Abstract
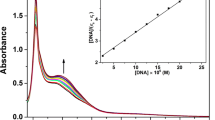
The product of human fragile histidine triad (FHIT) gene is a tumor suppressor protein of still largely unknown cellular background. We have shown previously that it binds protoporphyrin IX (a photosensitizer) which alters its enzymatic activity in vitro. Fhit, diadenosine triphosphate (Ap3A) hydrolase, possesses the active site with histidine triad His-φ-His-φ-His-φφ. So-called histidine Fhit mutants (His94Asn, His96Asn and His98Asn) exhibit highly reduced activity in vitro, however, their antitumor function has not been fully described yet. In this work we have cloned the cDNAs of histidine mutants into pPROEX-1 vector allowing the production of His6-fusion proteins. The mutated proteins: Fhit-H94N, Fhit-H96N and Fhit-H98N, were expressed in Escherichia coli BL21(DE3) and purified (up to 95%) by an improved, one-step affinity chromatography on Ni-nitrilotriacetate resin. The final yield was 2 mg homogenous proteins from 1 g bacteria (wet wt). The activity of purified proteins was assessed by previously described assay. The same purification procedure yielded 0.8 mg/ml and highly active wild-type Fhit protein (K m value for Ap3A of 5.7 μM). Importantly, purified mutant forms of Fhit also interact with a photosensitizer, protoporphyrin IX in vitro.




Similar content being viewed by others
References
Abend A, Garrison PN, Barnes LD et al (1999) Stereochemical retention of the configuration in the action of Fhit on phosphorus-chiral substrates. Biochemistry 38:3668–3676
Barnes LD, Garrison PN, Siprashvili Z et al (1996) Fhit, a putative tumor suppressor in humans, is a dinucleoside 5′,5″-P1,P3-triphosphate hydrolase. Biochemistry 35:11529–11535
Brenner C, Pace HC, Garrison PN et al (1997) Purification and crystallization of complexes modeling the active state of the fragile histidine triad protein. Protein Eng 10(12):1461–1463
Croce CM, Sozzi G, Huebner K et al (1999) Role of FHIT in human cancer. J Clin Oncol 17:1618–1624
Draganescu A, Hodawadekar SC, Gee KR et al (2000) Fhit-nucleotide specificity probed with novel fluorescent and fluorogenic substrates. J Biol Chem 275:4555–4560
Huebner K, Garrison PN, Barnes LD et al (1998) The role of the FHIT/FRA3B locus in cancer. Annu Rev Genet 32:7–31
Kowalska A, Kwiek P, Milosz E et al (2003) Application of electrophoretic methods for detection of protein–porphyrin complexes. 50(4):1155–1163
Kuroki T, Trapasso F, Yendamuri S et al (2003) Allelic loss on chromosome 3p21.3 and promoter hypermethylation of semaphorin 3B in non-small cell lung cancer. Cancer Res 63:3724–3728
Lima CD, Klein MG, Hendrickson WA (1997) Structure-based analysis of catalysis and substrate definition in the HIT protein family. Science 278:286–290
Ohta M, Inoue H, Cotticelli et al (1996) The FHIT gene, spanning the chromosome 3p14.2 fragile site and renal carcinoma-associated t(3;8) breakpoint, is abnormal in digestive tract cancers. Cell 84(4):587–597
Pace HC, Garrison PN, Robinson AK et al (1998) Genetic, biochemical, and crystallographic characterization of Fhit–substrate complexes as the active signalling form of Fhit. Proc Natl Acad Sci USA 95:5484–5489
Pawelczyk T, Kowara R, Golebiowski F et al (2000) Expression in Escherichia coli and simple purification of human Fhit protein. Protein Expr Purif 18(3):320–326
Sambrook J, Fritsh EF, Maniatis T (1989) Molecular cloning: a laboratory manual. Cold Spring Harbor Lab Press 3
Siprashvili Z, Sozzi G, Barnes LD et al (1997) Replacement of Fhit in cancer cells suppresses tumorigenicity. Proc Natl Acad Sci USA 94:13771–13776
Sozzi G, Veronese ML, Negrini M et al (1996) The FHIT gene 3p14.2 is abnormal in lung cancer. Cell 85(1):17–26
Trapasso R, Krakowiak A, Cesari R et al (2003) Designed FHIT alleles establish that Fhit-induced apoptosis in cancer cells is limited by substrate binding. Proc Natl Acad Sci USA 100(4):1592–1597
Werner NS, Siprashvili Z, Fong LYY et al (2000) Differential susceptibility of renal carcinoma cell lines to tumor suppression by exogenous Fhit expression. Cancer Res 60:2780–2785
Zawacka-Pankau J, Kowalska A, Issaeva N et al (2007) Tumor suppressor Fhit protein interacts with protoporphyrin IX in vitro and enhances the response of HeLa cells to photodynamic therapy. J Photochem Photobiol B 86:35–42
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zawacka-Pankau, J., Podhajska, A.J. Expression and simple, one-step purification of fragile histidine triad (Fhit) tumor suppressor mutant forms in Escherichia coli and their interaction with protoporphyrin IX. Biotechnol Lett 29, 877–883 (2007). https://doi.org/10.1007/s10529-007-9322-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-007-9322-9