Abstract
The effectiveness of augmentative biological control using parasitoids often depends on their physiological state and the pest population density at the time of release. Tamarixia triozae (Burks) (Hymenoptera: Eulophidae) is a primary host-feeding parasitoid of a serious invasive pest Bactericera cockerelli (Šulc) (Hemiptera: Triozidae). Here we investigated the effects of adult diets (honey, water, yeast, and hosts) and timing of their provision on T. triozae fitness and oviposition patterns, providing knowledge for enhancement of its biological control potential. Adults fed with honey for four days with no access to hosts or with water or yeast for one day followed by host feeding for three days had similar longevity and lifetime pest killing ability. Adults fed with only water for one day before release had significantly greater intrinsic rate of increase, shorter doubling time, and higher daily fecundity peak. Adults fed with honey or yeast for one day followed by host feeding for three days significantly flattened their daily oviposition curves. These findings have several implications for augmentative biological control using T. triozae. First, honey diet may allow at least four days for successful shipment of host-deprived adults without compromising biological control effectiveness. Second, the release of host-deprived adults with one-day water feeding may achieve rapid pest suppression when the pest population density is high. Finally, releasing host-deprived adults with one-day honey or yeast feeding followed by three-day host feeding can increase their establishment success and reduce the risk of massive removal of hosts when the pest population density is low.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Augmentative release of natural enemies, particularly parasitoids, is one of the most widely used approaches to pest biological control in annual crops and greenhouses (van Lenteren and Bueno 2003; Hoy 2008; Amadou et al. 2019; Bueno et al. 2020; Kazak et al. 2020). It is also commonly applied to control resurgent pests resulting from disruptions of natural enemy populations by seasonal factors and harvesting activities (Hajek and Eilenberg 2018; Stenberg et al. 2021) or insecticide applications (Coppel and Mertins 1977; DeBach and Rosen 1991). Evidence shows that adult host feeding is important for synovigenic parasitoids (Jervis and Kidd 1986; Giron et al. 2004; Burger et al. 2005; Jervis et al. 2008; Kapranas and Luck 2008; Liu et al. 2015; Benelli et al. 2017; Hanan et al. 2017; Gebiola et al. 2018), and supplementary adult diets such as honey and honeydew (Bezemer et al. 2005; Wade et al. 2008; Hossain and Haque 2015; Benelli et al. 2017; Dong et al. 2018; Picciau et al. 2019) and yeast (Bartlett 1964; Heimpel and Rosenheim 1995) can increase parasitoid fecundity and longevity. Therefore, provision of adult parasitoid diets before release can not only improve parasitoids’ ability to establish at release sites (Tena et al. 2017; Stahl et al. 2019) but also increase their biological control effectiveness after release (Hougardy et al. 2005; Hougardy and Mills 2006, 2007; Benelli et al. 2017).
Several studies report that adult diets can change parasitoids’ lifetime oviposition patterns (Bai and Smith 1993; Wade et al. 2008; Hill et al. 2020). This suggests that we may be able to modify parasitoids’ lifetime oviposition trajectory by tailoring their adult diets before release to achieve effective augmentative biological control under different circumstances. For example, when the pest population density is high, we may aim to achieve quick pest suppression (Karacaoğlu et al. 2018) where the parasitoids are expected to perform maximum host killing through host feeding and parasitization immediately after release. When the pest population density is low, we may want to delay their oviposition peaks and flatten their oviposition curves (Stahl et al. 2019) where the released parasitoids are expected to spread their oviposition and host feeding more evenly or move these activities toward their later life. Nevertheless, it is not clear how alteration of timing and type of diets provided for adult parasitoids before release can change their life history traits towards our advantage. It is also unclear whether such manipulation would compromise their overall pest killing ability in terms of fecundity and host feeding. This knowledge is crucial for the development of successful augmentative biological control programs using parasitoids.
The tomato-potato psyllid (TPP), Bactericera cockerelli (Šulc) (Hemiptera: Triozidae), is a severe invasive pest of solanaceous crops such as potatoes, tomatoes, eggplants and peppers in the USA, Mexico and New Zealand (Cranshaw 1994; Teulon et al. 2009; Crosslin et al. 2010; Butler and Trumble 2012; Rojas et al. 2015), causing significant economic losses. It has also invaded Australia, Canada and Central and South America where its economic importance is under evaluation (FAO 2017; WADPIRD 2018; Castillo Carrillo et al. 2019; Olaniyan et al. 2020). Tamarixia triozae (Burks) (Hymenoptera: Eulophidae) is an important synovigenic parasitoid of TPP (Bravo and López 2007; Rojas et al. 2015; Yang et al. 2015). It kills the pest by both host feeding and parasitization (Martinez et al. 2015). However, it is still unknown how provision of different adult diets and temporary deprivation of hosts could affect reproductive fitness and lifetime feeding and oviposition patterns in T. triozae. This information is of vital importance for development of strategies to optimize augmentative biological control of TPP using T. triozae.
In the present study, we tested the effects of adult diets with or without temporary host deprivation on survival, lifetime fecundity, host feeding, oviposition patterns and life table parameters in T. triozae. Knowledge presented in this work could help enhance the success of augmentative biological control using T. triozae.
Materials and methods
Insects
We obtained B. cockerelli and T. triozae from BioForce Limited, Auckland, New Zealand, and established the colonies in the laboratory. We maintained the psyllid colony on five two-month-old bell pepper plants, each of which was grown in a pot (9.5 cm in height × 10.5 cm in top diameter × 8.5 cm in bottom diameter) with potting mix. The potted plants were placed in an aluminium-framed cage (43 × 42 × 40 cm) with a fine metal mesh (aperture size = 0.25 × 0.25 mm) on the back and both sides and Perspex on the top and front and aluminium alloy on the bottom. We kept the parasitoid colony on the 3rd–5th instar psyllid nymphs feeding on five potted plants in another aluminum-framed cage of the same size. We provided 10% honey solution in a plastic tube (7.5 cm in length × 1 cm in diameter) fitted with cotton wick (3.5 cm in length × 1 cm in diameter) for parasitoid adults as supplementary food in the cage. To ensure the psyllid colony was parasite-free for experiments, we kept psyllid and parasitoid colonies separate in two climate rooms at 25 ± 1 °C, 40–60% RH and a L:D 14:10 photoperiod. We carried out all experiments under the above environmental conditions.
We transferred about 400 psyllid adults from the colony onto four two-month-old un-infested pepper plants in an aforementioned cage for oviposition. We transferred the infested plants into another cage and replaced them with the same number of un-infested plants once every two days. We obtained a total of 84 infested plants, observed them daily, and collected the 4th instar nymphs for experiments. We used transparent plastic cylinders consisting of two identical containers for production of experimental parasitoids. Briefly, we transferred about 100 fourth instar nymphs onto a bell pepper leaf, inserted the leaf petiole through a hole (1.0 cm in diameter) in the lid of a container (6.5 cm in diameter × 8.5 cm in height) filled with tap water. We then introduced five female wasps from the colony to the infested leaf and covered the container with another container upside down. We allowed these wasps to oviposit in the cylinder for 24 h before removing them. The top container had two holes (3 cm in diameter) covered by a metal mesh (aperture size = 0.25 mm) at the opposite sides of the wall for ventilation. We collected parasitoid pupae seven days later and placed them individually in glass vials (5 cm in height × 1.5 cm in diameter) plugged with cotton wool until emergence. We set up 20 such cylinders to obtain sufficient wasps for experiments.
Treatments
To determine how adult diets and temporary host deprivation affected parasitoid longevity, lifetime fecundity, daughter production, oviposition and host feeding patterns, and life table parameters, we carried out two phases of treatments. In the first phase, we provided one of the following three diets for wasps: (1) Honey—10% honey solution made of 10 g honey + 90 ml distilled water, (2) Yeast—5% yeast solution consisting of 5 g yeast extract + 95 ml distilled water, and (3) Water—distilled water only. For each diet treatment, we individually introduced 60 newly emerged female and 60 newly emerged male wasps into glass vials (5 cm in height × 1.5 cm in diameter). Each vial was provided with a diet saturated in a cotton ball (0.5 cm in diameter) and plugged with cotton wool. Because one-day-old wasps can successfully copulate within one hour after encountering a mate (Chen et al. 2020), we kept wasps in their vials for 24 h, and then individually paired males and females that fed on the same diet in glass vials and allowed 2 h for mating to occur. We obtained 28, 30, and 28 mated pairs that fed on honey, yeast, and water diets, respectively, for the second phase of treatments.
In the second phase, we divided the above pairs into two groups and maintained them in one of the following two conditions: (1) no host deprivation (+ Host)—each pair was released into a Petri dish (8.5 cm in diameter × 2.4 cm in height) containing the same adult diet saturated in a cotton ball and 24 4th instar psyllid nymphs feeding on a bell pepper leaf (5 cm in diameter, upside down) with its petiole embedded in water-saturated cotton wool and wrapped with parafilm. The lid of the Petri dish had two holes (1 cm in diameter), one plugged with a cotton wool for introducing wasps and one covered with metal mesh for ventilation. We allowed the pair to stay in the dish for 24 h, and then transferred them to another dish with the same diet and fresh hosts once every day until both male and female died. We replaced the adult diet with fresh one once every day. (2) Temporary host deprivation (− Host)—each pair was released into a Petri dish of the same size containing the same adult diet but no hosts. Three days later (live wasps were now four days old), we provided them with the same adult diet and 24 4th instar psyllid nymphs as above and allowed each pair to stay in the Petri dish for 24 h. We then transferred the pair into another Petri dish with the same adult diet and fresh hosts once every day until both male and female died. We replaced the adult diet with fresh one once every day. We tested 15, 13, 13, 13, 17 and 15 pairs for treatments Honey + Host, Yeast + Host, Water + Host, Honey − Host, Yeast − Host and Water − Host, respectively.
Effect of adult diets and temporary host deprivation on survival, reproduction, host feeding and life table parameters
We started recording daily survival of T. triozae adults after we transferred one-day-old, mated wasps to Petri dishes. We commenced to count the daily number of hosts fed and parasitized one day after hosts were provided, i.e., when wasps were two days old for + Host treatments and five days old for − Host treatments. We did not record reproduction data for treatments Yeast − Host and Water − Host because all females died before we provided them with hosts. We examined host feeding and parasitism under a stereomicroscope (Leica MZ12, Germany). Host feeding was identified by bleeding that occurred from the nymph (Martinez et al. 2015) or an inverted V-shape mark on the hollowed body (Morales et al. 2013). Because T. triozae females deposit eggs under the nymphs (Martinez et al. 2015), we turned all nymphs over and placed them on the surface of 1% agar to determine parasitism. We then transferred all parasitized nymphs from each dish onto a fresh bell pepper leaf in an above-mentioned plastic cylinder. We collected the parasitoid pupae seven days after parasitization and individually placed them in above-mentioned glass vials plugged with cotton wool until emergence. We recorded the developmental time from egg to adult and sex of each emerged adult. We used the data collected for life table calculations.
Statistical analysis
We conducted all data analyses using SAS software (SAS 9.3, SAS Institute Inc., NC, USA). We analyzed the data on adult survival using the Kaplan–Meier method. We compared the survival curves between treatments with a non-parametric Wilcoxon test (LIFETEST procedure). Data on lifetime host feeding were normally distributed (Shapiro–Wilk test, UNIVARIATE procedure) and analyzed using an ANOVA (GLM procedure) followed by a Tukey's Studentized (HSD) range test for multiple comparisons. Data on lifetime fecundity were not normally distributed even after transformation, and thus analyzed using a Kruskal–Wallis test.
We developed two Gaussian functional models according to Archontoulis and Miguez (2015): y = a \({e}^{{(-0.5\{[(x-c)-({x}_{0}-c)]/b\}}^{2})}\) to fit the daily host feeding and y = a \({e}^{{(-0.5\{\mathrm{ln}[(x-c)/({x}_{0}-c)]/b\}}^{2})}\) to fit the daily oviposition and daughter production for each treatment, where x is the age of female wasps (days), a is the peak at time x0 (wasp age), b is the coefficient controlling the width of the peak, and c is the period (days) before the females were exposed to hosts (i.e., c = 1 and 4 days for the + Host and − Host treatments, respectively). These models allowed us to compare the host feeding and reproduction patterns between treatments. If the 95% CLs of a given parameter overlap, then there is no significant difference between treatments.
To estimate how adult diets and temporary host deprivation affected population growth, we calculated the life table parameters (Jervis et al. 2005) using the data on daily survival and daughter production of each T. triozae female. The intrinsic rate of increase (r, daughters per female per day) was calculated by solving the Lotka-Euler equation: \(\sum {e}^{-rx}{\mathrm{l}}_{x}{\mathrm{m}}_{x}\) = 1, where x is the pivotal age, \({\mathrm{l}}_{x}\) is the proportion of females surviving to age x, and \({\mathrm{m}}_{x}\) is the number of daughters produced per female at age x. Other life table parameters included the net reproductive rate (R0 = \(\sum {\mathrm{l}}_{x}{\mathrm{m}}_{x}\), daughters per female per generation), doubling time [Dt = \({\mathrm{log}}_{e}(2)/r\), days] and generation time [T = \({\mathrm{log}}_{e}({R}_{0})/r\), days]. For each treatment, we used the bootstrap method with 100,000 bootstrap samples to calculate the pseudo-values of life table parameters (Huang and Chi 2012; Yu et al. 2013). We then employed a paired-bootstrap test (MULTTEST procedure) for multiple comparisons between treatments (Efron and Tibshirani 1993; Mou et al. 2015; Reddy and Chi 2015). Because multiple comparisons raise the Type I error (Noble 2009), the overall P value was adjusted by the Bonferroni correction (MULTTEST procedure) (Gravandian et al. 2022).
Results
Effect of adult diets and temporary host deprivation on survival and lifetime reproductive outputs
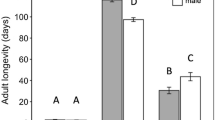
Our results reveal that most wasps in treatments Yeast − Host and Water − Host died within two days after emergence. These wasps lived significantly shorter than those in other treatments (\({x}_{5}^{2}\) = 94.56 and 93.35 for females and males, respectively; P < 0.0001) (Fig. 1). Wasps in other treatments including either hosts or honey lived for about 21 days (Fig. 1), where females had similar survival curves and males in treatment Honey + Host lived significantly longer. As shown in Fig. 2, female wasps in treatments Honey + Host, Yeast + Host, Water + Host and Honey − Host had similar lifetime host feeding and fecundity (F3,50 = 0.36, P = 0.7802 for host feeding; \({x}_{3}^{2}\) = 2.97, P = 0.3961 for fecundity).
Number of hosts fed (a) and eggs laid (b) by Tamarixia triozae females in different treatments. Each box plot shows the mean ( ×), median line, the upper and lower quartiles (i.e., the range where 25% of scores fall above and 25% fall below the median), and scores outside the 50% middle (upper ‘ ’ and lower ‘
’ and lower ‘ ’). Columns followed by the same letters are not significantly different (P > 0.05)
’). Columns followed by the same letters are not significantly different (P > 0.05)
Treatments significantly affected all life table parameters measured in this study (Table 1). Wasps in treatment Honey − Host had greatest net reproductive rate (R0) and longest generation time (T) while those in treatment Water + Host had highest intrinsic rate of increase (r) and shortest doubling time (Dt).
Effect of adult diets and temporary host deprivation on lifetime host feeding and reproductive patterns
When we provided one-day-old wasps with hosts immediately after their mating, daily host feeding (Fig. 3a) peaked between eight and 13 days after emergence, and daily fecundity (Fig. 3b) and daughter production (Fig. 3c) peaked between five and eight days after emergence. Host deprivation for three days after mating delayed the peaks of daily host feeding, fecundity and daughter production for three to four days (Fig. 3). There was no significant difference in the width of daily feeding (Fig. 3a) and daughter production (Fig. 3c) peaks between treatments. However, daily fecundity peak in Yeast + Host was significantly wider than in Honey + Host and Water + Host (Fig. 3b). Treatment had no significant effect on the height of daily host feeding peaks (Fig. 3a). Daily fecundity peak in Water + Host was significantly higher than in Honey + Host and Yeast + Host, and that in Honey − Host significantly higher than in Honey + Host (Fig. 3b). Daily daughter production peak was the highest in Honey − Host (Fig. 3c). Detailed statistical data are provided in Supplementary Table S1.
Daily number of hosts fed (a), eggs laid (b) and daughters produced (c) in Tamarixia triozae females that fed on different diets with or without host deprivation. Diet + Host = both adult diets and hosts were provided after mating, and Diet − Host = adult diets were provided but hosts were unavailable for three days after mating. For each parameter, treatments followed by the same letters in a column are not significantly different (overlapped 95% CLs of coefficients)
Discussion
In augmentative release programs, entomologists sometimes ship parasitoid pupae or adults (e.g., van Lenteren and Tommasini 1999; Yokoyama et al. 2010; Cancino and López-Arriaga 2016) from their production sites to the release sites in the absence of hosts, which can last days, depending on distance and transport methods. Like other synovigenic parasitoids (e.g., Giron et al. 2004; Liu et al. 2015; Gebiola et al. 2018), adult feeding on honey and/or hosts was essential for the survival of T. triozae because carbohydrates in these diets provided energy to prolong their longevity (e.g., Jervis et al. 2008; Picciau et al. 2019). However, our results do not support previous findings about the beneficial effect of yeast feeding on parasitoid fitness (Bartlett 1964; Heimpel and Rosenheim 1995) because T. triozae adults died in about two days if they only fed on yeast solution (Yeast − Host) or water (Water − Host) after emergence. Our findings suggest that honey diet should be provided for T. triozae adults immediately after emergence regardless of whether hosts are present, or for pupae during shipment to ensure newly emerged adults can get access to honey food in the absence of hosts.
We demonstrate that adult diets and temporary host deprivation altered daily feeding and oviposition patterns of T. triozae females. This information can be used to develop strategies for TPP biological control. For example, we could have four days to successfully ship honey-fed and host-deprived females from production to release sites without compromising their longevity and lifetime host-killing ability. Wasps in treatments Water + Host and Honey − Host had highest fecundity peaks, suggesting that when the pest density is high, we can quickly suppress the pest population by releasing wasps that have fed on water for 24 h or on honey for four days in the absence of hosts. The current study shows that wasps that fed on honey or yeast without temporary host deprivation (Honey + Host and Yeast + Host) flattened their daily oviposition curves, which may reduce the risk of massive removal of hosts and increase establishment success of wasps in the field when the pest population density is low (Eggenkamp-Rotteveel Mansveld et al. 1982). Furthermore, wasps that fed on honey for four days without access to hosts (Honey − Host) had highest peak of daughter production, suggesting that soon after release to the field (hosts become available), these wasps are not only able to quickly suppress the pest population but also increase their own.
In the absence of hosts for four days after emergence, honey-fed wasps had longest generation time (T) and relatively low intrinsic rate of increase (r) and long doubling time (Dt) due to delayed oviposition. However, these host-deprived and honey-fed adults achieved greatest net reproductive rate (R0), i.e., produced highest number of daughters, promoting future population growth (Sabbatini Peverieri et al. 2012). These findings suggest that treatment Honey − Host can lead to better and more sustainable control when the pest density is low at the time of release. On the other hand, wasps in treatment Water + Host had the highest r and shortest Dt, the second highest R0 and relatively short T. Therefore, if we aim to achieve immediate control of the pest when its population density is high, we should feed newly emerged wasps with water for 24 h and then immediately release them into the infested crops. The released wasps can kill more pest individuals within a shorter time period and quickly build up their own population. The life table parameters also provide references for producers to adjust their mass-rearing programs depending on whether they want to yield more parasitoids quickly or to slow production down.
In conclusion, adult diets can affect survival and reproductive patterns of T. triozae. In the absence of hosts at emergence, wasps feeding on water or yeast diet cannot live for more than two days, which is not long enough for successful shipment. However, adult T. triozae feeding on honey solutions for four days or other diets for one day followed by provision of hosts can survive for about 21 days with similar lifetime pest killing ability. Furthermore, wasps feeding on water for one day before access to hosts have greater intrinsic rate of increase, shorter doubling time, and higher daily fecundity peak while those fed with honey or yeast for one day followed by host feeding for three days flatten their daily oviposition curves. These findings have three implications: (1) honey diet can allow at least four days for successful shipment of host-deprived adults without compromising host-killing ability; (2) releasing host-deprived wasps with one-day water feeding can achieve rapid pest suppression when the pest population density is high, and (3) releasing host-deprived adults with one-day honey or yeast feeding followed by three-day host feeding can increase the establishment success and reduce the risk of massive removal of hosts when the pest population density is low. These implications can be tested under field conditions.
Data availability
The datasets from the current study are available from the corresponding author on request.
References
Amadou L, Ba MN, Baoua I, Muniappan R (2019) Timing of releases of the parasitoid Habrobracon hebetor and numbers needed in augmentative biological control against the millet head miner Heliocheilus albipunctella. BioControl 64:573–581
Archontoulis SV, Miguez FE (2015) Nonlinear regression models and applications in agricultural research. Agron J 107:786–798
Bai B, Smith SM (1993) Effect of host availability on reproduction and survival of the parasitoid wasp Trichogramma minutum. Ecol Entomol 18:279–286
Bartlett BR (1964) Patterns in the host-feeding habit of adult parasitic Hymenoptera. Ann Entomol Soc Am 57:344–350
Benelli G, Giunti G, Tena A, Desneux N, Caselli A, Canale A (2017) The impact of adult diet on parasitoid reproductive performance. J Pest Sci 90:807–823
Bezemer TM, Harvey JA, Mills NJ (2005) Influence of adult nutrition on the relationship between body size and reproductive parameters in a parasitoid wasp. Ecol Entomol 30:571–580
Bravo ME, López LP (2007) Principales plagas del chile de agua en los valles centrales de Oaxaca. Agroproduce Fundación Produce Oaxaca AC 7:12–15
Bueno AF, Braz ÉC, Favetti BM, França-Neto JB, Silva GV (2020) Release of the egg parasitoid Telenomus podisi to manage the Neotropical brown stink bug, Euschistus heros, in soybean production. Crop Prot 137:105310
Burger JMS, Kormany A, van Lenteren JC, Vet LEM (2005) Importance of host feeding for parasitoids that attack honeydew-producing hosts. Entomol Exp Appl 117:147–154
Butler CD, Trumble JT (2012) The potato psyllid, Bactericera cockerelli (Šulc) (Hemiptera: Triozidae): life history, relationship to plant diseases, and management strategies. Terr Arthropod Rev 5:87–111
Cancino J, López-Arriaga F (2016) Effect of hypoxia and its repercussions in packing pupae of the parasitoid Diachasmimorpha longicaudata (Hymenoptera: Braconidae) for shipment. Biocontrol Sci Technol 26:665–677
Castillo Carrillo C, Fu Z, Burckhardt D (2019) First record of the tomato potato psyllid Bactericera cockerelli from South America. Bull Insectol 72:85–91
Chen C, He XZ, Zhou P, Wang Q (2020) Tamarixia triozae, an important parasitoid of Bactericera cockerelli: circadian rhythms and their implications in pest management. BioControl 65:537–546
Coppel HC, Mertins JW (1977) Biological insect pest suppression. Springer, Berlin
Cranshaw WS (1994) The potato (tomato) psyllid, Paratrioza cockerelli (Šulc), as a pest of potatoes. In: Zehnder GW, Powelson ML, Jansson RK, Raman KV (eds) Advances in potato pest biology and management. APS Press, St. Paul, pp 83–95
Crosslin JM, Munyaneza JE, Brown JK, Liefting LW (2010) A history in the making: potato zebra chip disease associated with a new psyllid-borne bacterium - a tale of striped potatoes. https://www.apsnet.org/edcenter/apsnetfeatures/Pages/PotatoZebraChip.aspx. Accessed 11 Oct 2021
DeBach P, Rosen D (1991) Biological control by natural enemies. Cambridge University Press, Cambridge
Dong YC, Han P, Niu CY, Zappalà L, Amiens-Desneux E, Bearez P, Lavoir AV, Biondi A, Desneux N (2018) Nitrogen and water inputs to tomato plant do not trigger bottom-up effects on a leafminer parasitoid through host and non-host exposures. Pest Manag Sci 74:516–522
Efron B, Tibshirani RJ (1993) An introduction to the bootstrap. Chapman and Hall, New York
Eggenkamp-Rotteveel Mansveld MH, van Lenteren JC, Ellenbroek FJM, Woets J (1982) The parasite-host relationship between Encarsia formosa (Hymenoptera: Aphelinidae) and Trialeurodes vaporariorum (Homoptera: Aleyrodidae). XII. Population dynamics of parasite and host in a large, commercial glasshouse and test of the parasite-introduction method used in the Netherlands. Z Angew Entomol 93:258–279
FAO (2017) Detection of Bactericera cockerelli (tomato-potato psyllid) in Western Australia. https://www.ippc.int/en/countries/australia/pestreports/2017/02/detection-of-bactericera-cockerelli-tomato-potato-psyllid-in-western-australia/. Accessed 11 Oct 2021
Gebiola M, Gomez-Marco F, Simmons GS, Stouthamer R (2018) Effect of host feeding on life history traits of Tamarixia radiata, parasitoid of the Asian citrus psyllid, Diaphorina citri. BioControl 63:763–771
Giron D, Pincebourde S, Casas J (2004) Lifetime gains of host-feeding in a synovigenic parasitic wasp. Physiol Entomol 29:436–442
Gravandian M, Fathipour Y, Hajiqanbar H, Riahi E, Riddick EW (2022) Long-term effects of cattail Typha latifolia pollen on development, reproduction, and predation capacity of Neoseiulus cucumeris, a predator of Tetranychus urticae. BioControl. https://doi.org/10.1007/s10526-021-10116-4
Hajek AE, Eilenberg J (2018) Natural enemies: an introduction to biological control. Cambridge University Press, Cambridge
Hanan A, He XZ, Wang Q (2017) Insight into the success of whitefly biological control using parasitoids: evidence from the Eretmocerus warrae-Trialeurodes vaporariorum system. Pest Manag Sci 73:2294–2301
Heimpel GE, Rosenheim JA (1995) Dynamic host feeding by the parasitoid Aphytis melinus: the balance between current and future reproduction. J Anim Ecol 64:153–167
Hill JG, Aguirre MB, Bruzzone OA, Virla EG, Luft Albarracin E (2020) Influence of adult diet on fitness and reproductive traits of the egg parasitoid Anagrus virlai (Hymenoptera: Mymaridae), a potential biocontrol agent against the corn leafhopper. J Appl Entomol 144:578–588
Hossain MA, Haque MA (2015) Influence of food supplements on the reproductive potential of the parasitoid, Dinarmus basalis (Rondani) (Hymenoptera: Pteromalidae) on Callosobruchus chinensis (L.) (Coleoptera: Bruchidae). Afr Entomol 23:88–93
Hougardy E, Mills NJ (2006) The influence of host deprivation and egg expenditure on the rate of dispersal of a parasitoid following field release. Biol Control 37:206–213
Hougardy E, Mills NJ (2007) Influence of host deprivation and egg expenditure on the patch and host-finding behavior of the parasitoid wasp Mastrus ridibundus. J Insect Behav 20:229–246
Hougardy E, Bezemer TM, Mills NJ (2005) Effects of host deprivation and egg expenditure on the reproductive capacity of Mastrus ridibundus, an introduced parasitoid for the biological control of codling moth in California. Biol Control 33:96–106
Hoy MA (2008) Augmentative biological control. In: Capinera JL (ed) Encyclopedia of entomology. Springer, Dordrecht, pp 327–334
Huang YB, Chi H (2012) Assessing the application of the jackknife and bootstrap techniques to the estimation of the variability of the net reproductive rate and gross reproductive rate: a case study in Bactrocera cucurbitae (Coquillett) (Diptera: Tephritidae). J Agric For 61:37–45
Jervis MA, Kidd NAC (1986) Host-feeding strategies in hymenopteran parasitoids. Biol Rev 61:395–434
Jervis MA, Copland MJW, Harvey JA (2005) The life cycle. In: Jervis MA (ed) Insects as natural anemies: a practical perspective. Springer, Dordrecht, pp 73–165
Jervis MA, Ellers J, Harvey JA (2008) Resource acquisition, allocation, and utilization in parasitoid reproductive strategies. Annu Rev Entomol 53:361–385
Kapranas A, Luck RF (2008) Egg maturation, host feeding, and longevity in two Metaphycus parasitoids of soft scale insects. Biol Control 47:147–153
Karacaoğlu M, Keçeci M, Yarpuzlu F, Kütük H (2018) The effect of releasing the parasitoid, Aphidius colemani (Hymenoptera: Braconidae) on suppression of Myzus persicae (Hemiptera: Aphididae) populations on eggplants grown in the greenhouse. Int J Agric Biol 20:1741–1744
Kazak C, Döker İ, Karaca MM, Karut K (2020) Effect of cold storage on performance of Eretmocerus mundus, larval parasitoid of Bemisia tabaci in a conventional tomato growing greenhouse. Crop Prot 137:105293
Liu WX, Wang WX, Zhang YB, Wang W, Lu SL, Wan FH (2015) Adult diet affects the life history and host-killing behavior of a host-feeding parasitoid. Biol Control 81:58–64
Martinez AM, Chavarrieta JM, Morales SI, Caudillo KB, Figueroa JI, Diaz O, Bujanos R, Gomez B, Viñuela E, Pineda S (2015) Behavior of Tamarixia triozae females (Hymenoptera: Eulophidae) attacking Bactericera cockerelli (Hemiptera: Triozidae) and effects of three pesticides on this parasitoid. Environ Entomol 44:3–11
Morales ASI, Martínez AM, Figueroa JI, Espino AM, Chavarrieta JM, Ortíz R, Rodríguez CL, Pineda S (2013) Life parameters of the synovigenic parasitoid Tamarixia triozea (Hymenoptera. Eulophidae). Rev Colomb Entomol 39:243–249
Mou DF, Lee CC, Smith CL, Chi H (2015) Using viable eggs to accurately determine the demographic and predation potential of Harmonia dimidiata (Coleoptera: Coccinellidae). J Appl Entomol 139:579–591
Noble WS (2009) How does multiple testing correction work? Nat Biotechnol 27:1135–1137
Olaniyan O, Rodríguez-Gasol N, Cayla N, Michaud E, Wratten SD (2020) Bactericera cockerelli (Šulc), a potential threat to China’s potato industry. J Integr Agric 19:338–349
Picciau L, Alma A, Ferracini C (2019) Effect of different feeding sources on lifespan and fecundity in the biocontrol agent Torymus sinensis. Biol Control 134:45–52
Reddy GVP, Chi H (2015) Demographic comparison of sweetpotato weevil reared on a major host, Ipomoea batatas, and an alternative host, I. Triloba. Sci Rep 5:11871
Rojas P, Rodríguez-Leyva E, Lomeli-Flores JR, Liu TX (2015) Biology and life history of Tamarixia triozae, a parasitoid of the potato psyllid Bactericera cockerelli. BioControl 60:27–35
Sabbatini Peverieri G, Furlan P, Simoni S, Strong WB, Roversi PF (2012) Laboratory evaluation of Gryon pennsylvanicum (Ashmead) (Hymenoptera, Platygastridae) as a biological control agent of Leptoglossus occidentalis Heidemann (Heteroptera, Coreidae). Biol Control 61:104–111
Stahl JM, Babendreier D, Haye T (2019) Life history of Anastatus bifasciatus, a potential biological control agent of the brown marmorated stink bug in Europe. Biol Control 129:178–186
Stenberg JA, Sundh I, Becher PG, Björkman C, Dubey M, Egan PA, Friberg H, Gil JF, Jensen DF, Jonsson M, Karlsson M, Khalil S, Ninkovic V, Rehermann G, Vetukuri RR, Viketoft M (2021) When is it biological control? A framework of definitions, mechanisms, and classifications. J Pest Sci 94:665–676
Tena A, Stouthamer R, Hoddle MS (2017) Effect of host deprivation on the foraging behavior of the Asian citrus psyllid parasitoid Tamarixia radiata: observations from the laboratory and the field. Entomol Exp Appl 163:51–59
Teulon DAJ, Workman PJ, Thomas KL, Nielsen MC (2009) Bactericera cockerelli: incursion, dispersal and current distribution on vegetable crops in New Zealand. N Z Plant Prot 62:136–144
van Lenteren JC, Bueno VHP (2003) Augmentative biological control of arthropods in Latin America. BioControl 48:123–139
van Lenteren JC, Tommasini MG (1999) Mass production, storage, shipment and quality control of natural enemies. In: Albajes R, Gullino ML, van Lenteren JC, Elad Y (eds) Integrated pest and disease management in greenhouse crops. Springer, Dordrecht, pp 276–294
Wade MR, Hopkinson JE, Zalucki MP (2008) Influence of food supplementation on the fitness of two biological control agents: a predatory nabid bug and a bollworm pupal parasitoid. J Pest Sci 81:99–107
WADPIRD (Western Australian Department of Primary Industries and Regional Development) (2018) Tomato potato psyllid (TPP). https://www.agric.wa.gov.au/tomato-potato-psyllid-tpp. Accessed 11 Oct 2021
Yang XB, Campos-Figueroa M, Silva A, Henne DC (2015) Functional response, prey stage preference, and mutual interference of the Tamarixia triozae (Hymenoptera: Eulophidae) on tomato and bell pepper. J Econ Entomol 108:414–424
Yokoyama VY, Cáceres CE, Kuenen LPS, Wang XG, Rendón PA, Johnson MW, Daane KM (2010) Field performance and fitness of an olive fruit fly parasitoid, Psyttalia humilis (Hymenoptera: Braconidae), mass reared on irradiated Medfly. Biol Control 54:90–99
Yu LY, Chen ZZ, Zheng FQ, Shi AJ, Guo TT, Yeh BH, Chi H, Xu YY (2013) Demographic analysis, a comparison of the jackknife and bootstrap methods, and predation projection: A case study of Chrysopa pallens (Neuroptera: Chrysopidae). J Econ Entomol 106:1–9
Acknowledgements
We thank Mr. B. Gatimel and Mr. J. Thompson of Bioforce Ltd., Auckland, New Zealand, for providing the insects for this study and sharing experiences in their rearing. We also thank the handling editor, Dr. Eric W. Riddick, editor-in-chief, Dr. Eric Wajnberg, and two anonymous reviewers for their constructive comments, which have significantly improved the paper. This study was supported by the China Scholarship Council-Massey University Joint Scholarship Program to C.C., the New Zealand-China Doctoral Research Scholarships Programme to P.Z., and a Massey University Research Fund to Q.W.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. This study was partially funded by a China Scholarship Council-Massey University Joint Scholarship Program to C.C., a New Zealand-China Doctoral Research Scholarships Programme to P.Z., and a Massey University Research Fund to Q.W.
Author information
Authors and Affiliations
Contributions
CC, XZH and QW conceived and designed the study; CC and PZ collected the data. All author contributed to data analysis and manuscript preparation. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflicts of interest.
Research involving human participants and/or animals
No humans and/or animals were used in this study that required informed consent or submission to animal welfare committee for evaluation.
Additional information
Handling Editor: Eric Riddick.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, C., He, X.Z., Zhou, P. et al. Diets for Tamarixia triozae adults before releasing in augmentative biological control. BioControl 67, 297–306 (2022). https://doi.org/10.1007/s10526-022-10136-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-022-10136-8