Abstract
Entomopathogenic nematodes (EPNs) are commonly used biocontrol agents of insect pests, with a wide range of commercially available isolates targeting specific pests. New isolates are, however, required to improve pest control across a wider range of environmental conditions for target pests, including emerging threats. We assessed the effect of temperature on survival and virulence of 17 Australian isolates of five EPN species (Heterorhabditis bacteriophora, Heterorhabditis indica, Heterorhabditis marelatus, Heterorhabditis zealandica and Steinernema feltiae) against larvae and pupae of the Queensland fruit fly, Bactrocera tryoni. All isolates still infected and killed larvae after infective juveniles (IJ) had been kept without insect hosts at 15 °C, 25 °C or 30 °C for two weeks, indicating their potential to remain viable under field conditions. However, the mean LD50 value ranged from 35 to 150 and was generally lower at 15 °C than at 25 °C and 30 °C. Similarly, after IJs had been kept at 25 °C for 1–3 weeks without insect hosts, all isolates infected B. tryoni larvae, with mean LD50 values ranging from 25 to 144. Interestingly, 15 isolates infected and killed B. tryoni pupae after one week, with a mean LD50 value between 130 and 209, but only two isolates after two weeks, with a mean LD50 value between 229 to 209. No pupal mortality was seen after three weeks. In absence of hosts, EPNs survived longer at 15 °C and 25 °C than at 30 °C. Complete EPN mortality occurred after nine weeks at 30 °C, and after 18 weeks at 15 °C and 25 °C, except for some survival in one S. feltiae isolate (Sf.ECCS). Overall, six isolates of H. indica (Hi.HRN2, Hi.LMI2, Hi.QF6), H. bacteriophora (Hb.HIE), H. zealandica (Hz.NAR1) and S. feltiae (Sf.ECCS) performed best and need further testing as potential biocontrol agents against B. tryoni under semi-field and field conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The family of tephritid fruit flies (Diptera: Tephritidae) contains many important and widespread pest species (White and Elson-Harris 1992). They can cause substantial losses in fruit and fruiting vegetable crops, and limit market access of fresh produce because of biosecurity restrictions imposed by many countries (Clarke 2019). The Queensland fruit fly, Bactrocera tryoni (Frogatt), is the most destructive horticultural pest in Australia, and has a high invasion potential, being one of the most polyphagous tephritids (Clarke et al. 2011) with a high climatic adaptability (Popa-Báez et al. 2020). Adult females lay their eggs into the fruit where the larvae develop causing the fruit to rot. The eggs and developing larvae are protected within the fruit and therefore more difficult to target in pest control. Thus the focus of fruit fly control is mostly on adult fruit fly and reproduction. However, late instar larvae leave the fruit prior to the pre-pupal stage and then pupate within a few hours in the top layer (1 to 5 cm) of the soil (Hulthen and Clarke 2006), and adult flies emerge from the pupae and then crawl to the soil surface (Bateman 1972). These developmental stages outside the fruit and in the soil can be targeted by soil-borne natural enemies and biological control agents, including entomopathogenic nematodes (EPNs).
EPNs of the families Heterorhabditidae and Steinernematidae with their symbiotic bacteria Photorhabdus (in Heterorhabditis spp.) and Xenorhabdus (in Steinernema spp.) are well studied and effective biological control agents of diverse pest insects (Kaya and Gaugler 1993; Tailliez et al. 2010). The dauer juveniles, also known as infective juveniles (IJs), leave infected insect cadavers after the depletion of resources, and can survive a short time in the soil while seeking new insect hosts. The symbiotic bacteria of EPNs provide IJs with the capacity to kill an insect host in which the EPNs can then develop (Waterfield et al. 2009). While EPNs are successful biological control agents of a diversity of pest insects with developmental stages in the soil, they have also been proven effective against several fruit fly pests (Sirjani et al. 2009; Godjo et al. 2018), including B. tryoni (Langford et al. 2014; Aryal et al. 2022b). However, for effective biological control there is a need for EPN strains that can survive and remain virulent under local conditions.
In general, EPNs have limited ability to survive in the soil without insect hosts, and, therefore, ability of EPNs to survive and remain infective in the soil before finding new insect hosts remains a major constraint of EPN field applications (Strong 2002). IJs survival in the absence of hosts depends largely on temperature, moisture, soil type and pH (Kung et al. 1990a, b; Griffin 1993; Koppenhöfer and Fuzy 2007), the susceptibility of the targeted insect pest species and its developmental stages (Bedding et al. 1993). Moreover, survival and infectivity differ between nematode species and strains (Georgis and Gaugler 1991), and are also influenced by bacterial strain identity (Grewal et al. 1997), bioassay methods (Grewal et al. 1994) and lipid reserves, with higher lipid content allowing IJs to survive longer (Patel et al. 1997; Hass et al. 2002; Fitters and Griffin 2004). Thus increased long term survival and infectivity improve the efficiency of EPN-based biological control, allow lower application doses and rates (Bedding et al. 1993) and, therefore, reduce the associated cost.
A challenge in the development of EPNs as a biocontrol agent of fruit flies is that fruit flies can vary considerably in abundance and distribution across seasons. For example, B. tryoni has several generations per year and a diverse host range, and can respond to variable resource availability (Tasnin et al. 2021). Furthermore, earlier studies have shown that larval and adult stages of fruit flies are more susceptible to EPN infection than pupae (Yee and Lacey 2003; Kamali et al. 2013; Langford et al. 2014), yet larvae and adults are less likely to be exposed to EPNs because they spend most time above ground. In contrast, the pupal stage is the longest developmental stage of fruit flies in the soil (Bateman 1972), but may be less susceptible to EPNs (Langford et al. 2014; Aryal et al. 2022b). Therefore, in order to have an effect on fruit fly populations, EPNs must be able to survive when no fruit fly individuals are in the soil and maintain high levels of infectivity, or infect alternative hosts in the meantime (Kurtz et al. 2007; Susurluk and Ehlers 2008). IJs may also need to be able to infect individuals quickly as the available time window of infection of larvae (prior to pupation) and adults (after emergence) in the soil is with a few hours in B. tryoni (Hulthen and Clarke 2006) relatively short. Alternatively, IJs should have the capacity to also infect pupae (Aryal et al. 2022b). Furthermore, EPN isolates with longer survival might need to be applied less frequently and may be more economic. Likewise, more virulent isolates might allow application of lower doses.
We investigated the survival and virulence of EPN isolates at different temperatures to identify potential candidates for the biological control of B. tryoni. We assessed these traits in 15 EPN isolates that have recently been isolated from Australian soils and molecularly characterized (Aryal et al. 2022a, 2022b). We also compared these traits between the new EPN isolates and two commercially available EPN strains previously isolated from Australian soils but since then kept in long-term laboratory culture. We further aimed to identify EPN isolates that have superior survival and virulence and are less affected by variation in temperature.
Materials and methods
EPN production and storage
This study used 15 EPN isolates recently isolated from eastern Australia (Aryal et al. 2022a) and two commercial isolates obtained from Ecogrow Environment Pty Ltd (Table 1). The EPNs were reared on T. molitor larvae kept at 25 °C and were harvested from white traps as described previously (Aryal et al. 2022a) and stored in Ringer’s solution (9.0 g NaCl, 0.42 g KCl, 0.37 g CaCl2 × 2H2O and 0.2 g NaHCO3 dissolved in 1 l of distilled water) at a concentration of 1000 IJs ml−1 in a culture flask at 15 °C until use.
Insect rearing
We used a laboratory population of B. tryoni (HAC), established from flies collected on the Hawkesbury campus of Western Sydney University in Richmond, New South Wales (NSW), in summer 2009 (Morrow et al. 2015), and maintained in a glasshouse chamber at 25 °C and 70% RH as described previously (Langford et al. 2014). Eggs were collected from adult flies kept in cages (30 cm each side) by offering them a 120 ml oviposition cup filled with larval diet covered with perforated parafilm as described previously (Meats et al. 2004). The larval diet cups with eggs were then placed on a thin layer of sterile (autoclaved) washed sand contained within a container with fly mesh on top. Late third instar larvae left the larval diet cup for pupation on the sand layer. Larvae which had recently jumped from the larval diet cup (less than 4 h ago) and were still moving, and dark brown pupae (approximately three days old) were chosen for the laboratory assays.
Effect of temperature on EPN survival and virulence against fruit fly larvae
The effect of three temperatures (15 °C, 25 °C and 30 °C) on EPN survival and virulence against B. tryoni larvae was assessed using a sand plate assay. These temperatures include the temperature range of top soil in Australia throughout the year (Bureau of Meteorology), and a majority of sites previously assessed across southeastern Australia have been categorized with a thermic soil temperature regime (Watson 1980). Washed sand was autoclaved, oven dried and maintained at 10% moisture (w/w). Petri dishes (150 mm) were filled with 250 g of sand. Each EPN strain was tested as five replicates over time, with IJs for each replicate harvested from a different white trap. IJs were harvested from white traps, and, prior to inoculation of the sand, were stored in Ringer’s solution at 15 °C, 25 °C and 30 °C for two weeks. Then, solutions containing 50, 100, 200, 500 and 1000 IJs were prepared in 1 ml of Ringer’s solution and inoculated at the center of each plate. The control received 1 ml of Ringer’s solution without IJs. Immediately after EPN inoculation, 20 B. tryoni larvae were added to the center of each plate on top of the sand, and the larvae then dispersed across the plate. The plates were then incubated in the dark at the same temperature as prior inoculation. Insect mortality due to EPN infection was recorded after seven days, and EPN infection of individuals confirmed by dissection. The LD50 was calculated for each replicate to characterize the variation for each EPN isolate.
Effect of storage time at 25 °C on EPN survival and virulence against fruit fly larvae and pupae
The ability of EPNs to infect and kill B. tryoni larvae and pupae after one, two and three weeks at 25 °C and without the provision of an insect host during this time was assessed using another sand plate assay. Petri dishes (150 mm) were filled with 250 g sterile washed sand and maintained at 10% moisture as described earlier. Each EPN isolate was tested as five replicates over time, with IJs for each replicate harvested from a different white trap. EPN solutions containing 50, 100, 200, 500 and 1000 IJs in 1 ml of Ringer’s solution were inoculated at the center of the plates and incubated at 25 °C. After one, two and three weeks of storage, 20 B. tryoni larvae were added to the center of each plate (and larvae then dispersed across the plate), or 20 pupae were evenly distributed across each plate. The plates were then incubated at 25 °C. Insect mortality due to EPN infection was recorded after one week, and EPN infection of individuals confirmed by dissection. The LD50 was calculated for each replicate to characterize the variation for each EPN isolate.
Effect of temperature on IJ survival
Survival of IJs of the 17 EPN isolates was assessed in 24-well plates. For each isolate, 10,000 IJs in 2 ml Ringer’s solution were placed into each well and stored at 15 °C, 25 °C and 30 °C. Each isolate was tested as five replicates over time, with IJs for each replicate harvested from a different white trap. Dead nematodes were counted every week for 18 weeks or till 100% mortality was observed. Confirmation of IJ mortality was done by probing IJs with a fine needle to check for nematode movement (Kaya and Stock 1997). Before counting, the IJ solutions were homogenously mixed. Then, IJs contained in 100 µl were counted for each well. This was repeated three times to obtain the average mortality. The counted EPNs were placed back into their respective well and the volume was maintained at 2 ml after each count by adding additional Ringer’s solution.
Statistical analysis
The dose required to kill 50% of the insects (LD50) for each replicate was calculated and the averages were compared between different isolates (Strauch et al. 2004). The LD50 values were calculated by fitting a saturation model (mortality = a [1-ln(-bx)] + c) to the mortality data by minimization of the χ2 value for the comparison of the theoretical and observed distribution using Microsoft Excel Solver. In the model a is equal to the total number of insects corrected by the control mortality, b is the slope, c is the control mortality, and x is the dose used per insect. The data for the temperature effect on survival and virulence (LD50) were analysed for normality with the Shapiro–Wilk test. For normally distributed data, two-way ANOVA and Tukey's Honestly Significant Difference (HSD) test for multiple comparisons were performed. For non-normal distributed data (% survival), a Kruskal–Wallis test with a post-hoc Dunn’s test for multiple comparisons was used. All data analyses were performed in R version 4.0.2 (R Core Team 2020).
Results
Effect of temperature on EPN survival and virulence against B. tryoni larvae
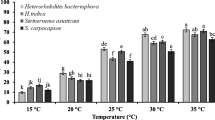
After two weeks in Ringer’s solution at 15 °C, 25 °C and 30 °C, all 17 EPN isolates were able to infect B. tryoni larvae at these temperatures (Fig. 1). The larval mortality caused by EPNs were significantly different among the temperatures (F2, 255 = 536.9; p < 0.0001), isolates (F16, 255 = 7.9; p < 0.0001) and their interaction (F32, 255 = 3.1; p < 0.0001). Across all isolates, the mean LD50 value at 15 °C was lower than at 25 and 30 °C and ranged from 34.56 IJs (± 4.6) to 62.44 (± 9.74) IJs per insect, 48.26 IJs (± 3.1) to 85.26 (± 4.86) IJs per insect, and 82.16 (± 12.54) to 149.92 IJs (± 19.79) IJs per insect, respectively. The lowest and highest LD50 were found for Hi.LMI2 and Hm.GOS1 at 15 °C, for Hz.NAR1 and Hm.ENCBF2 at 25 °C, and for Hz.NAR1 and Hm.ENCBF2 at 30 °C. The commercial strain Hz.EG was more virulent than Hm.GOS1 at 15 °C and 30 °C, and more virulent than Hm.ENCBF2 at 25 °C. In contrast, the commercial strain Sf.EG was less virulent than Hi.QF6, Hi.HRN2 and Hi.LMI2 at 15 °C, and less virulent than Hz.BB1, Hz.BB3, Hi.QF6 and Hz.NAR1 at 25 °C.
LD50 of EPN isolates (a) Heterorhabditis bacteriophora, (b) Heterorhabditis indica, (c) Heterorhabditis marelatus, (d) Heterorhabditis zealandica and (e) Steinernema feltiae tested against Bactrocera tryoni larvae after IJs had been kept for two weeks without insect hosts at 15 °C, 25 °C and 30 °C. Error bars indicate SD across five replicates. Different letters on top of the error bars indicate that means are significantly different from each other among all isolates and temperatures as per Tukey’s HSD test (p < 0.05). Asterisks on top of the braces denote statistically significant differences among EPN isolates at different temperatures (***p < 0.0001), and N.S stands for non-significant (p>0.05). Lower LD50 values correspond to higher virulence
IJ survival and virulence in sand against B. tryoni larvae and pupae
All EPN isolates were still able to infect and kill B. tryoni larvae after IJs had been kept at 25 °C without hosts for one, two and three weeks (Fig. 2), while 15 and two isolates still infected and killed B. tryoni pupae after one and two weeks, respectively (Fig. 3). However, no pupae were infected by EPN isolates after three weeks. Overall, the larval mortality caused by EPNs kept at 25 °C without hosts for one, two and three weeks was significantly different among the isolates (F16, 255 = 27.6; p < 0.0001), the time after EPN inoculation (F2, 255 = 778.7; p < 0.0001) and their interaction (F32, 255 = 4.6; p < 0.0001). The mean LD50 value against B. tryoni larvae ranged from 24.72 (± 3.29) to 70.82 (± 10.6) IJs per insect, 51.02 (± 4.57) to 117.24 (± 20.71) IJs per insect and 84.62 (± 12.45) to 144.02 (± 15.93) IJs per insect after IJs had been kept at 25 °C without hosts for one, two and three weeks, respectively.
LD50 of EPN isolates tested against Bactrocera tryoni larvae after (a) one week, (b) two weeks and (c) three weeks of incubation of IJs without insect hosts at 25 °C. Error bars indicate SD across five replicates. Different letters next to the error bars indicate that means are significantly different from each other as per Tukey’s HSD test (p < 0.05). Lower LD50 values correspond to higher virulence
LD50 of EPN isolates tested against Bactrocera tryoni pupae after (a) one week and (b) two weeks of incubation of IJs without insect hosts at 25 °C. IJs incubated for three weeks at 25 °C were not able to infect pupae (and are therefore not shown). Error bars indicate SD across five replicates. Different letters next to the error bars indicate that means are significantly different from each other as per Tukey’s HSD test (p < 0.05). Lower LD50 values resemble higher virulence
Furthermore, the pupal mortality caused by IJs of EPN isolates kept at 25 °C without hosts for different time periods was also significantly different among isolates (F16, 255 = 38.2; p < 0.0001), the time after EPN inoculation (F2, 255 = 1220.1; p < 0.0001) and their interaction (F32, 255 = 30.78; p < 0.0001). The pupal mortality caused by EPN isolates after one week without hosts at 25 °C ranged from 129.64 (± 16.5) to 209.28 (± 46.49) IJs per insect. Two EPN isolates, Hz.NAR1 (LD50 228.82 ± 27.8 IJs per insect) and Hi.ECCH (LD50 209.42 ± 20.48 IJs per insect), killed pupae after two weeks without hosts at 25 °C. The Hb.HIE isolate was more virulent than both commercial strains after all three time periods without hosts at 25 °C.
Long-term survival of IJs in Ringer’s solution
Temperature had a significant effect on the survival of tested EPN isolates (Fig. 4, Table 2). All isolates survived without hosts for up to 16 weeks at 15 and 25 °C, and nine weeks at 30 °C. The S. feltiae isolates had the highest survival and were the only species to survive ≥ 18 weeks at 15 °C and 25 °C and nine weeks at 30 °C. Among the Steinernema isolates, Sf.ECCS had the highest survival with over 17 weeks at 15 °C (54.28 ± 5.26) and 25 °C (56.6 ± 7.5), and with over eight weeks at 30 °C (11.78 ± 2.91). Among the Heterorhabditis isolates, Hi.ECCH had the highest survival, with up to 18 weeks at 15 °C (8.28 ± 5.07) and 25 °C (14.2 ± 1.92), and Hz.BB1, with up to nine weeks at 30 °C (15.2 ± 4.64).
Survival (%) of the EPN isolates of (a) Heterorhabditis bacteriophora, (b-g) Heterorhabditis indica, (h-i) Heterorhabditis marelatus, (j-m) Heterorhabditis zealandica and (n-q) Steinernema feltiae at 15 °C (black filled squares), 25 °C (grey filled squares) and 30 °C (open squares) across time. The name and origin of the isolates are listed in Table 1. Error bars indicate SD across five replicates
Discussion
We found that IJs of Australian EPN isolates can survive without hosts and remain virulent at different temperatures for a considerable time. This situation can commonly arise in EPN applications, but also naturally in the soil. Specifically, IJs of all isolates survived and remained virulent after two weeks without a host at all temperatures (15 °C, 25 °C and 30 °C). Furthermore, at 25 °C IJs survived for three weeks without a host to still infect larvae, and two weeks to still infect pupae. In the absence of hosts, we recorded higher IJ survival rates at lower temperatures, indicating that IJs may be better able to preserve energy stores at the lower temperatures. Similarly, IJs survived without hosts for a maximum of nine weeks at 30 °C and up to 18 weeks at 15 °C and 25 °C. After IJs had been kept at 15 °C for two weeks without hosts and then provided with B. tryoni larvae, the H. indica isolates were the most virulent followed by the H. zealandica isolates. At 25 °C, the H. zealandica isolates were the most virulent followed by the H. indica isolates, and at 30 °C, the H. zealandica isolates were the most virulent followed by the H. bacteriophora isolates. The H. marelatus isolates were the least virulent at all temperatures. After they had been kept without hosts for two weeks at the three temperatures, IJs of all isolates were able to infect B. tryoni larvae, but virulence was highest after they had been kept at 15 °C, whereas there was a reduction in virulence with increase in storage temperature. All isolates were still able to infect B. tryoni larvae after IJs had been kept at 25 °C for one, two and three weeks prior to the provision of hosts. However, we found higher infection rates after one week than two and three weeks.
Our results demonstrated that EPNs stored at lower temperatures and for shorter time periods showed higher survival and virulence against B. tryoni. Therefore, storage temperature and time prior to experiments can affect the virulence of EPN strains. Previous studies found that ageing of EPNs reduced the ability to locate and penetrate insect hosts, thereby reducing EPN infectivity and virulence (Yoder et al. 2004; Lee et al. 2016; Alonso et al. 2018). Furthermore, the EPN isolates tested in our study had been collected from warm-temperate and subtropical regions of eastern Australia, and, therefore, we expected them to be well adapted to these climatic conditions. Irrespective of this, and based on our study, we can imply that EPN isolates originating from warmer regions survive well at cool temperatures and can still infect hosts after a considerable time. A similar result was obtained by El Khoury et al. (2018) who recorded higher mortality of G. mellonella caused by S. feltiae and H. bacteriophora at 15 °C and 20 °C than at temperatures of up to 35 °C. An important contributing factor may be lipid reserves which are the EPNs’ sole energy source until they find a new host (Patel et al. 1997; Andaló et al. 2011). Higher temperatures might result in increased physiological activity leading to consumption of stored energy resulting in restricted movement and mortality of EPNs in absence of host resources (Smits, 1996; Hass et al. 2002). Conversely, in the presence of host, many studies reported increased EPN infectivity at higher temperatures (Yul et al. 2002; Hussaini et al. 2005; Rohde et al. 2010). For example, Rohde et al. (2010) observed an increase in EPN-caused mortality of C. capitata with increases in temperature, with highest mortality seen at 31 °C and lowest mortality at 19 °C. Similarly, Kepenekci et al. (2015) found that temperature had a significant effect on EPN virulence, with higher virulence at higher temperatures. Previous reports suggested that higher temperature not only affects virulence, but also progeny development. Hazir et al. (2001) recorded 100% mortality of G. mellonella at temperatures between 8 and 28 °C but no progeny was found at 28 °C, even with tropical S. feltiae isolates. Similarly, Lankin et al. (2020) found absolute mortality of the potato cutworm (Agrotis deprivata) due to S. feltiae at 28 °C, but no EPN progeny was obtained.
Control of pests in the soil can be challenging, thus EPN isolates with ability to survive for longer periods in the soil are better biocontrol agent candidates. In our study EPNs were still able to kill B. tryoni larvae after three weeks at 25 °C without host availability, and some isolates were able to still kill pupae after two weeks at 25 °C without host availability. In contrast, many previous studies reported no pupal infection with EPNs (Yee and Lacey, 2003; Langford et al. 2014), while other studies found lower infection rates in pupae than larvae (Aryal et al. 2022b). Several studies have been carried out to find EPN isolates with better persistence in soil after field application. Blatt et al. (2021) showed that S. feltiae and H. bacteriophora survived and remained infective in soil without irrigation for nine weeks. We found that the survival rate of Steinernema was higher than that of Heterorhabditis, and this corroborates previous studies (Abate et al. 2019). The higher survival rate of Steinernema might be the result of higher lipid reserves in this species when compared to Heterorhabditis (Selvan et al. 1993). Morever, EPNs can persist for up to 150 days (21.4 weeks) in the field, with higher persistence of H. bacteriophora than S. feltiae (Şahin and Gözel, 2021). Furthermore, two years after EPN application, Harvey and Griffin (2016) recovered S. carpocapsae from soil and underneath the bark of EPN treated tree stumps, and EPN presence in treated tree stumps was correlated with the number of insect hosts emerging from untreated tree stumps. This indicates the importance of host availability for EPN persistence in ecosystems.
EPN isolates may be adapted to the environmental conditions at their original location of isolation, and, in those environments, may be more virulent compared to introduced or commercial isolates (Abate et al. 2019). As the efficacy of EPNs can depend on several abiotic and biotic factors (including EPN species and target pest species), proper bioassays are important, in particular ones that consider the specific life cycle aspects of the target pest in the soil. EPN persistence can also be improved by managing the soil environment to be more suitable for EPN survival and dispersal. For example maintaining a neutral to acidic soil pH, adequate soil moisture and increasing soil organic matter can improve EPN persistence (Campos-Herrera et al. 2019). Besides environmental factors, species interactions with soil bacteria, fungi, mites and collembolans can also be important as several of these can act antagonistically and cause mortality in EPNs (Karthik Raja et al. 2020). Moreover, to improve the survival and persistence of EPNs after application, application of EPN-infected cadavers in place of aqueous suspension as suggested by Gulzar et al. (2020) may be useful. Furthermore, adult fruit flies could also play an important role for EPN dispersal in the field as indicated by Garriga et al. (2020) who found that around 21% of EPN infected adults of Drosophila suzukii could fly, therefore aiding EPN dispersal. Hence, more detailed research on the role of adult flies for the dispersal and persistence of EPNs in the field will be important.
In conclusion, high temperature in the absence of hosts significantly reduced the survival and virulence of EPNs against B. tryoni, and EPN virulence was higher in larvae than in pupae. Overall, based on our research, H. indica (Hi.HRN2, Hi.LMI2, Hi.QF6), H. zealandica (Hz.NAR1), H. bacteriophora (Hb.HIE) and S. feltiae (Sf.ECCS) are potential candidates for the control of B. tryoni. Next, the best performing isolates should be evaluated in their efficacy against B. tryoni under semi-field and field conditions, as well as their potential for large scale production, before the development of cost-effective application strategies can begin.
Data availability
All data is contained within the manuscript.
References
Abate BA, Slippers B, Wingfield MJ, Conlong DE, Burger DA, Hurley BP (2019) Virulence and survival of native entomopathogenic nematodes for the management of white grubs in South Africa. Biol Control 137:104043
Alonso V, Nasrolahi S, Dillman AR (2018) Host-specific activation of entomopathogenic nematode infective juveniles. Insects 9:59
Andaló V, Moino A Jr, Cleber M, Campos VP, Mendonça LA (2011) Influence of temperature and duration of storage on the lipid reserves of entomopathogenic nematodes. Rev Colomb Entomol 37:203–209
Aryal S, Nielsen UN, Sumaya NH, De Faveri S, Wilson C, Riegler M (2022a) Isolation and molecular characterization of five entomopathogenic nematode species and their bacterial symbionts from eastern Australia. BioControl 67:63–74
Aryal S, Nielsen UN, Sumaya NH, Wilson C, Riegler M (2022b) Virulence, penetration rate and reproductive potential of entomopathogenic nematodes from eastern Australia in Queensland fruit fly Bactrocera Tryoni. Biol Control 169:104871
Bateman M (1972) The ecology of fruit flies. Annu Rev Entomol 17:493–518
Bedding RA, Akhurst RJ, Kaya HK (1993) Nematodes and the biological control of insect pests. CSIRO Publishing, Australia
Blatt S, McDonald MR, Mlynarek J (2021) Entomopathogenic nematodes for control of carrot weevil: efficacy and longevity in muck and mineral soils. Pest Manag Sci 77:2433–2443
Campos-Herrera R, Blanco-Pérez R, Bueno-Pallero FÁ, Duarte A, Nolasco G, Sommer RJ, Martín JAR (2019) Vegetation drives assemblages of entomopathogenic nematodes and other soil organisms: evidence from the Algarve, Portugal. Soil Biol and Biochem 128:150–163
Clarke AR (2019) Biology and management of Bactrocera and related fruit flies. CABI Publishing, Wallingford, UK
Clarke AR, Powell KS, Weldon CW, Taylor PW (2011) The ecology of Bactrocera tryoni (Diptera: Tephritidae): what do we know to assist pest management? Ann Appl Biol 158:26–54
El Khoury YE, Oreste M, Noujeim E, Nemer N, Tarasco E (2018) Effect of temperature on the pathogenicity of Mediterranean native entomopathogenic nematodes (Steinernematidae and Heterorhabditidae) from natural ecosystems. Redia 101:123–127
Fitters PFL, Griffin C (2004) Spontaneous and induced activity of Heterorhabditis megidis infective juveniles during storage. Nematology 6:911–917
Garriga A, Morton A, Ribes A, Garcia-del-Pino F (2020) Soil emergence of Drosophila suzukii adults: a susceptible period for entomopathogenic nematodes infection. J Pest Sci 93:639–646
Georgis R, Gaugler R (1991) Predictability in biological control using entomopathogenic nematodes. J Econ Entomol 84:713–720
Godjo A, Zadji L, Decraemer W, Willems A, Afouda L (2018) Pathogenicity of indigenous entomopathogenic nematodes from Benin against mango fruit fly (Bactrocera dorsalis) under laboratory conditions. Biol Control 117:68–77
Grewal PS, Selvan S, Gaugler R (1994) Nematodes: niche breadth for infection, establishment, and reproduction. J Therm Biol 19:245–253
Grewal P, Matsuura M, Converse V (1997) Mechanisms of specificity of association between the nematode Steinernema scapterisci and its symbiotic bacterium. Parasitol 114:483–488
Griffin CT (1993) Temperature responses of entomopathogenic nematodes: implications for the success of biological control programmes. In: Bedding R, Akhurst R, Kaya H (eds) Nematodes and the biological control of insect pests. CSIRO Publishing, Australia, pp 115–126
Gulzar S, Usman M, Wakil W, Gulcu B, Hazir C, Karagoz M, Hazir S, Shapiro-Ilan DI (2020) Environmental tolerance of entomopathogenic nematodes differs among nematodes arising from host cadavers versus aqueous suspension. J Invertebr Pathol 175:107452
Harvey CD, Griffin CT (2016) Local host-dependent persistence of the entomopathogenic nematode Steinernema carpocapsae used to control the large pine weevil Hylobius abietis. BioControl 61:185–193
Hass B, Downes M, Griffin C (2002) Persistence of four Heterorhabditis spp. isolates in soil: role of lipid reserves. J Nematol 34:151–158
Hazir S, Stock SP, Kaya HK, Koppenhöfer AM, Keskin N (2001) Developmental temperature effects on five geographic isolates of the entomopathogenic nematode Steinernema feltiae (Nematoda: Steinernematidae). J Invertebr Pathol 77:243–250
Hulthen AD, Clarke AR (2006) The influence of soil type and moisture on pupal survival of Bactrocera tryoni (Froggatt) (Diptera: Tephritidae). Aust J Entomol 45:16–19
Hussaini S, Shakeela V, Dar M (2005) Influence of temperature on infectivity of entomopathogenic nematodes against black cutworm, Agrotis ipsilon (Hufnagel) and greater wax moth, Galleria mellonella (Linnaeus) larvae. J Biol Control 19:51–57
Kamali S, Karimi J, Hosseini M, Campos-Herrera R, Duncan LW (2013) Biocontrol potential of the entomopathogenic nematodes Heterorhabditis bacteriophora and Steinernema carpocapsae on cucurbit fly, Dacus ciliatus (Diptera: Tephritidae). Biocontrol Sci Technol 23:1307–1323
Karthik Raja R, Arun A, Touray M, Gulsen SH, Cimen H, Gulcu B, Hazir C, Aiswarya D, Ulug D, Cakmak I (2020) Antagonists and defense mechanisms of entomopathogenic nematodes and their mutualistic bacteria. Biol Control 152:104452
Kaya HK, Gaugler R (1993) Entomopathogenic nematodes. Annu Rev Entomol 38:181–206
Kaya HK, Stock SP (1997) Techniques in insect nematology. In: Lacey LA (ed) Manual of techniques in insect pathology. Academic Press, pp 281–324
Kepenekci I, Hazir S, Özdem A (2015) Evaluation of native entomopathogenic nematodes for the control of the European cherry fruit fly Rhagoletis cerasi L. (Diptera: Tephritidae) larvae in soil. Turk J Agric for 39:74–79
Koppenhöfer AM, Fuzy EM (2007) Soil moisture effects on infectivity and persistence of the entomopathogenic nematodes Steinernema scarabaei, S. glaseri, Heterorhabditis zealandica, and H. bacteriophora. Appl Soil Ecol 35:128–139
Kung S-P, Gaugler R, Kaya HK (1990a) Influence of soil pH and oxygen on persistence of Steinernema spp. J Nematol 22:440–445
Kung S-P, Gaugler R, Kaya HK (1990b) Soil type and entomopathogenic nematode persistence. J Invertebr Pathol 55:401–406
Kurtz B, Toepfer S, Ehlers RU, Kuhlmann U (2007) Assessment of establishment and persistence of entomopathogenic nematodes for biological control of western corn rootworm. J Appl Entomol 131:420–425
Langford EA, Nielsen UN, Johnson SN, Riegler M (2014) Susceptibility of Queensland fruit fly, Bactrocera tryoni (Froggatt) (Diptera: Tephritidae), to entomopathogenic nematodes. Biol Control 69:34–39
Lankin G, Castaneda-Alvarez C, Vidal-Retes G, Aballay E (2020) Biological control of the potato cutworm Agrotis deprivata (Lepidoptera: Noctuidae) with Steinernema feltiae LR (Nematoda: Steinernematidae): Influence of the temperature, host developmental stage, and application mode on its survival and infectivity. Biol Control 144:104219
Lee JH, Dillman AR, Hallem EA (2016) Temperature-dependent changes in the host-seeking behaviors of parasitic nematodes. BMC Biol 14:36
Meats A, Holmes H, Kelly G (2004) Laboratory adaptation of Bactrocera tryoni (Diptera: Tephritidae) decreases mating age and increases protein consumption and number of eggs produced per milligram of protein. Bull Entomol Res 94:517–524
Morrow JL, Frommer M, Shearman DC, Riegler M (2015) The microbiome of field-caught and laboratory-adapted Australian tephritid fruit fly species with different host plant use and specialisation. Microb Ecol 70:498–508
Patel M, Stolinski M, Wright D (1997) Neutral lipids and the assessment of infectivity in entomopathogenic nematodes: observations on four Steinernema species. Parasitol 114:489–496
Popa-Báez Á-D, Lee SF, Yeap HL, Prasad SS, Schiffer M, Mourant RG, Castro-Vargas C, Edwards OR, Taylor PW, Oakeshott JG (2020) Climate stress resistance in male Queensland fruit fly varies among populations of diverse geographic origins and changes during domestication. BMC Genet 21:135
R Core Team (2020) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.r-project.org/
Rohde C, Moino A Jr, da Silva MA, Carvalho FD, Ferreira CS (2010) Influence of soil temperature and moisture on the infectivity of entomopathogenic nematodes (Rhabditida: Heterorhabditidae, Steinernematidae) against larvae of Ceratitis capitata (Wiedemann) (Diptera: Tephritidae). Neotrop Entomol 39:608–611
Şahin Ç, Gözel U (2021) Efficacy and persistence of native entomopathogenic nematodes against Capnodis tenebrionis in peach (Prunus persica) orchard in Turkey. Phytoparasitica 49:613–621
Selvan S, Gaugler R, Lewis EE (1993) Biochemical energy reserves of entomopathogenic nematodes. J Parasitol 79:167–172
Sirjani FO, Lewis EE, Kaya HK (2009) Evaluation of entomopathogenic nematodes against the olive fruit fly, Bactrocera oleae (Diptera: Tephritidae). Biol Control 48:274–280
Smits PH (1996) Post-application persistence of entomopathogenic nematodes. Biocontrol Sci Technol 6:379–388
Strauch O, Oestergaard J, Hollmer S, Ehlers R-U (2004) Genetic improvement of the desiccation tolerance of the entomopathogenic nematode Heterorhabditis bacteriophora through selective breeding. Biol Control 31:218–226
Strong DR (2002) Populations of entomopathogenic nematodes in foodwebs. In: Gaugler R (ed) Entomopathogenic nematology. CABI Publishing, Wallingford, UK, pp 225–240
Susurluk A, Ehlers R-U (2008) Field persistence of the entomopathogenic nematode Heterorhabditis bacteriophora in different crops. BioControl 53:627–641
Tailliez P, Laroui C, Ginibre N, Paule A, Pagès S, Boemare N (2010) Phylogeny of Photorhabdus and Xenorhabdus based on universally conserved protein-coding sequences and implications for the taxonomy of these two genera. Proposal of new taxa: X. vietnamensis sp. nov., P. luminescens subsp. caribbeanensis subsp. nov., P. luminescens subsp. hainanensis subsp. nov., P. temperata subsp. khanii subsp. nov., P. temperata subsp. tasmaniensis subsp. nov., and the reclassification of P. luminescens subsp. thracensis as P. temperata subsp. thracensis comb. nov. Int J Syst Evol Microbiol 60:1921–1937
Tasnin MS, Bode M, Merkel K, Clarke AR (2021) A polyphagous, tropical insect herbivore shows strong seasonality in age-structure and longevity independent of temperature and host availability. Sci Rep 11:11410
Waterfield NR, Ciche T, Clarke D (2009) Photorhabdus and a host of hosts. Annu Rev Microbiol 63:557–574
Watson C (1980) Seasonal soil temperature regimes in South-Eastern Australia. Soil Res 18:325–331
White IM, Elson-Harris MM (1992) Fruit flies of economic significance: their identification and bionomics. CABI Publishing, Wallingford UK
Yee WL, Lacey LA (2003) Stage-specific mortality of Rhagoletis indifferens (Diptera: Tephritidae) exposed to three species of Steinernema nematodes. Biol Control 27:349–356
Yoder CA, Grewal P, Taylor RAJ (2004) Rapid age-related changes in infection behavior of entomopathogenic nematodes. J Parasitol 90:1229–1234
Yul C, Wonn L, Sook Y, Myeong L, Thi H (2002) Effects of temperature and nematodes concentration on pathogenicity and reproduction of entomopathogenic nematode, Steinernema carpocapsae Pocheon strain (Nematode: Steinernematidae). Korean J Appl Entomol 41:269–277
Acknowledgements
We thank Geraldine Tilden for technical support with fruit fly rearing.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. This research was supported by the Australian Research Council Industrial Transformation Training Centre (ARC-ITTC) Fruit Fly Biosecurity Innovation (IC150100026), with a PhD scholarship to SA, and the Department of Agriculture, Water and the Environment’s Strengthening Australia’s Fruit Fly System Research Program; project: A national biocontrol program to manage pest fruit flies in Australia (4-EKSH327).
Author information
Authors and Affiliations
Contributions
SA, MR and UNN conceptualized and designed the experimental work. SA and MR collected material. CW provided additional material. SA performed the experiments, collected and analyzed the data, under guidance of MR and UNN, and advice of NHS and CW. MR was responsible for research funding. SA wrote the manuscript together with MR and UNN and with input of all other authors. All authors agree with the submission of the manuscript.
Corresponding authors
Ethics declarations
Conflicts of interest
The authors do not have a conflict of interest, except that CW works at Ecogrow, a company that supplied two EPN isolates for this study.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aryal, S., Nielsen, U.N., Sumaya, N.H. et al. Effect of temperature on survival of Australian entomopathogenic nematodes and their virulence against the Queensland fruit fly, Bactrocera tryoni. BioControl 67, 617–628 (2022). https://doi.org/10.1007/s10526-022-10166-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-022-10166-2