Abstract
The alfalfa weevil (Hypera postica Gyllenhal, Coleoptera: Curculionidae) is a major pest of alfalfa crops. Chemical control measures are inefficient, but the larvae are often infested by parasitoid wasps of the genus Bathyplectes Förster (Hymenoptera: Ichneumonidae), which offer a potential biological control strategy. The development of effective biological control requires the identification of parasitoid species, but conventional methods involve the rearing of parasitoids to the puparium stage for morphological confirmation. Here we designed a PCR method in which two pairs of primers are used to detect and identify Bathyplectes curculionis Thomson and Bathyplectes anura Thomson larvae in a faster way. We compared conventional rearing to the new method as a means to determine the parasitism rates caused by each species in Spain during the 2019 and 2020 seasons. In 2019, the PCR method detected five times as many B. curculionis events and twice as many B. anura events. Similarly, in 2020, the PCR method detected seven times as many B. curculionis events and twice as many B. anura events. High mortality of H. postica larvae was recorded in 2020 due to an epizootic attack by the fungus Zoophthora phytonomi Arthur (Zygomycetes: Entomophthorales), explaining the lower overall rate of parasitism and the widespread death of the larvae. Statistical analysis confirmed that the PCR method is more sensitive than conventional rearing for the detection of target parasitoids. However, only rearing can confirm the effective parasitism or the presence of unexpected species. We therefore recommend the use of both methods in parallel when evaluating host–parasitoid systems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alfalfa (Medicago sativa L.) is the most valuable cultivated forage crop in the world (Orloff 1997). In Spain, it is a traditional component of crop rotations covering 250,000 ha, accounting for ~ 20% of the alfalfa land area in Europe (Delgado and Lloveras 2020). Alfalfa is also an important reservoir for pest insects that infest alfalfa as well as surrounding crops (Pons and Nuñez 2020; Madeira et al. 2022).
The alfalfa weevil (Hypera postica Gyllenhal; Coleoptera: Curculionidae) is native to Eurasia (Hoffmann 1963) but has spread globally and is now one of the most destructive alfalfa pests (Goosey 2012; Hoff et al. 2002; Pons and Nuñez 2020; Saeidi and Moharramipour 2017; Soroka et al. 2019). In Spain, the weevil larvae cause serious damage to the first cutting (from March to the end of April). There is little information in the scientific literature about the ecology and pest status of this species in Europe. Natural enemies can reduce alfalfa weevil populations (Soroka et al. 2020). Alfalfa weevil larvae are parasitized by solitary endoparasitoid wasps of the genus Bathyplectes (Hymenoptera: Ichneumonidae) which is native to the Old World (Kingsley et al. 1993; Kuhar et al. 1999; Radcliffe and Flanders 1998). B. anura and B. curculionis were introduced to North America with remarkable success as a weevil control strategy (Radcliffe and Flanders 1998; Rand 2013). Although eight Bathyplectes species have been recorded in Spain (Ribes 2012), only B. anura Thomson and B. curculionis Thomson are associated with alfalfa. The identification of adult wasps is challenging because there are only slight morphological differences between species (Pons and Nuñez 2020), especially in the male (Soroka et al. 2020). The puparia are easier to distinguish because B. anura forms a hard, dark-brown puparium with a narrow, raised, white horizontal band, whereas the B. curculionis puparium is light brown with a flat, diffuse, white horizontal band (Day 1970; Dysart and Day 1976). For definitive parasitoid identification, each H. postica larva must therefore be reared until pupation, which requires optimal environmental conditions and feeding, and the avoidance of other natural factors that cause mortality. Using this approach, the rates of parasitism recorded in Spain are generally low but highly variable (Levi-Mourao et al. 2021; Pons & Nuñez 2020).
In contrast to the delayed results from rearing experiments, DNA analysis allows parasitism to be followed in real time, and does not require the use of captive insects in controlled-environment chambers (Liang et al. 2015, 2018; Wolf et al. 2018; Agustí et al. 2020; Molina et al. 2021). The high sensitivity and fidelity of molecular methods also facilitate detailed studies of trophic interactions that are otherwise inaccessible (Traugott et al. 2013). Such methods require the development of specific molecular probes to detect target organisms. In arthropods, the evolution of mitochondrial genes has been studied in detail, and divergent sequences in related populations provide a source of species-specific PCR primers (Black et al. 1989; Simon et al. 1994). Several studies have used cytochrome C oxidase subunit I (COI) mitochondrial DNA fragments as targets to increase the specificity of detection (Agustí et al. 2003a, 2005). Here we report for the first time the development of COI primers to detect and identify the main parasitoids of alfalfa weevil (B. curculionis and B. anura) in order to estimate the rate of parasitism in H. postica larvae compared to the classical rearing method.
Materials and methods
Insect rearing
Adult specimens of H. postica were collected from a commercial alfalfa field in Lleida, North-East Spain, in spring 2018. They were reared in 2000-ml glass jars covered with mousseline for proper ventilation, and kept at 20 °C, 60–70% relative humidity with an 8–16 (L:D) photoperiod. Fresh alfalfa stems were provided daily for egg laying and feeding. These were placed in a glass vial filled with water and sealed with parafilm to prevent dehydration of the plants and the drowning of adult insects. All non-parasitized H. postica larvae individuals used in the experiments were derived from this laboratory population.
For B. anura and B. curculionis adults obtaining, H. postica larvae were collected from the field. They were kept in rearing polyethylene cages of 500 ml capacity (maximum 50 larvae/cage), covered by a mesh to facilitate aeration. Fresh alfalfa was provided in a daily basis. Larvae were maintained in a climatic chamber at 22 °C, 8:16 (L:D) photoperiod and 50% relative humidity until pupation. In the case they were parasitized, parasitoid puparia were used for morphological identification. The resulting B. anura and B. curculionis adults were frozen at –80 °C for posterior DNA extraction.
Primer design and testing
Fragments of the conserved COI gene from 10 individuals of B. anura and B. curculionis and the host H. postica were amplified by PCR using the universal forward primer C1-J-1718 (5′-GGA GGA TTT GGA AAT TGA TTA GTT CC-3′) and the universal reverse primer C1-N-2191 (5′-CCC GGT AAA ATT AAA ATA TAA ACT TC-3′) (Simon et al. 1994). Each 20-µl amplification reaction contained 1 µl (3–50 ng/µl) resuspended individual DNA, 7.5 µl pure water, 9.5 µl 2.0 Taq RED Master Mix kit, 1.5 mM MgCl2 (Apex Bioresearch, Genesee Scientific) and 1 µl of each forward and reverse primer (10 µM). Samples were denatured at 94 °C for 3 min followed by 35 cycles of 94 °C for 30 s, 58 °C (for 30 s, and 72 °C for 1 min, and then a final extension at 72 °C for 3 min. The parasitoid amplicons were purified using the QIAquick PCR Purification Kit (Qiagen, Düsseldorf, Germany) and were transferred to the vector pGEM-T easy. Escherichia coli DH5α Scompetent cells were transformed with the vector using an adapted heat shock method (Froger and Hall 2007) and cultivated in lysogeny broth (LB) supplemented with 1 mg/ml ampicillin. Transformants were plated on LB agar supplemented with 20 mg/ml 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-gal) and 80 mg/ml isopropyl β-d-1-thiogalactopyranoside (IPTG) for blue-white selection. We transferred 50 white colonies, indicating the presence of an insert to 7 ml LB supplemented with 100 µg/ml ampicillin. Plasmid DNA was extracted for PCR with the primers shown above. Thirty DNA samples yielding the anticipated band sizes for B. anura (442 bp) and B. curculionis (465 bp) were analyzed by Sanger sequencing (Stab vida, Portugal) using SP6 forward primer 5′-ATT TAG GTG ACA CTA TAG-3′ and M13 reverse primer 5′-CAG GAA ACA GCT ATG AC-3′. Sequences were aligned in UGENE for each construct and non-matching nucleotides between sequences were used to design specific primers (following Innis and Gelfand 1990 and Saiki 1990).
DNA was extracted from homogenized non-parasitized H. postica larvae and individual field parasitoid adults from each species using the BioSprint 96 DNA Blood Kit (Qiagen) and the samples were stored at− 20 °C. Each 20-µl reaction contained 1 µl (3–50 ng/µl) resuspended larval DNA, 7.5 µl pure water, 9.5 µl 2.0 Taq RED Master Mix kit, 1.5 mM MgCl2 (Apex Bioresearch, Genesee Scientific) and 1 µl of each newly forward and reverse primer (10 µM). Samples were denatured at 94 °C for 3 min followed by 35 cycles of 94 °C for 30 s, 65 °C (for B. curculionis) or 60 °C (for B. anura) for 30 s, and 72 °C for 1 min, and then a final extension at 72 °C for 5 min. PCR products were separated by 2.0% agarose gel electrophoresis and stained with SYBR safe (Thermo Fisher Scientific, Waltham, MA, USA). We have tested 10 individuals of each species following this protocol.
Sensitivity and primer species specificity
Sensitivity of the assay was determined for both primer pairs by testing serial dilutions of parasitoid DNA. The original parasitoid DNA samples (~ 3 ng/µl) were serially diluted to 600, 120, 24, 4.8, 0.9 and 0.19 pg/µl. The specificity of each pair of primers was tested on 10 B. anura and B. curculionis individuals and on 10 H. postica larvae as negative controls. 10 Microctonus sp. Wesmael (Hymenoptera: Braconidae), an endoparasitoid of H. postica adults, were also tested. DNA from seven random H. postica larvae was mixed with B. anura and B. curculionis DNA to ensure efficiently detection. Products were detected using a NanoDrop spectrophotometer (Thermo Fisher Scientific).
Field samples
Samples were collected in the Ebro basin region (north-east Spain), where 60% of Spanish alfalfa is cultivated (Delgado and Lloveras 2020). We selected 35 commercial fields located along four different counties (1-Urgell, 2-Segrià, 3-Baja Cinca and 4-Monegros) during the 2019 and 2020 seasons. All fields were insecticide-free during the study period. Larvae were collected by 20 sweeps, each of 180°, with a 38-cm diameter net (Bio-quip, Rancho Dominguez, CA USA) and were separated into three groups over the next 24 h.
Field rates of parasitism detected by classical rearing
The first set of 20–300 larvae per field (L3–L4) were kept in 500-ml polyethylene cages (maximum 50 larvae per cage) and provided with fresh alfalfa every 2 days. Cages were maintained in a climatic chamber at 22° C, 8:16 (light: dark) photoperiod and 50% relative humidity until pupation. We counted H. postica pupae, Bathyplectes puparia and dead larvae on a daily basis, and removed them from the cage. Bathyplectes anura and B. curculionis were distinguished by the characteristics of the puparia. We also recorded (1) the total number of H. postica adults that emerged; (2) the number of larvae showing symptoms of infection by Z. phytonomi (brown or black color and soft body) according to Los and Allen (1982); and (3) the number of parasitoid adults that emerged from puparia. These puparia were kept under laboratory conditions (24 ± 3 °C) for 12 months. The rate of parasitism and the effective rate (percentage of parasitoid adults that emerged among field parasitized reared H. postica larvae) by each species were calculated.
Field rates of parasitism detected by molecular analysis
A second set of 50 larvae per field (L3–L4) was immediately frozen at − 80 °C and used to estimate the rate of parasitism by PCR with the newly designed primers. A third set of 150 larvae in total was used to detect parasitoid DNA in different larval instars. We therefore collected a similar number of L2, L3 and L4 larvae, which were immediately frozen at − 80 °C. Larvae from both sets were 3% bleach rinsed and stored in individual Eppendorf before frozen. Individual larvae were squashed prior to extraction using sterile pestles. Total DNA was extracted from each larva using the DNeasy 96 Blood Kit (Qiagen). PCR was carried out to detect the presence of B. curculionis and/or B. anura as described above, with three technical replicates per sample. Products were separated by 2% agarose electrophoresis, and analyzed using a ChemiDoc transilluminator (Bio-Rad Laboratories, Hercules, CA, USA). Samples with the anticipated band sizes were recorded as positives. Parasitism rates were calculated by dividing the total number of individuals with anticipated bands by the total number of samples.
Data analysis
The Shapiro–Wilk test was used to determine whether or not the original data was normally distributed. If not, data were arcsin-transformed before analysis. To evaluate differences between results from both methods (classical and molecular), in the case of normally distributed data, a t-test was performed. For data that did not satisfy the condition of normality required for parametric tests, a Wilcoxon signed rank test was used. The same analysis was performed to compare the rate of parasitism within each larval instar between B. anura and B. curculionis. Statistical analysis was carried out using JMP Pro15 (SAS Institute, Cary, NC, USA).
Results
Design of COI primers specific for B. anura and B. curculionis
COI DNA fragments were successfully amplified from total B. anura (OM641987), B. curculionis (OL413497) and H. postica (OL413498) DNA. The fragments were cloned, and the corresponding sequences were confirmed against GenBank. The sequences were aligned in UGENE to identify non-matching bases suitable for the design of unique primers (Table 1). Accordingly, the Bcfw3 forward primer and Bcrv3 reverse primer were designed to amplify a COI fragment specific for B. curculionis, whereas the 2Bafw2 forward primer and 2Barv2 reverse primer were designed to amplify a COI fragment specific for B. anura (Fig. 1).
Analysis of COI amplicons by 2% agarose gel electrophoresis. The primers specific for B. curculionis (Bcfw3 and Bcrv3) generate a 422-bp product. The primers specific for B. anura (2Bafw2 and 2Barv2) generate a 448-bp product. The left-hand lane in both gels shows 100-bp size markers. Bc , Bathyplectes curculionis; Ba , Bathyplectes anura; Hp, Hypera postica negative control; Msp, Microctonus sp. and NT , no template control (without DNA)
Sensitivity and primer species specificity
Sensitivity of the new PCR method was tested on 1:5 serial dilutions of DNA from each parasitoid species. Successful amplification was achieved using as little as 3.2 pg of B. curculionis DNA or 15 pg of B. anura DNA, whereas no amplification products were detected when using H. postica or Microctonus sp. DNA. We then mixed DNA from seven random H. postica samples with B. curculionis or B. anura DNA, and found that the parasitoid DNA was still detected efficiently even with a large excess of H. postica DNA.
Rates of parasitism detected by classical rearing
The total mortality of H. postica larvae varied during the 2019 and 2020 seasons. In 2019, mortality ranged from 0% to almost 30% due to unknown causes, whereas in 2020 up to 100% of the H. postica larvae in some fields were killed by the entomopathogenic fungus Zoophthora phytonomi (Arthur) (Zygomycetes: Entomophthorales) (Table 2). Yearly parasitism rates per field ranged from less than 2% to more than 30%, with mean values of 15.4 ± 2.3% in 2019 and 5.1 ± 1.6% in 2020 (Table 2). The parasitism rate by B. anura was higher in 2019 (12.0 ± 2.6%) than 2020 (4.2 ± 1.3%). Similarly, the parasitism rate by B. curculionis was higher in 2019 (3.4 ± 1.6%) than 2020 (0.9 ± 0.3%). In one field during 2019, the parasitism rate exceeded 17 ± 2.6% (Table 2a). The effective parasitism rates in 2019 were 6.9 ± 1.0% for B. anura and 3.5 ± 2.6% for B. curculionis, but in 2020 both species achieved effective parasitism rates of only 1 ± 0.4%.
Rates of parasitism detected by molecular analysis
A total of 1750 H. postica larvae were tested using this protocol. The PCR test detected much greater variability in the parasitism rate per field compared to classical analysis, ranging from 0% to more than 60.4%. The mean parasitism rate due to B. anura was 20.7 ± 2.7% in 2019 and 10.7 ± 1.7% in 2020, and the equivalent values for B. curculionis were 18.0 ± 5.1% in 2019 and 12.4 ± 2.6% in 2020. Accordingly, B. curculionis achieved a slightly lower parasitism rate than B. anura in 2019 but the ranking was reversed in 2020 (Table 2b). DNA from both parasitoid species was not detected in the same H. postica larvae.
Yearly statistical tests showed significant differences between the classical and molecular methods for both parasitoids. The PCR assay detected a significantly greater rate of parasitism than the classical method in 2019 for B. anura (t = –3.63, df = 13, p = 0.0031) and for B. curculionis (z = 3.14, p = 0.0016), and the same was true for 2020 (B. anura z = 3.00, p = 0.0026; B. curculionis z = 3.60, p = 0.0003).
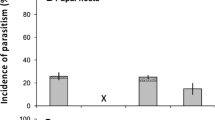
The analysis of parasitized instars revealed that B. curculionis and B. anura DNA was found at a similar rate in L3 larvae (~ 15% of specimens) and L4 larvae (~ 10% of specimens), but that only B. curculionis parasitized L2 larvae (~ 20% of specimens). These results indicate that B. anura prefers to parasitize later-instars since no DNA was detected in L2 H. postica larvae (Fig. 2).
Discussion
We have developed a new molecular method based on the amplification of the mitochondrial COI gene for the quick detection of B. anura and B. curculionis in H. postica larvae. The direct analysis of parasitoid DNA is an alternative to the time-consuming morphological analysis of puparia, which requires the rearing of insects in containment. The mitochondrial COI gene has proven useful for the reliable identification of other morphologically similar species (Nanini et al. 2019; Solà et al. 2018; Traugott and Symondson 2008). We therefore designed specific primers that discriminate between the B. curculionis and B. anura COI genes, a strategy that has been successful for other parasitoids and predators (Agustí et al. 2003b, 2005). The new strategy can be used to investigate interactions between H. postica and its larval parasitoids in more detail.
The classical technique revealed a larger number of emerging adults for B. anura compared to B. curculionis and thus a greater rate of parasitism, suggesting that B. anura is more prevalent than B. curculionis in the study area, as previously reported (Pons and Nuñez 2020). In regions of the New World where the two species are colocalized, B. anura is generally dominant over B. curculionis and can even displace it completely due to greater reproductive capacity and aggression, and more successful host finding (Harcourt 1990). Our new molecular method detected up to seven times as many parasitism events as the classical technique, indicating greater sensitivity and thus more reliability when estimating the rate of parasitism, as suggested for other species (Agustí et al. 2005; Gariepy et al. 2008; Gomez-Polo et al. 2014). Furthermore, the molecular method suggested for the first time that the rate of parasitism caused by B. anura and B. curculionis does not differ by so wide a margin as suggested by the classical method (Pons and Nuñez 2020; Levi-Mourao et al. 2021). The mean rate of parasitism was similar for both species, suggesting that the two species coexist in the alfalfa crops of north-east Spain. Moreover, the classical and molecular methods both showed that B. anura was the prevalent species in March, when H. postica begins to attack alfalfa crops, whereas B.curculionis was slightly more prevalent during April. This indicates a succession from one species to the other, as recently reported (Levi-Mourao et al. 2021) and may explain the absence of multiparasitism by both species.
The major and most effective parasitoid of H. postica in some regions of North America is thought to be B. curculionis (Berberet and Bisges 1998; Radcliffe and Flanders 1998; Soroka et al. 2019). However, its effectiveness is often comprised by the encapsulation of the parasitoid egg by hemocytes in the host hemocoel (Berberet et al. 2003; Salt and van den Bosch 1967; Shoubu et al. 2005). H. postica L1 larvae have little defense against parasitism, but 30–50% of the L2–L4 instars survive as a result of encapsulation (Berberet et al. 1987; van Den Bosch and Dietrick 1959). This may explain why we detected a larger number of B. curculionis parasitism events by PCR compared to conventional rearing. PCR-based methods can overestimate the rate of parasitism because they detect parasitoids that are already neutralized by the host immune system, whereas the classical method allows the direct measurement of parasitoid survival (Traugott et al. 2006). On the other hand, classical rearing techniques are influenced by the mortality of parasitoids under laboratory conditions, which can result in partial data loss (Tilmon et al. 2000). Furthermore, the puparia of each species have different environmental requirements to complete their life cycle, which can also influence the results. For example, B. curculionis can extend its diapause up to 10–12 months in an unfavorable environment (Radcliffe and Flanders 1998), but high emergence rates were achieved by placing the puparia in a refrigerator for 4–6 months before transfer to an environment set at 21 °C with a 12-h photoperiod (Jacob and Evans 2000; 2004). The low effective rate of parasitism we observed in the case of B. curculionis may be due to the maintenance of the puparia in rearing cages under laboratory conditions. In contrast to B. curculionis, B. anura eggs are almost never encapsulated by H. postica (Maund and Hsiao 1991; Puttler 1967). This may explain the correlation between the two methods during 2019, when there was no additional mortality caused by Z. phytonomi.
Our results also showed that B. anura females prefers L3 H. postica larvae because no DNA was found in L2 larvae, agreeing with previous findings (Bartell and Pass 1980; Dowell and Horn 1977). In contrast, B. curculionis targeted L2 larvae, concurring with reports showing that this species favors early-instar H. postica larvae and that this is strictly related to high parasitoid larval survival (Duodu and Davis 1974; Barney et al. 1978). The differences in larval instar preference probably reflects the length of the ovipositor, which is longer for B. curculionis and facilitates the utilization of early instars still hidden in unfolded leaves and buds (Dowell and Horn 1977). Our results also suggest that B. anura has a shorter larval development phase than B. curculionis.
Given the differences in parasitoid occurrence, development, and host instar preferences, alfalfa crop management during the first cutting could be optimized to enhance the survival and development of B. anura and B. curculionis. Bathyplectes anura can survive and complete its development during alfalfa weevil infestation because it appears earlier in the field, favors late-instar host larvae and develops more quickly (Levi-Mourao et al. 2021). In contrast, the survival of B. curculionis can be seriously compromised by the timing of first cutting because it appears later in the field (mainly during the second half of April, when most H. postica larvae have nearly completed development) and favors young larvae which are increasingly scarce by this time point (Levi-Mourao et al. 2021; Levi et al. in preparation). The commercial cutting of alfalfa at the end of April eliminates almost the entire H. postica population, so bringing this forward to reduce yield losses could severely limit the availability of hosts for B. curculionis. A delay in this practice can help to the survival of this parasitoid species. Beside this, a recent study in the Ebro Basin on the efficacy of a winter alfalfa cutting (Levi-Mourao et al. 2022) to reduce the egg population and to prevent the development of larvae of H. postica at the first alfalfa commercial cutting, shows that the larval density was significantly reduced, whereas the rate of parasitism increased, especially B. anura, the prevalent species at the beginning of the spring. Furthermore, the reduction of alfalfa weevil larvae below the economic thresholds, enhanced by the winter cutting, would allow delaying the date of the first commercial cutting and, in turn, B. curculionis survival.
Parasitism rates in 2020 were lower than in 2019 due to the presence of Z. phytonomi. Epizooties of this fungus occur in years with high rainfall (Barney and Armbrust 1981; Kuhar et al. 1999). This was the case in 2020 but not in 2019, which featured a dry winter. Although, Z. phytonomi kills H. postica larvae and is considered an important biological control agent (Harcourt and Guppy, 1991; Giles and Obricky, 1997), it also kills parasitoid larvae (Giles et al. 1994; Kuhar et al. 1999). Our results show that epizootic infections of this fungus disrupt the alfalfa weevil–parasitoid system under Spanish crop conditions. The lower B. anura parasitism rate in 2020 suggests that this species was probably the most affected by the fungus. B. curculionis appears later in the study area and therefore has an advantage over B. anura because the environmental conditions no longer favor the spread of the disease, increasing the likelihood of host survival until pupation and thus the survival of the parasitoid.
Parasitism rates in 2019 varied at the field level, with maximum values of 37% for B. anura and 17% for B. curculionis. This agrees with other studies in the same area, where variable rates were reported with a maximum of 30% (Pons and Nuñez 2020). These parasitoids were most effective when introduced into North America to control the alfalfa weevil. The rate of parasitism with B. curculionis tended to be high, at times exceeding 90% in the mild San Francisco Bay and Pleasanton areas, but this approach was much less effective in the hotter San Joaquin Valley (Radcliffe and Flanders 1998). Rearing studies conducted in south-western Canada revealed B. curculionis parasitism rates of up to 17% (Soroka et al. 2020). This suggests that environmental conditions play a key role in the success of parasitism, with hotter temperatures inhibiting parasitoid performance, and can explain our lower rates recorded in our study area. In other regions of North America, where B. anura tends to be the prevalent species (as is the case in Spain), the rate of parasitism was similar to our findings (Harcourt 1990; Berberet and Bisges 1998).
In conclusion, our new molecular strategy provides information about the ecology of B. anura and B. curculionis, reveals the prevalence of both species, and contributes to the development of biological control strategies in Europe. Our results show that specific primers can be used to detect and identify both endoparasitoid wasps in alfalfa weevil specimens, and provided an alternative way to estimate the rate of parasitism in the field. One drawback of the new method is its tendency to overestimate the rate of parasitism by counting unsuccessful events. Accordingly, we recommend that DNA analysis should be combined with conventional rearing to determine the effective rate of parasitism and also to accommodate interactions with other species that are not specifically targeted by the molecular assay. In spite of the potential of Bathyplectes sp. as a biological control agent for H. postica, it seems that the alfalfa crop management system currently performed in Spain may be unfavorable to their control capacity. However, the incorporation of a winter cutting and the delay of the first spring alfalfa cutting, which increase the rates of parasitism of B. anura and can help to the survival of B. curculionis, respectively, are tools that can be included in integrated pest management strategies in Spain and, potentially, in other Mediterranean countries.
Change history
12 June 2022
The original version of this article has been revised: The Handling Editor has been added.
References
Agustí N, Shayler SP, Harwood JD, Vaughan IP, Sunderland KD, Symondson WOC (2003a) Collembola as alternative prey sustaining spiders in arable ecosystems: Prey detection within predators using molecular markers. Mol Ecol 12:3467–3475
Agustí N, Unruh TR, Welter SC (2003b) Detecting Cacopsylla pyricola (Hemiptera: Psyllidae) in predator guts using COI mitochondrial markers. Bull Entomol Res 93:179–185
Agustí N, Bourguet D, Spataro T, Delos M, Eychenne N, Folcher L, Arditi R (2005) Detection, identification and geographical distribution of European corn borer larval parasitoids using molecular markers. Mol Ecol 14:3267–3274
Agustí N, Castañé C, Fraile I, Alomar O (2020) Development of a PCR-based method to monitor arthropod dispersal in agroecosystems: Macrolophus pygmaeus (Hemiptera: Miridae) from banker plants to tomato crops. Insect Sci 27:1125–1134
Barney RJ, Armbrust EJ (1981) List of the insect predators of the alfalfa weevil, Hypera postica (Coleoptera: Curculionidae). Bull Entomol Soc Am 27:241–244
Barney RJ, Armbrust EJ, Bartell DP, Goodrich MA (1978) Frequency occurrence of Bathyplectes curculionis within instars of Hypera postica larvae in Illinois. Environ Entomol 7:241–245
Bartell DP, Pass BC (1980) Morphology, development, and behavior of the immature stages of the parasite Bathyplectes anurus (Hymenoptera: Ichneumonidae). Can Entomol 112:481–487
Berberet RC, Bisges AD (1998) Potential for competition among natural enemies of larvae of Hypera postica (Coleoptera: Curculionidae) in the southern plains. Environ Entomol 27:743–751
Berberet RC, Willson LJ, Odejar M (1987) Probabilities for encapsulation of eggs of Bathyplectes curculionis (Hymenoptera: Ichneumonidae) by larvae of Hypera postica (Coleoptera: Curculionidae) and resulting reduction in effective parasitism. Ann Entomol Soc Am 80:483–485
Berberet RC, Zarrabi AA, Payton ME, Bisges AD (2003) Reduction in effective parasitism of Hypera postica (Coleoptera: Curculionidae) by Bathyplectes curculionis (Hymenoptera: Ichneumonidae) due to encapsulation. Environ Entomol 32:1123–1130
Black W, McLain D, Rai K (1989) Patterns of variation in the rDNA cistron within and among world populations of a mosquito, Aedes albopictus (Skuse). Genetics 121:539–550
Day WH (1970) The survival value of its jumping cocoons to Bathyplectes anurus, a parasite of the alfalfa weevil. J Econ Entomol 63:586–589
Delgado I, Lloveras J (2020) Historia y distribución de la alfalfa. In: Lloveras J, Delgado I, Chocarro C (eds) La alfalfa, agronomía y utilización, 1st edn. Edicions de la Universitat de Lleida, Lleida, Spain, pp 17–32
Dowell RV, Horn DJ (1977) Adaptive strategies of larval parasitoids of the alfalfa weevil (Coleoptera: Curculionidae). Can Entomol 109:641–648
Duodu YA, Davis DW (1974) Selection of alfalfa weevil larval instars by, and mortality due to, the parasite Bathyplectes curculionis (Thomson). Environ Entomol 3:549–552
Dysart RJ, Day WH (1976) Release and recovery of introduced parasites of the alfalfa weevil in Eastern North America, 1st edn. Agricultural Research Service - United States Department of Agriculture, Washington
Froger A, Hall JE (2007) Transformation of Plasmid DNA into E. coli using the heat shock method. J vis Exp 6:253
Gariepy T, Kuhlmann U, Gillott C, Erlandson M (2008) A large-scale comparison of conventional and molecular methods for the evaluation of host-parasitoid associations in non-target risk-assessment studies. J Appl Ecol 45:708–715
Giles KL, Obrycki JJ (1997) Reduced insecticide rates and strip-harvesting effects on alfalfa weevil (Coleoptera: Curculionidae) larval populations and prevalence of Zoophthora phytonomi (Entomophthorales: Entomophthoraceae). J Econ Entomol 90:933–944
Giles KL, Obrycki JJ, Degoover TA (1994) Prevalence of predators associated with Acyrthosiphon pisum (Homoptera: Aphididae) and Hypera postica Gyllenhal (Coleoptera: Curculionidae) during growth of the first crop of alfalfa. Biol Control 4:170–177
Gomez-Polo P, Traugott M, Alomar O, Castañé C, Rojo S, Agustí N (2014) Identification of the most common predatory hoverflies of Mediterranean vegetable crops and their parasitism using multiplex PCR. J Pest Sci 87:371–378
Goosey HB (2012) A degree-day model of sheep grazing influence on alfalfa weevil and crop characteristics. J Econ Entomol 105:102–112
Harcourt DG (1990) Displacement of Bathyplectes curculionis (Thoms.) (Hymenoptera: Ichneumonidae) by B. anurus (Thoms.) in eastern Ontario populations of the alfalfa weevil, Hypera postica (Gyll.) (Coleoptera: Curculionidae). Can Entomol 122:641–645
Harcourt DG, Guppy JC (1991) Numerical analysis of an outbreak of the alfalfa weevil (Coleoptera: Curculionidae) in eastern Ontario. Environ Entomol 20:217–223
Hoff KM, Brewer MJ, Blodgett SL (2002) Alfalfa weevil (Coleoptera: Curculionidae) larval sampling: comparison of shake-bucket and sweep-net methods and effect of training. J Econ Entomol 95:748–753
Hoffman A (1963) Sous famille des Curculionidae. Tribu des Hyperini. Les Hypera (Syn Phytonomus). In: Balachowsky A (ed) Entomologe appliquée a l’agriculture Tome I Coleoptères, second volume. París, Masson et Cie, pp 984–989
Innis M, Gelfand D (1990) Optimization of PCRs. In: Innis M, Gelfand D, Sninsky J, White T (eds) PCR protocols. Academic Press, San Diego, pp 3–12
Jacob HS, Evans EW (2000) Influence of carbohydrate foods and mating on longevity of the parasitoid Bathyplectes curculionis (Hymenoptera: Ichneumonidae). Environ Entomol 29:1088–1095
Jacob HS, Evans EW (2004) Influence of different sugars on the longevity of Bathyplectes curculionis (Hym., Ichneumonidae). J Appl Entomol 128:316–320
JMP, version Pro 15. SAS Institute Inc, Cary, NC, 1989–2021
Kingsley PC, Bryan MD, Day WH, Burger TL, Dysart RJ, Schwalbe CP (1993) Alfalfa weevil (Coleoptera: Curculionidae) biological control: spreading the benefits. Environ Entomol 22:1234–1250
Kuhar TP, Youngman RR, Laub CA (1999) Alfalfa weevil (Coleoptera: Curculionidae) pest status and incidence of Bathyplectes spp. (Hymenoptera: Ichneumonidae) and Zoophthora phytonomi (Zygomycetes: Entomophthorales) in Virginia. J Econ Entomol 92:1184–1189
Levi-Mourao A, Núñez E, García A, Meseguer R, Pons X (2022) Alfalfa winter cutting: Effectiveness against the alfalfa weevil, Hypera postica (Gyllenhal) (Coleoptera: Curculionidae) and effect on its rate of parasitism due to Bathyplectes spp. (Hymenoptera: Ichneumonidae). Crop Prot 152:105858
Levi-Mourao A, Meseguer R, Pons X (2021) Parasitism of the alfalfa weevil Hypera postica Gyllenhal by Bathyplectes sp in Spain, in Proceedings of the 1st International Electronic Conference on Entomology. 10505
Liang GH, Jang EB, Heller WP, Chang CL, Chen JH, Zhang FP, Geib SM (2015) A qPCR-based method for detecting parasitism of Fopius arisanus (Sonan) in oriental fruit flies, Bactrocera dorsalis (Hendel). Pest Manag Sci 71:1666–1674
Liang GH, Fu LQ, Zheng JX, Lin HY, Lin JH, Sim SB, Jang BJ, Heller WP, Geib SM (2018) Molecular characterization of interspecific competition of Diachasmimorpha longicaudata (Ashmead) and Fopius arisanus (Sonan) parasitizing the oriental fruit fly, Bactrocera dorsalis (Hendel). Biol Control 118:10–15
Los LM, Allen WA (1982) Visual technique for determining presence and stage of Zoophthora phytonomi in dead alfalfa weevil larvae. J Econ Entomol 75:375–377
Madeira F, Clemente-Orta G, Alomar O, Batuecas I, Sossai S, Albajes R (2022) Land use alters the abundance of herbivore and predatory insects on crops: the case of alfalfa. J Pest Sci 95:473–491
Maund CM, Hsiao TH (1991) Differential encapsulation of two Bathyplectes parasitoids among alfalfa weevil strains, Hypera postica (Gyllenhal). Can Entomol 123:197–203
Molina P, Martínez-Ferrer MT, Campos-Rivela JM, Riudavets J, Agustí N (2021) Development of a PCR-based method for the screening of potential predators of the African citrus psyllid Trioza erytreae (Del Guercio). Biol Control 160:104661
Nanini F, Maggio DH, Ferronato P, Rugno G, Yamamoto PT, Corrêa AS (2019) Molecular marker to identify Diaphorina citri (Hemiptera: Liviidae) DNA in gut content of predators. Neotrop Entomol 48:927–933
Orloff SB (1997) Introduction. In: Orloff SB, Karlson HL, Tauber LR (eds) Intermountain alfalfa management. University of California. Division of Agriculture and Natural Resources, Oakland, pp 2–3
Pons X, Nuñez E (2020) Plagas da la alfalfa: Importancia, daños y estrategias de control. In: Lloveras J, Delgado I, Chocarro C (eds) La alfalfa, agronomía y utilización, 1st edn. Edicions de la Universitat de Lleida, Lleida, Spain, pp 167–202
Puttler B (1967) Interrelationship of Hypera postica (Coleoptera: Curculionidae) and Bathyplectes curculionis (Hymenoptera: Ichneumonidae) in the Eastern United States with particular reference to encapsulation of the parasite eggs by the weevil larvae. Ann Entomol Soc Am 60:1031–1038
Radcliffe EB, Flanders KL (1998) Biological control of alfalfa weevil in North America. Integr Pest Manag Rev 3:225–242
Rand TA (2013) Host density drives spatial variation of the alfalfa weevil, Hypera postica, across dryland and irrigated alfalfa cropping systems. Environ Entomol 42:116–122
Ribes A (2012) Himenòpters de Ponent. http://ponent.atspace.org/fauna/ins/index.htm. Accessed 3 May 2021
Saeidi M, Moharramipour S (2017) Physiology of cold hardiness, seasonal fluctuations, and cryoprotectant contents in overwintering adults of Hypera postica (Coleoptera: Curculionidae). Environ Entomol 46:960–966
Saiki R (1990) Amplification of genomic DNA. In: Innis M, Gelfand D, Sninsky J, White T (eds) PCR protocols. Academic Press, San Diego, pp 13–20
Salt G, van den Bosch R (1967) The defense reactions of three species of Hypera (Coleoptera: Curculionidae) to an ichneumon wasp. J Invertebr Pathol 9:164–177
Shoubu M, Okumura M, Shiraishi A, Kimura H, Takagi M, Ueno T (2005) Establishment of Bathyplectes anurus (Hymenoptera: Ichneumonidae), a larval parasitoid of the alfalfa weevil, Hypera postica (Coleoptera: Curculionidae) in Japan. Biol Control 34:144–151
Simon C, Frati F, Beckenbach A, Crespi B, Liu H, Flook P (1994) Evolution, weighting, and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers. Ann Entomol Soc Am 87:651–701
Solà M, Riudavets J, Agustí N (2018) Detection and identification of five common internal grain insect pests by multiplex PCR. Food Control 84:246–254
Soroka J, Grenkow L, Cárcamo H, Meers S, Barkley S, Gavloski J (2019) An assessment of degree-day models to predict the phenology of alfalfa weevil (Coleoptera: Curculionidae) on the Canadian Prairies. Can Entomol 152:110–129
Soroka J, Bennett AMR, Kora C, Schwarzfeld MD (2020) Distribution of alfalfa weevil (Coleoptera: Curculionidae) and its parasitoids on the Canadian Prairies, with a key to described species of Nearctic Bathyplectes (Hymenoptera: Ichneumonidae). Can Entomol 152:663–701
Tilmon KJ, Danforth BN, Day WH, Hoffmann MP (2000) Determining parasitoid species composition in a host population: a molecular approach. Ann Entomol Soc Am 93:640–647
Traugott M, Symondson WOC (2008) Molecular analysis of predation on parasitized hosts. Bull Entomol Res 98:223–231
Traugott M, Zangerl P, Juen A, Schallhart N, Pfiffner L (2006) Detecting key parasitoids of lepidopteran pests by multiplex PCR. Biol Control 39:39–46
Traugott M, Kamenova S, Ruess L, Ruess L, Seeber J, Plantegenest M (2013) Empirically characterising trophic networks: what emerging DNA-based methods, stable isotope and fatty acid analyses can offer. Adv Ecol Res 49:177–224
Van Den Bosch R, Dietrick EJ (1959) The interrelationships of Hypera brunneipennis (Coleoptera: Curculionidae) and Bathyplectes curculionis (Hymenoptera: Ichneumonidae) in Southern California. Ann Entomol Soc Am 52:609–616
Wolf S, Zeisler C, Sint D, Romeis J, Traugott M, Collatz J (2018) A simple and cost-effective molecular method to track predation on Drosophila suzukii in the field. J Pest Sci 91:927–935
Acknowledgements
This study was funded by the Ministerio de Ciencia e Innovación of the Spanish Government (project AGL2017-84127-R). The authors would like to thank Dr. Nuria Agustí for all her suggestions during the experiments and her comments when writing this article. Dr. Addy García, Dr. Carmen Lopez and Marta Franch for support with the laboratory and field experiments. We also thank J. Piqué, Oses-Nafosa, Aldhara-Europe S.R.L., Granja San José and Cooperatives de Bell-lloc d’Urgell, and Verge de les Sogues—Bellvís for allowing sampling in their commercial alfalfa fields. We also thank Dr. Jaume Almacelles from the Laboratori de Sanitat Vegetal and Dr. Enrique Quesada from the Universidad de Córdoba. A.L-M. was funded by a Jade Plus Grant from the Universitat de Lleida, and R.M. was funded by an FPI Grant from the Ministerio de Ciencia e Innovación.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This study was funded by Ministerio de Ciencia, Innovación y Universidades, Spanish Government. Project AGL2017-84127-R: Arable crop management and landscape interactions for pest control. Alexandre Levi-Mourao was funded by a predoctoral JADE plus grant from the University of Lleida and Roberto Meseguer by a predoctoral grant FPI-PRE2018-083602, Ministerio de Ciencia, Innovación y Universidades.
Author information
Authors and Affiliations
Contributions
Conceptualization: XP; Methodology: AL-M; PM; PC-B; Formal analysis: AL-M; PC-B; XP. Investigation: AL-M; PC-B; RM and XP; Writing—original draft preparation: AL-M; PC-B; XP; Writing—review and editing: AL-M; RM PC-B; PM and XP; Funding acquisition: XP; Resources: XP; Supervision: PM; XP. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Handling Editor: Dirk Babendreier
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Levi-Mourao, A., Muñoz, P., Cerda-Bennasser, P. et al. Molecular and morphological identification of the alfalfa weevil larval parasitoids Bathyplectes anura and Bathyplectes curculionis to estimate the rate of parasitism. BioControl 67, 319–330 (2022). https://doi.org/10.1007/s10526-022-10141-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-022-10141-x