Abstract
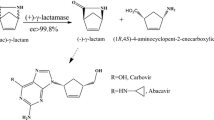
Mhg, a previously reported (−)-γ-lactamase from Microbacterium hydrocarbonoxydans, was identified to have perhydrolase activity by combining structure similarity search with activity assays. Kinetic studies illustrated that perhydrolysis was the native activity owing to lower K m and higher k cat/K m values. Experimental evidence showed that both hydrolysis and perhydrolysis reactions took place at the same active center. Engineering of the putative substrate-binding pocket revealed that Leu233 site played a vital role in the aspects of selective catalysis, soluble protein expression level and optimum temperature shift, etc. The mutants L233A, L233P, and L233T retained (−)-γ-lactamase activity but lost perhydrolase activity, while L233M only kept perhydrolase activity. Substitutions of Leu233 could dramatically influence the state of expressed protein. Computational analysis explicitly explained the relationships between mutations and γ-lactamase activity changes. Our investigations demonstrated that it was an efficient method to identify the enzyme catalytic promiscuity by combining 3D structure alignment with activity validations, and engineering of substrate-binding pocket could serve as a promising way to regulate activities of promiscuous enzymes.





Similar content being viewed by others
References
Anandarajah K, Kiefer PM, Donohoe BS, Copley SD (2000) Recruitment of a double bond isomerase to serve as a reductive dehalogenase during biodegradation of pentachlorophenol. Biochemistry 39(18):5303–5311
Beadle BM, Shoichet BK (2002) Structural bases of stability—function tradeoffs in enzymes. J Mol Biol 321(2):285–296
Bernhardt P, Hult K, Kazlauskas RJ (2005) Molecular basis of perhydrolase activity in serine hydrolases. Angew Chem Int Ed 44(18):2742–2746
Carbonell P, Faulon JL (2010) Molecular signatures-based prediction of enzyme promiscuity. Bioinformatics 26(16):2012–2019
Carbonell P, Nussinov R, del Sol A (2009) Energetic determinants of protein binding specificity: insights into protein interaction networks. Proteomics 9(7):1744–1753
Catrina I, O'Brien PJ, Purcell J, Nikolic-Hughes I, Zalatan JG, Hengge AC, Herschlag D (2007) Probing the origin of the compromised catalysis of E. coli alkaline phosphatase in its promiscuous sulfatase reaction. J Am Chem Soc 129(17):5760–5765
Chakraborty S, Rao BJ (2012) A measure of the promiscuity of proteins and characteristics of residues in the vicinity of the catalytic site that regulate promiscuity. PLoS One 7(2):e32011
Cheeseman JD, Tocilj A, Park S, Schrag JD, Kazlauskas RJ (2004) Structure of an aryl esterase from Pseudomonas fluorescens. Acta Crystallogr Sect D: Biol Crystallogr 60(7):1237–1243
Copley S (2003) Enzymes with extra talents: moonlighting functions and catalytic promiscuity. Curr Opin Chem Biol 7(2):265–272
Counago R, Wilson CJ, Pena MI, Wittung-Stafshede P, Shamoo Y (2008) An adaptive mutation in adenylate kinase that increases organismal fitness is linked to stability-activity trade-offs. Protein Eng Des Sel 21(1):19–27
Gomez A, Domedel N, Cedano J, Pinol J, Querol E (2003) Do current sequence analysis algorithms disclose multifunctional (moonlighting) proteins? Bioinformatics 19(7):895–896
Gonsalvez IS, Isupov MN, Littlechild JA (2001) Crystallization and preliminary X-ray analysis of a gamma-lactamase. Acta Crystallogr Sect D: Biol Crystallogr 57(2):284–286
Holm L, Rosenstrom P (2010) Dali server: conservation mapping in 3D. Nucleic Acids Res 38(Web Server issue):W545–W549
Holm L, Kaariainen S, Rosenstrom P, Schenkel A (2008) Searching protein structure databases with DaliLite v. 3. Bioinformatics 24(23):2780–2781
Hult K, Berglund P (2007) Enzyme promiscuity: mechanism and applications. Trends Biotechnol 25(5):231–238
Kahakeaw D, Reetz MT (2008) A cell-based adrenaline assay for automated high-throughput activity screening of epoxide hydrolases. Chem Asian J 3(2):233–238
Kelly SM, Jess TJ, Price NC (2005) How to study proteins by circular dichroism. Biochim Biophys Acta 1751(2):119–139
Khersonsky O, Tawfik DS (2010) Enzyme promiscuity: a mechanistic and evolutionary perspective. Annu Rev Biochem 79:471–505
Krebsfänger N, Zocher F, Altenbuchner J, Bornscheuer U (1998) Characterization and enantioselectivity of a recombinant esterase from Pseudomonas fluorescens. Enzyme Microb Technol 22(7):641–646
Lawson AJ, Walker EA, White SA, Dafforn TR, Stewart PM, Ride JP (2009) Mutations of key hydrophobic surface residues of 11 β-hydroxysteroid dehydrogenase type 1 increase solubility and monodispersity in a bacterial expression system. Protein Sci 18(7):1552–1563
Line K, Isupov MN, Littlechild JA (2004) The crystal structure of a (-) gamma-lactamase from an Aureobacterium species reveals a tetrahedral intermediate in the active site. J Mol Biol 338(3):519–532
Little DB, Croteau RB (2002) Alteration of product formation by directed mutagenesis and truncation of the multiple-product sesquiterpene synthases δ-selinene synthase and γ-humulene synthase. Arch Biochem Biophys 402(1):120–135
Major DT, Weitman M (2012) Electrostatically guided dynamics—the root of fidelity in a promiscuous terpene synthase? J Am Chem Soc 134(47):19454–19462
Mosavi LK, Zy P (2003) Structure-based substitutions for increased solubility of a designed protein. Protein Eng 16(10):739–745
Mukaiyama A, Haruki M, Ota M, Koga Y, Takano K, Kanaya S (2006) A hyperthermophilic protein acquires function at the cost of stability. Biochemistry 45(42):12673–12679
Nastopoulos V, Vallone B, Politi L, Scotto d’Abusco A, Scandurra R, Tsernoglou D (2001) Crystallization and X-ray diffraction measurements of a thermophilic archaeal recombinant amidase from Sulfolobus solfataricus MT4. Acta Crystallogr Sect D: Biol Crystallogr 57(7):1036–1037
Nobeli I, Favia AD, Thornton JM (2009) Protein promiscuity and its implications for biotechnology. Nat Biotechnol 27(2):157–167
O'Brie PJ, Herschlag D (2001) Functional interrelationships in the alkaline phosphatase superfamily: phosphodiesterase activity of Escherichia coli alkaline phosphatase. Biochemistry 40(19):5691–5699
Overman RC, Green I, Truman CM, Read JA, Embrey KJ, McAlister MS, Attwood TK (2013) Stability and solubility engineering of the EphB4 tyrosine kinase catalytic domain using a rationally designed synthetic library. Protein Eng Des Sel 26(10):695–704
Poor CB, Andorfer MC, Lewis JC (2014) Improving the stability and catalyst lifetime of the halogenase RebH by directed evolution. Chembiochem 15(9):1286–1289
Roodveldt C, Tawfik DS (2005) Shared promiscuous activities and evolutionary features in various members of the amidohydrolase superfamily. Biochemistry 44(38):12728–12736
Singh R, Vince R (2012) 2-Azabicyclo[2.2.1]hept-5-en-3-one: chemical profile of a versatile synthetic building block and its impact on the development of therapeutics. Chem Rev 112(8):4642–4686
Song JK, Ahn HJ, Kim HS, Song BK (2006) Molecular cloning and expression of perhydrolase genes from Pseudomonas aeruginosa and Burkholderia cepacia in Escherichia coli. Biotechnol Lett 28(12):849–856
Steinkellner G, Gruber CC, Pavkov-Keller T, Binter A, Steiner K, Winkler C, Lyskowski A, Schwamberger O, Oberer M, Schwab H, Faber K, Macheroux P, Gruber K (2014) Identification of promiscuous ene-reductase activity by mining structural databases using active site constellations. Nat Commun 5:4150
Taylor Ringia EA, Garrett JB, Thoden JB, Holden HM, Rayment I, Gerlt JA (2004) Evolution of enzymatic activity in the enolase superfamily: functional studies of the promiscuous o-succinylbenzoate synthase from Amycolatopsis. Biochemistry 43(1):224–229
Taylor SJ, Brown RC, Keene PA, Taylor IN (1999) Novel screening methods-the key to cloning commercially successful biocatalysts. Bioorg Med Chem 7(10):2163–2168
Wang J, Guo X, Zheng G, Wen C (2009) Purification and characterization of a novel (-) gamma-lactamase from Microbacterium hydrocarbonoxydans. Ann Microbiol 59(2):345–348
Wang J, Zhao G, Zhang Z, Liang Q, Min C, Wu S (2014a) Autodisplay of an archaeal γ-lactamase on the cell surface of Escherichia coli using Xcc_Est as an anchoring scaffold and its application for preparation of the enantiopure antiviral drug intermediate (-)-γ-lactam. Appl Microbiol Biotechnol 98(6):6991–7001
Wang J, Zhu J, Wu S (2014b) Immobilization on macroporous resin makes E. coli RutB a robust catalyst for production of (-) Vince lactam. Appl Microbiol Biotechnol. doi:10.1007/s00253-014-6247-9
Wang J, Zhu Y, Zhao G, Zhu J, Wu S (2014c) Characterization of a recombinant (+)-γ-lactamase from Microbacterium hydrocarbonoxydans which provides evidence that two enantiocomplementary γ-lactamases are in the strain. Appl Microbiol Biotechnol. doi:10.1007/s00253-014-6114-8
Yang M, Gao Q, Wu S, Wang J, Zheng G (2012) Characterization of a recombinant (-)-γ-lactamase from Microbacterium hydrocarbonoxydans. Biotechnol Lett 34(2):275–279
Yoshikuni Y, Ferrin TE, Keasling JD (2006) Designed divergent evolution of enzyme function. Nature 440(7087):1078–1082
Zhao H, Caflisch A (2014) Discovery of dual ZAP70 and Syk kinases inhibitors by docking into a rare C-helix-out conformation of Syk. Bioorg Med Chem Lett 24(6):1523–1527
Zou J, Hallberg BM, Bergfors T, Oesch F, Arand M, Mowbray SL, Jones TA (2000) Structure of Aspergillus niger epoxide hydrolase at 1.8Å resolution: implications for the structure and function of the mammalian microsomal class of epoxide hydrolases. Structure 8(2):111–122
Acknowledgments
We thank the financial support from the State Key Laboratory of Microbial Resources, Institute of Microbiology, Chinese Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 368 kb)
Rights and permissions
About this article
Cite this article
Sun, Y., Zhao, H., Wang, J. et al. Identification and regulation of the catalytic promiscuity of (−)-γ-lactamase from Microbacterium hydrocarbonoxydans . Appl Microbiol Biotechnol 99, 7559–7568 (2015). https://doi.org/10.1007/s00253-015-6503-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-015-6503-7