Abstract
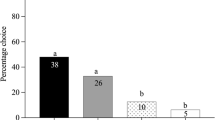
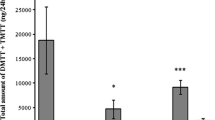
Plants emit a wide array of complex blends of volatile organic compounds that can be involved in plant communication with herbivores and their natural enemies. Bracon vulgaris Ashmead (Hymenoptera: Braconidae) is a gregarious larval ectoparasitoid that attacks the boll weevil larvae, Anthonomus grandis Boheman (Coleoptera: Curculionidae), an important pest in cotton plantations in Brazil. This parasitoid species has been studied as a potential biological control agent of A. grandis. However, little is known about B. vulgaris host foraging behaviour. We have previously demonstrated that female wasps respond to host-associated cues (boll weevil’s aggregation pheromone) and host habitat odours, such as cotton volatiles induced by the presence of the boll weevil’s pheromone. In the current study, we evaluated the electrophysiological and behavioural responses of B. vulgaris to constitutive and herbivore-induced plant volatiles (HIPVs) emitted by A. grandis-infested cotton plants at different phenological stages. The results demonstrated that B. vulgaris recognizes and responds to reproductive cotton HIPVs and that polar compounds might not be essential for its attraction. Electroantennogram (EAG) recordings and bioassays suggested that the compounds β-myrcene, (E)-ocimene, (E)-4,8-dimethylnona-1,3,7-triene (DMNT), (E)-(1R,9S)-caryophyllene, and (E,E)-4,8,12-trimethyltrideca-1,3,7,11-tetraene (TMTT), as well as other minor components of cotton blend, can be used by B. vulgaris wasps in its host foraging behaviour. Our results show an important role of terpenoids in cotton indirect defence, which is discussed relative to the role of other minor plant volatiles.






Similar content being viewed by others
References
Adams CH, Cross W, Mitchell HC (1969) Biology of Bracon mellitor, a parasite of the boll weevil. J Econ Entomol 62:889–896
Allmann S, Baldwin IT (2010) Insects betray themselves in nature to predators by rapid isomerization of green leaf volatiles. Science 329:1075–1078
Alves TJSA, Wanderley-Teixeira V, Teixeira AAC, Silva-Torres CSA, Malaquias JB, Pereira BF, Cunha FM (2014) Parasitoid–host interaction: sensory structures involved in the parasitism behavior of Bracon vulgaris (Hymenoptera: Braconidae). Anim Biol 64:365–381
Alves TJS, Silva-Torres CSA, Wanderley-Teixeira V, Teixeira AAC, Torres JB, Lima TA, Ramalho FS (2015) Behavioral studies of the parasitoid Bracon vulagris Ahsmead (Hymenoptera: Braconidae). J Insect Behav 28:604–617
Blassioli-Moraes MC, Borges M, Laumann RA (2013) The application of chemical cues in arthropod pest management for arable crops. In: Wajnberg E, Colazza S (eds) Chemical Ecology of insect parasitoids. Wiley-Blackwell, Oxford, pp 225–244
Blassioli-Moraes MC, Borges M, Michereff MFF, Magalhães DM, Laumann RA (2016) Semiochemicals from plants and insects on the foraging behavior of Platygastridae egg parasitoids. Pesq Agropec Bras 41:454–464
Boncan DAT, Tsang SSK, Li C, Lee IHT, Lam HM, Chan TF, Hui JHL (2020) Terpenes and terpenoids in plants: interactions with environment and insects. J Mol Sci 32:7382
Bruce TJA, Pickett JA (2011) Perception of plant volatile blends by herbivorous insects—finding the right mix. Phytochem 72:1605–1611
Carvalho SL, Fernandes WD, Habib MEM, Patel PN (2002) Preference of Bracon vulgaris and adequacy of some hosts under laboratory conditions. Rev Agr 77:39–56
Clavijo-McCormick A, Unsicker SB, Gershenzon J (2012) The specificity of herbivore-induced plant volatiles in attracting herbivore enemies. Trends Plant Sci 17:303–310
Clavijo-McCormick A, Gershenzon J, Unsicker SB (2014) Little peaks with big effects: establishing the role of minor plant volatiles in plant-insect interactions. Plant Cell & Environ 37:1836–1844
Colazza S, Fucarino A, Peri E, Salerno G, Conti E, Bin F (2004) Insect oviposition induces volatile emission in herbaceous plants that attracts egg parasitoids. J Exp Biol 207:47–53
D’Alessandro M, Turlings TCJ (2005) In situ modification of herbivore induced plant odors: a novel approach to study the attractiveness of volatile organic compounds to parasitic wasps. Chem Senses 30:739–753
De Moraes CM, Lewis WJ, Tumlinson JH (2000) Examining plant-parasitoid interactions in tritrophic systems. An Soc Entomol Bras 29:189–203
Dicke M (1994) Local and systemic production of volatile herbivore-induced terpenoids: their role in plant-carnivore mutualism. J Plant Physiol 143:465–472
Dicke M, Lucas-Barbosa D (2020) Herbivore-induced plant volatiles as source of information in plant-inset networks. In: Pichersky E, Dudareva N (eds) Biology of plant volatiles. CRC Press, Boca Raton, pp 327–346
Dudareva N, Klempien A, Muhlemann JK, Kaplan I (2013) Biosynthesis, function and metabolic engineering of plant volatile organic compounds. New Phytol 198:16–32
Khan ZR, Midega CAO, Njuguna EM, Amudavi DM, Wanyama JM, Pickett JA (2008) Economic performance of ‘push–pull’ technology for stemborer and Striga control in smallholder farming systems in western Kenya. Crop Prot 27:1084–1097
Magalhães DM, Borges M, Laumann RA, Sujii ER, Mayor P, Caulfield JC, Midega CA, Khan ZR, Pickett JA, Birkett MA, Blassioli-Moraes MC (2012) Semiochemicals from herbivory induced cotton plants enhance the foraging behaviour of the cotton boll weevil, Anthonomus grandis. J Chem Ecol 38:1528–1538
Magalhães DM, Borges M, Laumann RA, Woodcock CM, Pickett JA, Blassioli-Moraes MC (2016) Influence of two acyclic homoterpenes (Tetranorterpenes) on the foraging behavior of Anthonomus grandis Boh. J Chem Ecol 42:305–313
Magalhães DM, Borges M, Laumann RA, Woodcock CM, Withall DM, Pickett JA, Birkett MA, Blassioli-Moraes MC (2018) Identification of volatile compounds involved in host location by Anthonomus grandis (Coleoptera: Curculionidae). Front Ecol Evol 6:98
Magalhães DM, Silva ITFA, Borges M, Laumann RA, Blassioli-Moraes MC (2019) Anthonomus grandis aggregation pheromone induces cotton defence and attracts the parasitic wasp Bracon vulgaris. J Exp Bot 70:1891–1901
Magalhães DM, Borges M, Laumann RA, Caulfield JC, Birkett MA, Blassioli-Moraes MC (2020) Inefficient weapon—the role of plant secondary metabolites in cotton defence against the boll weevil. Planta 252:94
Neves RCS, Torres JB, Barros EM, Vivan LC (2018) Boll weevil season and off-season activity monitored using a pheromone-and-glue reusable tube trap. Sci Agr 75:313–320
Optiz S, Kunert G, Gersehenzon J (2008) Increased terpenoid accumulation in cotton (Gossypium hirsutum) foliage is a general wound response. J Chem Ecol 34:508–522
Pallini A, Silvie P, Monnerat RG, De Ramalho FS, Songa JM, Birch ANE (2006) Non-target and biodiversity impacts on parasitoids. In: Angelika H, Andow DA, Fontes EMG (eds) Environmental risk assessment of genetically modified organisms: methodologies for assessing Bt cotton in Brazil. CABI Publishing, Wallingford, UK, pp 200–224
Pickett JA, Khan ZR (2016) Plant volatile-mediated signalling and its application in agriculture: successes and challenges. New Phytol 212:856–870
Pierik R, Ballaré CL, Dicke M (2014) Ecology of plant volatiles: taking a plant community perspective. Plant, Cell & Environ 37:1845–1853
R Core Team (2020) R: A Language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.r-project.org/.
Roh GH, Kim J, Park CG (2017) Possible antagonism of (Z)-2-hexenyl (E)-3-hexenoate against the attractiveness of (E)-2-hexenyl (Z)-3-hexenoate to Ooencyrtus nezarae (Hymenoptera: Encyrtidae). J Appl Entomol 142:327–332
Rutledge CE (1996) A survey of identified kairomones and synomones used by insect parasitoids. Chemoecology, 7:121–131
Schmidt FGV, Monnerat R, Borges M, Carvalho R (2001) Criação de insetos para avaliação de agentes entomopatogênicos e semioquímicos. Embrapa Recursos Genéticos e Biotecnologia, Circular Técnica 11:1–20
Showler AT (2008) Longevity and egg development of adult female boll weevils fed exclusively on different parts and stages of cotton fruiting bodies. Entomol Exp App 127:125–132
Smid HM, Vet LEM (2016) The complexity of learning, memory and neural processes in an evolutionary ecological context. Curr Opin Insect Sci 15:61–69
Steidle JLM, van Loon JJA (2003) Dietary specialization and infochemical use in carnivorous arthropods: testing a concept. Entomol Exp Appl 108:133–148
Toscano LC, Carvalho SL (2000a) Parasitismo em Pectinophora gossypiella Saunders (Lepidoptera: Gelechiidae) e Anagasta Kuhniella Zeller (Lepidoptera: Pyralidae) por Bracon vulgaris Ashmead (Hymenoptera: Braconidae). Braz J Ecol 12:23–28
Toscano LC, Carvalho SL (2000b) Parasitismo em Anthonomus grandis Boheman, 1843 por Bracon vulgaris, Ashmead em cultura de algodão sem medidas de controle na região de Ilha Solteira-SP. Braz J Ecol 12:123–127
Turlings TCJ, Erb M (2018) Tritrophic interactions mediated by herbivore-induced plant volatiles: Mechanisms, ecological relevance, and application potential. Annu Rev Entomol 63:433–452
van Wijk M, de Bruijn PJA, Sabelis MW (2011) Complex odor from plants under attack: herbivore’s enemies react to the whole, not its parts. PLoS ONE 6(7):e21742
Vinson SB (1976) Host selection by insect parasitoids. Annu Rev Entomol 21:109–133
Wanderley PA, Ramalho FS (1996) Biology and thermal requirements of Catolaccus grandis (Burks) (Hymenoptera: Pteromalidae) parasitoid of cotton boll weevil. Pesq Agropec Bras 31:237–247
Acknowledgements
We thank Isabela Grisi, Sulian Gomes de Azevedo and Helio Moreira da Silva for helping with laboratory rearing of the insects. This work received financial support from the Coordination of Superior Level Staff Improving (CAPES) through a grant to Izabela Thais Alves da Silva, the National Council for Scientific and Technological Development (CNPq), the Federal District Research Foundation (FAP-DF) and the Brazilian Corporation of Agricultural Research (EMBRAPA, project number 20.19.03.016.00.02.007).
Funding
This work was funded by the Empresa Brasileira de Pesquisa Agropecuaria, 20.19.03.016.00.02.007 (for Maria Carolina Blassioli Moraes), the Coordenacao de Aperfeicoamento de Pessoal de Nivel Superior, 88881.1317661/2014–01 (for Izabela Thais Fidelis Alves da Silva), and the Conselho Nacional de Desenvolvimento Cientifico e Tecnologico, 304329/2017–7 (for Maria Carolina Blassioli Moraes).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent to participate
All authors declared that no competing interests exist. All authors and relevant institutions have read the submitted version of the manuscript and approve its submission.
Consent for publication
The work as submitted has not been published or accepted for publication, nor is being considered for publication elsewhere, either in whole or substantial part.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Handling Editor: Stefano Colazza.
Rights and permissions
About this article
Cite this article
Da Silva, I.T.F.A., Magalhães, D.M., Borges, M. et al. Exploitation of herbivore-induced cotton volatiles by the parasitic wasp Bracon vulgaris reveals a dominant chemotactic effect of terpenoids. BioControl 67, 135–148 (2022). https://doi.org/10.1007/s10526-022-10135-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-022-10135-9