Abstract
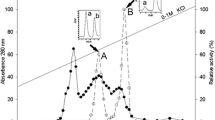
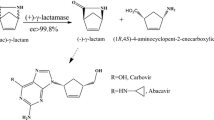
A (−)γ-lactamase, Mhg, from Microbacterium hydrocarbonoxydans was over-expressed in E. coli and was characterized after purification. The maximum activity was at pH 8.0 and 60°C and the half life of Mhg was ~30 min at 75°C. The enzyme was activated by DTT. The catalytic triad of the (−)γ-lactamase is comprised of residues Ser98, Asp230, and His259 and an oxyanion hole was formed by Tyr32 and Met99 according to the alignment results. Under native conditions, the (−)γ-lactamase consists of two 31 kDa homodimers.


Similar content being viewed by others
References
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Chapouthier G, Venault P (2001) A pharmacological link between epilepsy and anxiety. Trend Pharmacol Sci 22:491–493
Chen CS, Fujimoto Y, Girdaukas G, Sih CJ (1982) Quantitative analyses of biochemical kinetic resolutions of enantiomers. J Amer Chem Soc 10:7294–7299
Foster AC, Kemp JA (2006) Glutamate and GABA-based CNS therapeutics. Curr Opin Pharmacol 6:7–17
Kobayashi M, Komeda H, Nagasawa T, Nishiyama M, Horinouchi S, Beppu T, Yamada H, Shimizu S (1993) Amidase coupled with low-molecular-mass nitrile hydratase from Rhodococcus rhodochrous J1. Eur J Biochem 217:327–336
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Li J, Liang S (2002) C-terminal sequence analysis of peptides using triphenylgermanyl isothiocyanate. Anal Biochem 302:108–113
Li K, Xu E (2008) The role and the mechanism of gamma-aminobutyric acid during central nervous system development. Neurosci Bull 24:195–200
Line K, Isupov MN, Littlechild JA (2004) The crystal structure of a (−) gamma-lactamase from an Aureobacterium species reveals a tetrahedral intermediate in the active site. J Mol Biol 338:519–532
Taylor SJC, Sutherland AG, Lee C, Wisdom R, Thomas S, Roberts SM, Evans C (1991) ChemInform abstract: chemoenzymatic synthesis of (−)-carbovir utilizing a whole cell-catalyzed resolution of 2-azabicyclo(2.2.1)hept-5-en-3-one. ChemInform 22(7)
Taylor SJC, McCague R, Wisdom R, Lee C et al (1993) ChemInform abstract: development of the biocatalytic resolution of 2-azabicyclo(2.2.1)hept-5-en-3-one as an entry to single-enantiomer carbocyclic nucleosides. ChemInform 24(41)
Toogood HS, Brown RC, Line K, Keene PA, Taylor SJC, McCague R, Littlechild JA (2004) The use of a thermostable signature amidase in the resolution of the bicyclic synthon (rac)-[gamma]-lactam. Tetrahedron 60(3):711–716
Wang J, Guo X, Zheng G, Wen C (2009) Purification and characterization of a novel (−) gamma-lactamase from Microbacterium hydrocarbonoxydans. Ann Microbiol 59:345–348
Wang J, Zhang X, Min C, Wu S, Zheng G (2011) Single-step purification and immobilization of [gamma]-lactamase and on-column transformation of 2-azabicyclo [2.2.1] hept-5-en-3-one. Proc Biochem 46:81–87
Acknowledgment
This work was supported by Youth Fund of State Key Laboratory of Microbial Resources, Institute of Microbiology, Chinese Academy of Sciences and Open fund of State Key Laboratory of Microbial Resources, Institute of Microbiology, Chinese Academy of Sciences.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yang, M., Gao, Q., Wu, S. et al. Characterization of a recombinant (−)γ-lactamase from Microbacterium hydrocarbonoxydans . Biotechnol Lett 34, 275–279 (2012). https://doi.org/10.1007/s10529-011-0758-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-011-0758-6