Abstract
Entomopathogenic nematodes (EPNs) are effective against the immature stages of the large pine weevil Hylobius abietis. In three field trials we compared the efficacy of the application method of EPN for weevil suppression below the suggested threshold of 20 weevils per stump: applying the EPN suspension in the top edges of the stumps (‘top’) vs. drenching the soil around stumps (‘standard’). For Steinernema carpocapsae, weevil suppression was below the targeted threshold only when suspension was applied in the standard way (two of the three sites). On the other hand, weevil suppression was provided in all three cases of ‘top’ application of Heterorhabditis downesi suspension, whereas suppression in ‘standard’ application was observed in one site. Percentage parasitism of developing weevils in relation to depth and distance help explain EPN movement post-application. Weevil suppression relative to suggested thresholds can be improved by altering the method of EPN application depending on the nematode species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Entomopathogenic nematodes (EPNs) have been successfully used for biological control of soil pests (Lacey and Georgis 2012; Campos-Herrera 2015). However, the persistence of EPNs following application rapidly declines due to biotic and abiotic factors and therefore large numbers of EPNs are typically applied (Griffin 2015). Entomopathogenic nematode efficacy in soils is highly dependent on several factors such as soil texture, moisture, temperature and chemistry (Stuart et al. 2015). Thus applications of EPNs should take into account all these abiotic factors to warrant success in providing adequate control. Application methods aim to maximize first EPN survival in soils and secondly movement towards their hosts, e.g., application early or late in the day to avoid damaging levels of UV is recommended (Cabanillas and Raulston 1995). When application of EPN is directed on the soil, adequate moisture for survival and movement is required, and thus irrigation is recommended (Shetlar et al. 1988; Downing 1994; Koppenhöfer et al. 1995). Other ways for improving efficacy of EPN applications are related to equipment modification (e.g., nozzles, pumps, spray distribution) for enhanced survival and dispersion (Shapiro-Ilan et al. 2006; Brusselman et al. 2012). EPN are also used successfully for controlling tree-boring pests, and in such cases the application method can be more sophisticated, using for instance gels and injections, rather than just drenching in the soil around the tree (De altube et al. 2008; Dembilio et al. 2010; Morton and García Del Pino 2008; El-borai et al. 2012; Shapiro-Ilan et al. 2010, 2015, 2016a, b), but little is known about dispersal and host-finding in the tree environment (Santhi et al., 2015).
Biological control of the large pine weevil Hylobius abietis (Coleoptera: Curculionidae) (LPW) using entomopathogenic nematodes (EPNs) has become a feasible sustainable method over the last decade (Torr et al. 2005; Brixey et al. 2006; Dillon et al. 2006, 2007, 2008; Williams et al. 2013a; Kapranas et al. 2017). The LPW is the most important pest of tree seedlings in replanted coniferous forests in Northern Europe (Evans et al. 2015). The weevils are attracted to clear-felled areas by volatile chemicals emitted by the stumps of recently felled trees. They oviposit in the stumps and immature weevils develop under the bark (Leather et al. 1999). Upon emergence, adult weevils feed on young seedlings and can cause significant mortality: a single adult can damage or kill several young plants (Eidmann and Lindelow 1997, Heritage and Moore 2001) and thus even a low number of adults emerging from stumps can have a significant impact on sites that have been replanted. Current approaches to management of LPW are moving away from pesticide applications and there is a need for more environmentally sustainable approaches, besides delaying of restocking (Leather et al. 1999; Örlander and Nilsson 1999; Örlander and Nordlander 2003).
EPNs are the most promising biological control agents of LPW applied in augmentative fashion (Torr et al. 2005; Brixey et al. 2006; Dillon et al. 2006, 2007, 2008; Williams et al. 2013a, b; Kapranas et al. 2017). It has been shown that EPN species with different foraging strategies give adequate control in particular with reference to accepted infestation thresholds of 20 weevils per stumps, in a variety of forest soils, including both peats and mineral soils (Wainhouse et al. 2007; Kapranas et al. 2017; unpublished note Coillte, Ireland). In most of the previous trials on the use of EPN, typically nematode suspension is poured in close proximity around the stump (drenching). Previous studies suggest that EPN reach their target, at least to some extent, by passive movement of the suspension liquid (Dillon et al. 2006; Williams et al. 2013a; Kapranas et al. 2017). It has been shown that artificial root-routeways are important for facilitating EPN movement towards their subterranean hosts (Ennis et al. 2010) and thus it is reasonable to hypothesize that EPN applied in closer contact with the roots will provide better defence against LPW. Moreover, Brixey et al. (2006) found that a subsurface drench, where nematodes were covered by litter, was more successful than a surface spraying, and attributed this to the EPN infective juveniles (IJs) being protected from damaging UV and desiccation. As tree stumps begin to dry and decay, the bark becomes loosened from the wood, providing a protected space in between them. Since this is also the zone in which feeding LPW larvae are found, we hypothesize that slight modification of the application method of the EPN suspension so that at least some volume can enter between the loosened bark and the wood and can follow the internal root-routeways of the stumps while being protected from UV and desiccation, would increase the efficacy of the application.
The main objective of this study was to test whether application of EPN suspension in the pine stumps in a way that facilitates their movement along the protected root-routeways between bark and wood provides a better means of controlling LPW in comparison to the more traditional approach of applying the EPN suspension on the soil in close proximity to the stump. In a previous study EPN application methods against LPW were compared, but in both methods EPN were applied either in or on the soil (Brixey et al. 2006). In this study we compare a surface drench around the stump with drenching on the top periphery of the stump.
Materials and methods
Field sites and nematode application
Overall we conducted three trials: in one site in 2014 (Cloondara) and in two sites in 2015 (Doon and Tigroney). All three sites were clear-felled lodgepole pine Pinus contorta Dougl. var. latifolia which supports a large number of weevils (Thorpe and Day 2002). Cloondara and Doon had a peat soil which is rich in organic matter whereas Tigroney had a mineral, lithosol type soil with a thinner organic topsoil layer. More details about the field sites are given by Kapranas et al. (2017).
Steinernema carpocapsae (EN03) and Heterorhabditis downesi (K122) used for the trials were provided by e-nema GmbH. Packages with EPN IJs were stored for less than a week at 9 °C until the day of application (first two weeks of June 2014 and 2015). The EPN dose applied to each stump was ca. 3.5 × 106 IJs in 500 ml of water. The EPN suspension was applied either in the standard fashion of drenching the soil close to the periphery of each stump (‘standard’; Fig. 1a) or by pouring the suspension primarily around the top edge of the stump such that the majority of the suspension entered the gap between bark and wood (‘top’; Fig. 1b). In each site there were 100 stumps with 20 stumps assigned to each of five treatments: 1) control, 2) H. downesi with the ‘standard’ method of application, 3) H. downesi applied on ‘top’, 4) S. carpocapsae ‘standard’ and 5) S. carpocapsae applied on ‘top’. Treatments were arranged in a randomized block design with each block bearing one stump for each treatment. For each treatment, half of the stumps (ten per treatment) were destructively sampled for assessment of parasitism rates and the other half (ten) were selected for monitoring emergence of weevils (placement of traps).
Parasitism rates and efficacy of EPN treatments
Parasitism rates of LPW were assessed by removing the bark of about one quarter of the stump with a chisel to a depth of at least 40 cm under the soil surface, and recording the stage (larva, pupa, adult), status (healthy, parasitized by nematode, parasitized by fungi, dead by undetermined reason) and location (depth relative to soil level and distance from bole) of each individual pine weevil. Healthy weevils were removed with clean forceps, placed in 24-well plates and transferred to the laboratory. They were then incubated at ~20 °C for another two weeks to check for post-sampling EPN mortality. Efficacy of treatments was assessed by collection of adult weevils in emergence traps (Moore 2001) that were erected about two weeks after EPN application. Traps were sampled every 2-4 weeks throughout the season, starting mid-July until weevil emergence ceased in November-December. General methods follow established protocols as described for previous studies (Williams et al. 2013a; Kapranas et al. 2017).
Statistical analysis
Analysis of factors influencing immature weevil parasitism rates and adult weevil emergence was performed with Generalized Linear Models (GLMs) (Crawley 1993, 2007). We assumed quasi-binomial error variance with a logit link for parasitism (proportional) data and after rescaling for potential over- or under-dispersion, significance of effects (site, nematode species and method of application) was assessed by the change in deviance when a variable was removed from the full model (the significance of explanatory variables was assessed by F ratio tests). We ran models for assessing effect significance both ‘on site’ and after ca. ten days of laboratory incubation. P-values assuming quasi-binomial errors are not exact but estimates and are asymptotically correct, thus when close to 0.05 should be interpreted with caution (Crawley 1993, 2007). For each site, we explored parasitism rates in relation to depth below soil surface and horizontal distance from the bole of the stump by using a mixed effect binary logistic regression and a logit link. Type of intervention which corresponds to nematode species and application method (a four levels factor), depth and distance were introduced as fixed effects, whereas each stump was introduced in the analysis as a random effect. For emergence data (cumulative trap collections over the season) we assumed a normal error variance and used a one-way ANOVA followed by a Fisher’s least significant difference test, to detect differences among means across all site and treatment combinations, with the controls included (α = 0.05). A complementary one-tailed t-test comparing trap catches with a mean of 20 which is the number of weevils per stump that are indicated as a threshold for chemical treatment as recommended by Coillte (Ireland’s national forestry company), was also performed.
Results
Parasitism rates assessed by destructive sampling of stumps
In general, parasitism rates of LPW either before or after laboratory incubation were influenced by site (Table 1; Fig. 2) but they were not influenced by EPN species (Table 1). Method of application did not significantly influence parasitism rates either before or after laboratory incubation. However there was an overall notable trend of application method being significant when parasitism rates were assessed after laboratory incubation (P = 0.058, Table 1), or having a nearly significant interaction with nematode species for parasitism rates recorded on site (P = 0.054, Table 1).
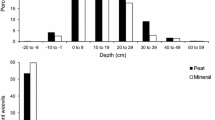
Percentage parasitism of Hylobius abietis by Heterorhabditis downesi and Steinernema carpocapsae in stumps at different sites. Parasitism rates shown are after laboratory incubation of healthy weevils for two weeks at ~20 °C to check for post-sampling EPN mortality (total parasitism rates). Bars show average values with asymmetrical, quasi-binomially distributed standard errors
Parasitism in relation to depth and distance from bole
In Cloondara, weevil parasitism declined with depth (F1,568 = 14.23, P < 0.001; Fig. 3a) and distance from bole (F1,568 = 40.85, P < 0.001; Fig. 3b). The type of intervention (nematode species and application method) significantly influenced parasitism rates (F3,568 = 2.64, P = 0.048). The interaction of intervention and distance was also significant (F3,568 = 3.08, P = 0.026), (Fig. 3b). In Doon, weevil parasitism declined with depth (F1,1281 = 63.49, P < 0.001; Fig. 3c) and distance from bole (F1,1281 = 164.76, P < 0.001; Fig. 3d). The type of intervention (nematode species and application method) did not significantly influence parasitism rates (F3,1281 = 1.83, P = 0.140). However, the interaction of intervention and depth was significant (F3,1281 = 6.91, P < 0.001; Fig. 3c). In Tigroney, weevil parasitism declined with depth (F1,394 =20.12, P < 0.001) and distance from bole (F1,394 = 70.46, P < 0.001). The type of intervention (nematode species and application method) did not significantly influence parasitism rates (F3,394 = 1.91, P = 0.126).
Parasitism of Hylobius abietis by Heterorhabditis downesi and Steinernema carpocapsae in relation to depth and distance when applied in different application methods at Cloondara and Doon sites. Negative values of depth correspond to weevils found in stumps above ground. Points show actual data and lines show the fitted logistic regression models. Parasitism rates shown are total parasitism rates after incubation of healthy weevils for two weeks at ~20°C
Efficacy of treatments assessed by trapped adult pine weevils
H. downesi-treated stumps always (all three sites) had fewer emerging adult weevils than control ones, whereas for S. carpocapsae, weevil numbers differed from control stumps in only two out of three sites. The method of application of nematode suspension did not affect weevil numbers for either nematode species at any site (Fig. 4). However, in some instances, the two different methods of application led to differential suppression with reference to the suggested threshold of 20 weevils per stump (Fig. 4). S. carpocapsae was successful in suppressing weevils below the suggested threshold only when the suspension was applied with the ‘standard’ method (two out of three times). On the other hand, H. downesi application was more frequently successful (all three times) when suspension was applied on top of the stump, compared to just once with the standard application.
Numbers of adult Hylobius abietis (average ± SE) emerging from control stumps and stumps treated with suspensions of entomopathogenic nematodes (Heterorhabditis downesi and Steinernema carpocapsae) that were applied in two different methods: ‘standard’ in the soil around the stump and ‘top’ on top edges of the stump (between bark and wood) at three sites. Different letters in the base of the bars show significantly different treatments within each site determined by post-hoc tests (Fisher’s least significant difference test, P < 0.05), and asterisks on top of the charts denote treatments wherein weevil numbers are less than 20 per stump (horizontal dashed line)
Discussion
Previous research has shown that application of either H. downesi or S. carpocapsae can provide significant suppression of LPW below acceptable thresholds (Kapranas et al. 2017). Our trials at seven different sites over two years showed that H. downesi gave slightly better results but not significantly different from S. carpocapsae (Kapranas et al. 2017), in contrast to earlier work showing that H. downesi was superior (Dillon et al. 2006; Williams et al. 2013a, b). Typically EPN suspension is applied directly to the soil around the stump (Dillon et al 2006; Williams et al. 2013a; Kapranas et al. 2017), but in our study we tested a modified method of application by applying the suspension on the top edges of the stump, targeting the gap between the bark and the wood (though some of the suspension will eventually make it to soil by spill over the stump). In the latter method it is likely that some nematodes were more exposed to UV light and desiccation and consequently died but, on the other hand, most of the nematodes entered the space between the wood and the bark and would have transferred, at least to some extent passively, along the rootways, thus reaching more quickly the weevils that are typically found there.
Steinernema carpocapsae performed better when applied by drenching the suspension around the stump, rather than when applied on top of the stumps: parasitism rates tended to be higher, but not statistically significant. However, weevil suppression was greater, in particular with reference to weevil infestation thresholds set at 20 weevils per stump. In fact, S. carpocapsae provided adequate control only when nematodes were drenched in the soil. One hypothesis to explain why S. carpocapsae does better when applied to soil rather than at the top is that IJs that are carried into the space between the bark and wood may be encouraged to sit and wait there. S. carpocapsae was detected in this location up to two years post-application, though this was assumed to include nematodes that had recycled in LPW (Harvey and Griffin, 2016). The host species with which S. carpocapsae has most frequently been associated is the codling moth, Cydia pomonella (Peters 1996). Codling moths spend most of their life cycle as larvae within fruits. Natural infections are of cocooned larvae located near the base of trees and close to the soil (Lacey et al. 2006). For a parasite targeting larvae moving from fruit to soil the ideal location to sit and wait would be the protected areas on the tree bark. Previous research, using S. carpocapsae in a peaty-gley, clear-felled area has also shown that application of this species provides better control when the applied nematodes were immediately covered by soil (subsurface drench) than when applied to the soil surface (Brixey et al. 2006). On the other hand H. downesi seems to be more efficacious in the ‘top’ vs. the ‘standard’ application. Specifically, weevil suppression with reference to the infestation threshold (20 weevils per stump) was greater when this species was applied on top in all three field trials, whereas adequate suppression when applied around the stump was observed in only one of them. These results can be explained by mutually non-exclusive hypotheses. Firstly, the space between bark and wood may provide suitable conditions for “cruise forager” H. downesi to detect and move towards pine weevil volatiles. Secondly, H. downesi might be more vulnerable to predation by collembolans, mites and nematophagous fungi found in the soil. For instance, in previous studies with H. bacteriophora, poor persistence was positively correlated with numbers of mites and collembola in plots where nematodes were surface-applied, but not in plots where they were subsurface-applied (Wilson and Gaugler 2004). However, other studies suggested that EPN natural enemies such as mites and nematophagous fungi show stronger responses in the presence of steinernematids rather than heterorhabditids (Duncan et al. 2007; Greenwood et al. 2011). Lastly it is possible that nematodes applied on the top edges of the stump more easily reach other host species (such as Pissodes spp.) that occur in between the wood and the bark, and that these hosts are more susceptible to H. downesi, thus improving recycling post-application. Thus, application of the EPN suspension on top of the stump where a proportion is carried down between the bark and wood might be beneficial, even to an extent that offsets increased mortality of the portion of the suspension that is subject to the detriments of UV light and desiccation if it remains on the outside of the stump.
Parasitism rates of weevils declined both in relation to depth and distance from the bole, which is in agreement with previous studies (Dillon et al. 2006; Williams et al. 2013a, b; Kapranas et al. 2017). However, these rates of decline are further influenced by the method of application. Our results show that at, Cloondara, ‘top’ application of H. downesi led to a steeper decline in parasitism in relation to distance from bole, suggesting that the nematodes stayed close to the bole and did not disperse to the more distant parts of the root system. Since most of the weevils at this site were close to the bole (ca. 72% of weevils were less than 10 cm distance from the bole compared to 49% at Doon and 51% at Tigroney), killing a high proportion in this region should result in higher parasitism overall and a drop off in percentage parasitism with distance might have not been important. However, application of nematode suspension on top of the stump can be more efficacious against the weevils that are found in the stump above ground level, as indicated by significant interaction of intervention and depth at Doon (Fig. 3c). Typically, only a small proportion of weevils (on average 10 %) is found above ground, but in sites with a higher proportion above ground a top application might be recommended. Kapranas et al. (2017) showed that pine weevil distribution within stumps, as influenced by soil properties, can explain patterns of parasitism and suppression by EPN. The results of the current trials indicate that weevil distribution may also help explain the relative success of different application methods. Results of these trials represent a rare insight into how application method may influence parasitism and hence efficacy of EPN applied against a tree-boring pest.
In conclusion our results show that even simple adjustment in the application of EPN suspension around the stumps of pine trees can have significant effects on weevil suppression to below economic damage thresholds. However, increased weevil suppression resulting from alternative methods of application depends on EPN species. While for S. carpocapsae better results are always achieved when the suspension is applied directly on the soil around the stump, for H. downesi it appears that LPW suppression can be anticipated more frequently when the EPN suspension is applied in top of the stump. Spray workers could direct the application of EPN suspension either in close proximity to the soil or on the top edges of stumps depending on the species being used, thus enhancing the efficacy of the EPN suspension.
References
Brixey JM, Moore R, Milner AD (2006) Effect of entomopathogenic nematode (Steinernema carpocapsae Weiser) application technique on the efficacy and distribution of infection of the large pine weevil (Hylobius abietis L.) in stumps of Sitka spruce (Picea sitchensis Carr.) created at different times. Forest Ecol Manag 226:161–172
Brusselman E, Beck B, Pollet S, Temmerman F, Spanoghe P, Moens M, Nuyttens D (2012) Effect of the spray application technique on the deposition of entomopathogenic nematodes in vegetables. Pest Manag Sci 68:444–453
Cabanillas HE, Raulston JR (1995) Impact of Steinernema riobravis (Rhabditida: Steinernematidae) on the control of Helicoverpa zea (Lepidoptera: Noctuidae) in corn. J Econ Entomol 88:58–64
Campos-Herrera R (2015) Nematode pathogenesis of insects and other pests - ecology and applied technologies for sustainable plant and crop protection. Springer International Publishing
Crawley M (1993) GLIM for ecologists. Blackwell Scientific, Oxford
Crawley M (2007) The R Book. Wiley (UK), Chichester
De Altube MM, Strauch O, De Castro GF, Pena AM (2008) Control of the flat–headed root borer Capnodis tenebrionis (Linne) (Coleoptera: Buprestidae) with the entomopathogenic nematode Steinernema carpocapsae (Weiser) (Nematoda: Steinernematidae) in a chitosan formulation in apricot orchards. BioControl 53:531–539
Dembilio O, Llacer E, De Altube MM, Jacas JA (2010) Field efficacy of imidacloprid and Steinernema carpocapsae in a chitosan formulation against the red palm weevil Rhynchophorus ferrugineus (Coleoptera: Curculionidae) in Phoenix canariensis. Pest Manag Sci 66:365–370
Dillon AB, Ward D, Downes MJ, Griffin CT (2006) Suppression of the large pine weevil Hylobius abietis (L.) (Coleoptera: Curculionidae) in pine stumps by entomopathogenic nematodes with different foraging strategies. Biol Control 38:217–226
Dillon AB, Downes MJ, Ward D, Griffin CT (2007) Optimizing application of entomopathogenic nematodes to manage large pine weevil, Hylobius abietis L. (Coleoptera: Curculionidae) populations developing in pine stumps, Pinus sylvestris. Biol Control 40:253–263
Dillon AB, Moore CP, Downes MJ, Griffin CT (2008) Evict or Infect? Managing populations of the large pine weevil, Hylobius abietis, using a bottom-up and top-down approach. Forest Ecol Manag 255:2634–2642
Downing AS (1994) Effect of irrigation and spray volume on efficacy of entomopathogenic nematodes (Rhabditida: Heterorhabditidae) against white grubs (Coleoptera: Scarabaeidae). J Econ Entomol 87:643–646
Duncan LW, Graham JH, Zellers J, Bright D, Dunn DC, El-Borai FE, Porazinska DL (2007) Food web responses to augmenting the entomopathogenic nematodes in bare and animal manure–mulched soil. J Nematol 39:176–189
Eidmann HH, Lindelow A (1997) Estimates and measurements of pine weevil feeding on conifer seedlings: their relationships and application. Can J Forest Res 27:1068–1073
El-Borai FE, Stuart RJ, Campos-Herrera R, Pathak E, Duncan LW (2012) Entomopathogenic nematodes, root weevil larvae, and dynamic interactions among soil texture, plant growth, herbivory, and predation. J Invertebr Pathol 109:134–142
Ennis DE, Dillon AB, Griffin CT (2010) Simulated roots and host feeding enhance infection of subterranean insects by the entomopathogenic nematode Steinernema carpocapsae. J Invertebr Pathol 103:140–143
Evans H, McAllister F, Saunders T, Moore R, Jenkins T, Butt T, Ansari M, Griffin C, Williams C, Teck R, Sweeney J (2015) The Impact project guide to Hylobius management 2015. Taliesin Communications. http://www.impactproject.eu/uploads/impact_hylobius_publication.pdf
Greenwood CM, Barbercheck ME, Brownie C (2011) Short term response of soil microinvertebrates to application of entomopathogenic nematode–infected insects in two tillage systems. Pedobiologia 54:177–186
Griffin CT (2015) Behaviour and population dynamics of entomopathogenic nematodes following application. In: Campos-Herrera R (ed.) Nematode pathogenesis of insects and other pests - ecology and applied technologies for sustainable plant and crop protection, Springer International Publishing, pp 57-96
Harvey CD, Griffin CT (2016) Local host-dependent persistence of the entomopathogenic nematode Steinernema carpocapsae used to control the large pine weevil Hylobius abietis. BioControl 61:185–193
Heritage S, Moore R (2001) The assessment of site characteristics as part of a management strategy to reduce damage by Hylobius. Forestry Commission Information Note 38, HMSO, UK.
Kapranas A, Malone B, Quinn S, McNamara L, Williams CD, O’Tuama P, Peters A, Griffin CT (2017) Efficacy of entomopathogenic nematodes for control of large pine weevil, Hylobius abietis: effects of soil type, pest density and spatial distribution. J Pest Sci 90:495–505
Koppenhöfer AM, Kaya HK, Taormino SP (1995) Infectivity of entomopathogenic nematodes (Rhabditida: Steinernematidae) at different soil depths and moistures. J Invertebr Pathol 65:193–199
Shapiro-Ilan DI, Gouge DH, Piggott SJ, Patterson Fife J (2006) Application technology and environmental considerations for use of entomopathogenic nematodes in biological control. Biol Control 38:124–133
Lacey LA, Arthurs SP, Unruh TR, Headrick HL, Fritts R Jr (2006) Entomopathogenic nematodes for control of codling moth (Lepidoptera: Tortricidae) in apple and pear orchards: effect of nematode species and seasonal temperatures, adjuvants, application equipment and post-application irrigation. Biol Control 37:214–223
Lacey LA, Georgis R (2012) Entomopathogenic nematodes for control of insect pests above and below ground with comments on commercial production. J Nematol 44:218–225
Leather SR, Day KR, Salisbury AN (1999) The biology and ecology of the large pine weevil, Hylobius abietis (Coleoptera: Curculionidae): a problem of dispersal? Bull Entomol Res 89:3–16
Moore R (2001) Emergence trap developed to capture adult large pine weevil Hylobius abietis (Coleoptera: Curculionidae) and its parasite Bracon hylobii (Hymenoptera: Braconidae). Bull Entomol Res 91:109–115
Morton A, García Del Pino F (2008) Field efficacy of the entomopathogenic nematode Steinernema feltiae against the Mediterranean flat-headed rootborer Capnodis tenebrionis. J Appl Entomol 132:632–637
Örlander GR, Nilsson U (1999) Effect of reforestation methods on pine weevil (Hylobius abietis) damage and seedling survival. Scand J For Res 14:341–354
Örlander GR, Nordlander GR (2003) Effects of field vegetation control on pine weevil (Hylobius abietis) damage to newly planted Norway spruce seedlings. Ann For Sci 60:667–671
Peters A (1996) The natural host range of Steinernema and Heterorhabditis spp. and their impact on insect populations. Biocontrol Sci Technol 6:389–402
Santhi VS, Salame L, Nakache Y, Koltai H, Soroker V, Glazer I (2015) Attraction of entomopathogenic nematodes Steinernema carpocapsae and Heterorhabditis bacteriophora to the red palm weevil (Rhynchophorus ferrugineus). Biol Control 83:75–81
Shapiro-Ilan DI, Cottrell TE, Mizell RF, Horton DL, Behle RW, Dunlap CA (2010) Efficacy of Steinernema carpocapsae for control of the lesser peachtree borer, Synanthedon pictipes: Improved aboveground suppression with a novel gel application. Biol Control 54:23–28
Shapiro-Ilan DI, Cottrell TE, Mizell RF, Horton DL, Zaid A (2015) Field suppression of the peachtree borer, Synanthedon exitiosa, using Steinernema carpocapsae: Effects of irrigation, a sprayable gel and application method. Biol Control 82:7–12
Shapiro-Ilan DI, Cottrell TE, Mizell RF, Horton DL (2016a) Efficacy of Steinernema carpocapsae plus fire gel applied as a single spray for control of the lesser peachtree borer, Synanthedon pictipes. Biol Control 94:33–36
Shapiro-Ilan DI, Cottrell TE, Mizell RF, Horton DL (2016b) curative control of peach treee borer with entomopathogneic nematodes. Journal of Nematol 48:170–176
Shetlar DJ, Suleman PE, Georgis R (1988) Irrigation and use of entomogenous nematodes, Neoaplectana spp. and Heterorhabditis heliothidis (Rhabditida: Steinernematidae and Heterorhabditidae), for control of Japanese beetle (Coleoptera: Scarabaeidae) grubs in turfgrass. J Econ Entomol 81:1318–1322
Stuart RJ, Barbercheck ME, Grewal PS (2015) Entomopathogenic nematodes in the soil environment: distributions, interactions and the influence of biotic and abiotic factors In: Campos-Herrera R (ed) Nematode pathogenesis of insects and other pests - ecology and applied technologies for sustainable plant and crop protection, Springer International Publishing, pp 97-137
Thorpe KV, Day KR (2002) The impact of host plant species on the larval development of the large pine weevil Hylobius abietis L. Agric Forest Entomol 4:187–194
Torr PS, Wilson MJ, Heritage S (2005) Forestry applications. In: Grewal PS, Ehlers R-U, Shapiro-Ilan DI (eds) Nematodes as biocontrol agents. CABI Publishing, Oxfordshire, pp 281–293
Wainhouse D, Brough S, Greenacre B (2007) Managing the pine weevil on lowland pine. Forestry Commission. https://www.forestry.gov.uk/pdf/fcpn014.pdf/$FILE/fcpn014.pdf
Williams CD, Dillon AB, Harvey CD, Hennessy R, McNamara L, Griffin CT (2013a) Control of a major pest of forestry, Hylobius abietis, with entomopathogenic nematodes and fungi using eradicant and prophylactic strategies. Forest Ecol Manag 305:212–222
Williams CD, Dillon AB, Girling RD, Griffin CT (2013b) Organic soils promote the efficacy of entomopathogenic nematodes, with different foraging strategies, in the control of a major forest pest: A meta-analysis of field trial data. Biol Control 65:357–364
Wilson M, Gaugler R (2004) Factors limiting short–term persistence of entomopathogenic nematodes. J Appl Entomol 128:250–253
Acknowledgements
We thank Gail Maher, Louise Mc Namara and Christopher Williams for help in the laboratory and the field and numerous forest managers and technicians from Coillte Ireland for providing access to sites and helping with trials. This research was funded by the European Union FP7 under grant agreement no 612713 (BIOCOMES project).
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Ralf Ehlers.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Kapranas, A., Malone, B., Quinn, S. et al. Optimizing the application method of entomopathogenic nematode suspension for biological control of large pine weevil Hylobius abietis . BioControl 62, 659–667 (2017). https://doi.org/10.1007/s10526-017-9824-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-017-9824-x