Abstract
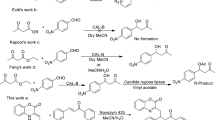
Regioselective undecylenoylation of purine nucleosides as potential dual prodrugs was achieved by Candida antarctica lipase B using adenosine as a model reactant. The optimum organic solvent, molar ratio of vinyl ester to nucleoside, enzyme dosage, reaction temperature and molecular sieve amount were anhydrous THF, 5:1, 20 U/ml, 45°C and 75 mg/ml, respectively. Under the optimum conditions, the initial reaction rate, yield and 5′-regioselectivity were 1.1 mM/h, 90% and >99%, respectively. The enzymatic acylation of various nucleosides furnished the desired 5′-ester derivatives with the yields of 60–95% and 5′-regioselectivities of >99%. In addition, the lipase displayed excellent operational stability in THF, and retained 96% of its initial activity after reused for five batches.



Similar content being viewed by others
References
De Clercq E, Field HJ (2006) Antiviral prodrugs––the development of successful prodrug strategies for antiviral chemotherapy. Br J Pharmacol 147:1–11
Diaz-Rodriguez A, Fernandez S, Lavandera I, Ferrero M, Gotor V (2005) Novel and efficient regioselective enzymatic approach to 3′-, 5′- and 3′,5′-di-O-crotonyl 2′-deoxynucleoside derivatives. Tetrahedron Lett 46:5835–5838
Ferrero M, Gotor V (2000) Biocatalytic selective modifications of conventional nucleosides, carbocyclic nucleosides, and C-nucleosides. Chem Rev 100:4319–4347
Galmarini CM, Mackey JR, Dumontet C (2002) Nucleoside analogues and nucleobases in cancer treatment. Lancet Oncol 3:415–424
Garcia J, Fernandez S, Ferrero M, Sanghvi YS, Gotor V (2003) Novel enzymatic synthesis of levulinyl protected nucleosides useful for solution phase synthesis of oligonucleotides. Tetrahedron Asymmetry 14:3533–3540
Lavandera I, Fernandez S, Magdalena J, Ferrero M, Kazlauskas RJ, Gotor V (2005) An inverse substrate orientation for the regioselective acylation of 3′, 5′-diaminonucleosides catalyzed by Candida antarctica lipase B? ChemBioChem 6:1381–1390
Li XF, Zong MH, Wu H, Lou WY (2006) Markedly improving Novozym 435-mediated regioselective acylation of 1-β-D-arabinofuranosylcytosine by using co-solvent mixtures as the reaction media. J Biotechnol 124:552–560
Li N, Zong MH, Liu XM, Ma D (2007) Regioselective synthesis of 3′-O-caproyl-floxuridine catalyzed by Pseudomonas cepacia lipase. J Mol Catal B 47:6–12
Li N, Ma D, Zong MH (2008a) Enhancing the activity and regioselectivity of lipases for 3′-benzoylation of floxuridine and its analogs by using ionic liquid-containing systems. J Biotechnol 133:103–109
Li N, Zong MH, Ma D (2008b) Regioselective acylation of nucleosides catalyzed by Candida antarctica lipase B: enzyme substrate recognition. Eur J Org Chem 5375–5378
Li X, Wu Q, Zhang F, Lin XF (2008c) Chemoenzymatic synthesis, characterization, and controlled release of functional polymeric prodrugs with acyclovir as pendant. J Appl Polym Sci 108:431–437
Li N, Zeng QM, Zong MH (2009a) Substrate specificity of lipase from Burkholderia cepacia in the synthesis of 3′-arylaliphatic acid esters of floxuridine. J Biotechnol 142:267–270
Li N, Zong MH, Ma D (2009b) Regioselective acylation of nucleosides and their analogs catalyzed by Pseudomonas cepacia lipase: enzyme substrate recognition. Tetrahedron 65:1063–1068
Li N, Zong MH, Ma D (2009c) Thermomyces lanuginosus lipase-catalyzed regioselective acylation of nucleosides: Enzyme substrate recognition. J Biotechnol 140:250–253
Li N, Smith TJ, Zong MH (2010) Biocatalytic transformation of nucleoside derivatives. Biotechnol Adv 28:348–366
Mak KH, Yu CY, Kuan IC, Lee SL (2009) Immobilization of Pseudomonas cepacia lipase onto magnetic nanoparticles for biodiesel production. ISESCO Sci Technol Vision 5:19–23
Pleiss J, Fischer M, Schmid RD (1998) Anatomy of lipase binding sites: the scissile fatty acid binding site. Chem Phys Lipids 93:67–80
Plunkett W, Gandhi V (2001) Purine and pyrimidine nucleoside analogs. In: Giaccone G, Schilsky R, Sondel P (eds) Cancer chemotherapy and biological response modifiers: annual 19. Elsevier Science, Amsterdam, pp 21–46
Raku T, Kitagawa M, Shimakawa H, Tokiwa Y (2003) Enzymatic synthesis of hydrophilic undecylenic acid sugar esters and their biodegradability. Biotechnol Lett 25:161–166
Simeo Y, Sinisterra JV, Alcantara AR (2009) Regioselective enzymatic acylation of pharmacologically interesting nucleosides in 2-methyltetrahydrofuran, a greener substitute for THF. Green Chem 11:855–862
Tokiwa Y, Kitagawa M, Raku T (2007a) Enzymatic synthesis of arbutin undecylenic acid ester and its inhibitory effect on mushroom tyrosinase. Biotechnol Lett 29:481–486
Tokiwa Y, Kitagawa M, Raku T, Yanagitani S, Yoshino K (2007b) Enzymatic synthesis of arbutin undecylenic acid ester and its inhibitory effect on melanin synthesis. Bioorg Med Chem Lett 17:3105–3108
Van der Steen M, Stevens CV (2009) Undecylenic acid: a valuable and physiologically active renewable building block from castor oil. ChemSusChem 2:692–713
Wang ZY, Li N, Zong MH (2009) A simple procedure for the synthesis of potential 6-azauridine prodrugs by Thermomyces lanuginosus lipase. J Mol Catal B 59:212–219
Weber HK, Weber H, Kazlauskas RJ (1999) ‘Watching’ lipase-catalyzed acylations using 1H NMR: competing hydrolysis of vinyl acetate in dry organic solvents. Tetrahedron Asymmetry 10:2635–2638
Zhao ZL, Zong MH, Li N (2009) Efficient regioselective synthesis of 3′-O-crotonyl-floxuridine catalyzed by Pseudomonas cepacia lipase. Biotechnol Appl Biochem 52:45–51
Acknowledgments
We are grateful for financial support of this work by the National Natural Science Foundation of China (20906032) and the Fundamental Research Funds for the Central Universities, SCUT (2009zz0018).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Gao, WL., Li, N. & Zong, MH. Highly regioselective synthesis of undecylenic acid esters of purine nucleosides catalyzed by Candida antarctica lipase B. Biotechnol Lett 33, 2233–2240 (2011). https://doi.org/10.1007/s10529-011-0685-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-011-0685-6