage protein (CiVSP) from Cichorium intybus and its expression in the root and shoot in relation to nitrogen status and pathogen resistance. Physiol Plant 121:568–577
Moons A, Prinsen EIS, Bauw G, Montaguasi MV (1997) Antagonistic effects of abscisic acid and jasmonates on salt stress-inducible transcripts in rice roots. Plant Cell 9:2243–2259
Moreno M, Segura A, Garcia-Olmedo F (1994) Pseudothionin, a potato peptide active against potato pathogens. Eur J Biochem 223:135–139
Olli S, Kirti PB (2006) Cloning, characterization and antifungal activity of defensin Tfgd1 from Trigonella foenumgraecum L. J Biochem Mol Biol 39:278–283
Pan W, Shou J, Zhou X, Zha X, Guo T, Zhu M, Pan J (2011) Al-induced cell wall hydroxyproline-rich glycoprotein accumulation is involved in alleviating Al toxicity in rice. Acta Physiol Plant 33:601–608
Park HC, Kang YH, Chun HJ et al (2002) Characterization of a stamen-specific cDNA encoding a novel plant defensin in Chinese cabbage. Plant Mol Biol 50:59–69
Park JI, Kumar TS, Ahmed NU, Nou IS (2010) Construction of full-length cDNA library and investigation of potential genes in Brassica rapa. J Basic Life Res Sci 10:6–11
Radauer C, Lackner P, Breiteneder H (2008) The Betv1 fold: an ancient, versatile scaffold for binding of large, hydrophobic ligands. BMC Evol Biol 8:286
Rajam MV, Chandola N, Saiprasad GP, Singh D, Kashyap V, Choudhary ML, Sihachakr D (2007) Thaumatin gene confers resistance to fungal pathogens as well as tolerance to abiotic stresses in transgenic tobacco plants. Biol Plant 51(1):135–141
Ryals JA, Neuenschwander UH, Willits MG, Molina A, Steiner HY, Hunt MD (1996) Systemic acquired resistance. Plant Cell 8:1809–1819
Salunkhe DK, Kadam SS (1998) Handbook of vegetable science technology: production, composition, storage, and processing. Marcel Dekker Inc, New York
Selitrennikoff CP (2001) Antifungal proteins. Appl Env Microbiol 67:2883–2894
Senthil-Kumar M, Govind G, Kang L, Mysore KS, Udayakumar M (2007) Functional characterization of Nicotiana benthamiana homologs of peanut water deficit-induced genes by virus-induced gene silencing. Planta 225:523–539
Somssich IE, Schmelzer E, Bollmann J, Hahlbrock K (1986) Rapid activation by fungal elicitor of genes encoding “pathogenesis-related” proteins in cultured parsley cells. Proc Natl Acad Sci USA 83:2427–2430
Terras FRG, Eggermont K, Kovaleva V, Raikhel NV, Osborn RW, Kester A, Rees SB, Torrekens S, Van Leuven F, Vanderleyden J, Cammue BPA, Broekaert WF (1995) Small cysteine-rich antifungal proteins from radish: their role in host defense. Plant Cell 7:573–588
Van Loon LC (2001) The families of pathogenesis-related proteins. Abstracts of the 6th international workshop on PR-proteins, Spa, Belgium, 20–24 May, p 9
Van Loon LC, Van Kammen A (1970) Polyacrylamide disc electrophoresis of the soluble leaf proteins from Nicotiana tabacum var. ‘Samsun’ and ‘Samsun NN’ II. Changes in protein constitution after infection with tobacco mosaic virus. Virology 40:199–211
Van Loon LC, Van Strien EA (1999) The families of pathogenesis-related proteins, their activities, and comparative analysis of PR-1 type proteins. Physiol Mol Plant Pathol 55:85–97
Van Loon LC, Rep M, Pieterse CM (2006) Significance of inducible defense-related proteins in infected plants. Annu Rev Phytopathol 44:135–162
Warner SAJ, Scott R, Draper J (1993) Isolation of an asparagus intracellular PR gene (AoPR1) wound-responsive promoter by the inverse polymerase chain reaction and its characterization in transgenic tobacco. Plant J 3:191–201
Acknowledgments
This work was supported by the Cabbage Genomics assisted breeding supporting center research program funded by Ministry for Food, Agriculture, Forestry and Fisheries of the Korean Government.
Author information
Authors and Affiliations
Corresponding author
Additional information
Nasar Uddin Ahmed and Jong-In Park equally contributed to this work.
Rights and permissions
About this article
Cite this article
Ahmed, N.U., Park, JI., Jung, HJ. et al. Identification and characterization of stress resistance related genes of Brassica rapa . Biotechnol Lett 34, 979–987 (2012). https://doi.org/10.1007/s10529-012-0860-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-012-0860-4