Abstract
There is growing evidence that extracellular vesicles (EVs) play a functional role in tissue repair and anti-aging by transferring the contents of donor cells to recipient cells. We hypothesized that Dauer (C. elegans), known as “ageless” nematodes, can also secrete extracellular vesicles and influence the lifespan of C. elegans. Here, we isolated EVs of dauer larvae (dauer EVs). Dauer EVs were characterized using transmission electron microscopy, nanoparticle tracking analysis (NTA), and Western blot analysis. Wild-type C. elegans were fed in the presence or absence of dauer EVs and tested for a range of phenotypes, including longevity, mobility and reproductive capacity. Results showed that dauer EVs increased the average lifespan of nematodes by 15.74%, improved mobility, slowed age-related pigmentation as well as body length, and reduced the accumulation of reactive oxygen species and lipids, while not impairing nematode reproductive capacity. These findings suggest that dauer EVs can extend the lifespan of C. elegans as well as the healthy lifespan by reducing ROS accumulation, with potential anti-aging capacity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aging is a process involving the degeneration of physiological functions. Over time, this process eventually leads to death of the body as various diseases emerge. Aging is an irreversible process, but with the study of aging mechanisms, it has been revealed that the rate of aging can be regulated by various genetic and environmental manipulations (Zeng et al. 2020). Changing the rate of aging of the body is one strategy for increasing life expectancy while reducing the probability of disease. Therefore, there is a growing need for strategies to slow the aging process and prevent aging-related diseases (Shetty et al. 2018).
Extracellular vesicles (EVs) have been extensively studied as messengers of intercellular communication. EVs from stem cells, young individuals, and long-lived individuals have been found to play important roles in tissue repair and anti-aging strategies (Fafián-Labora et al. 2020; Roefs et al. 2020). Stem cell-derived EVs inherit the ability of stem cells to rescue target cells. Researchers revealed that triple-helix repeat-containing 1 and osteoprotegerin from urine-derived stem cell-derived EVs reduced age-related bone loss (Chen et al. 2019). EVs from human umbilical cord mesenchymal stem cells, adipose stem cells, and induced multifunctional stem cells carry antioxidant proteins (glutathione peroxidase 1, superoxide dismutase, and catalase) that influence cell proliferation, collagen biosynthesis, DNA damage repair by controlling mitochondrial membranes and scavenging reactive oxygen species (ROS) accumulated during aging, thereby exerting anti-photoaging effects (Choi et al. 2019; Deng et al. 2020; Oh et al. 2018). Further studies revealed that EVs isolated from the blood of young donors contained high levels of antioxidant proteins (nicotinamide phosphoribosyltransferase and glutathione-related protein) that facilitate the anti-aging treatment of target tissues (hypothalamus, pancreas, retina, and hippocampus) by enhancing NAD + biogenesis. The proteins also enhanced endogenous glutathione S-transferase activity, scavenged ROS, and prevented lipid peroxidation (Yoshida et al. 2019). EVs from long-lived individuals have higher antioxidant capacity, and they can alleviate chronic inflammation caused by aging (Storci et al. 2019). These results suggest that source-specific EVs can achieve the goals of repairing tissue damage, delaying aging, and extending longevity.
Almost all cells, especially eukaryotic cells, secrete EVs to the outside environment. The EVs of nematodes were isolated and purified using sterile liquid culture for 24 h. Nematode EVs were found to be associated with immunity and senescence in a histological study (Russell et al. 2020a, b). The nematode life cycle consists of the embryonic stage, four larval stages (L1–L4), and the adult stage. Under unfavorable conditions, larvae enter the dauer stage at L2, which allows them to survive for several months without feeding, and when conditions become favorable again, they end the dauer state and re-enter their life cycle from the L4 stage without affecting their subsequent life course (Karp & Greenwald 2013; Wirick et al. 2021). Dauer larvae are also known as “ageless” nematodes, and dauer nematodes are in the juvenile stage of the nematode life cycle (Ewald et al. 2018).
Over past decades, Caenorhabditis elegans has become one of the most typical animal models in the field of aging. As a multicellular organism, its short life cycle, transparent genetic characteristics, and various physiological and behavioral phenotypes that change with age make C. elegans an excellent in vivo model for studying the environmental and genetic basis of lifespan. Some of the seminal findings in the study of aging were first discovered in nematodes and later validated in other advanced species; for example, insulin/IGF‐1 signaling (IIS), which also demonstrated that signaling pathways associated with aging are highly conserved in evolutionary terms (Kenyon et al. 1993). In summary, C. elegans plays an important role as a “transit point” for the study of the basic biology of aging and the translational application of anti-aging strategies. Most current studies on the use of EVs for delaying aging and extending lifespan have been conducted at the tissue level because it is difficult to simply visualize the effects of EVs on the organism because of the long lifespan of mammals, and the complexity of the in vivo environment. Therefore, we propose to use L4 stage C. elegans and the EVs of dauer larvae as experimental subjects to investigate the effects of EVs on nematode senescence and longevity.
Based on prior findings, we hypothesized that dauer larvae secrete EVs and that these EVs have anti-aging-related effects. In this study, the role of dauer EVs in nematode longevity was investigated in L4 C. elegans. Dauer EVs induced dose-dependent increases in longevity. Healthy senescence-related phenotypes and motility were improved at optimal doses, whereas reproductive capacity was not significantly changed. Further testing of aging-related indicators revealed age-dependent improvements in lipofuscin accumulation, ROS levels, and adiposity levels at the optimal dose, tentatively suggesting that dauer EVs-mediated lifespan extension in nematodes is related to antioxidant capacity. The results of this study demonstrate the great potential of dauer EVs for extending the healthy lifespan, which will contribute to the further development of EVs for promoting healthy lifespan extension in individuals and provide ideas for studying the anti-aging effects of EVs.
Materials and methods
Strain, culture and handing procedure
The wild-type C. elegans strain N2 (ancestral) and uracil auxotroph Escherichia coli strain OP50 were obtained from the Caenorhabditis Genetics Center (University of Minnesota, Minneapolis, MN, USA). Nematode growth medium (NGM) was supplied by Shanghai Haohai Biological Technology Co. Ltd. In addition to the acquisition of dauer larvae, the other worms were cultured and analyzed on solid medium (NGM). E. coli OP50 was plated in NGM dishes. All other chemicals used were of analytical reagent grade.
Dauer larvae maintenance conditions and EV acquisition
To generate a sufficient biomass to extract EVs, we cultured a large number of dauer larvae in a liquid environment (Supplementary Methods, Dauer larvae culture) (Hibshman et al. 2021; Karp 2018; Russell et al. 2020a, b). Dauer larvae and culture supernatant were collected, dauer larvae were ground, and the supernatant was collected at 13,000 × g for 10 min. The supernatant of the two parts was filtered at 0.22 μm, and the precipitate was collected at 100,000 × g for 90 min (Beckman, US) (Crescitelli, et al. 2021). The collected precipitates were analyzed by electron microscopy, and the size of the precipitate was determined by nanoparticle tracking (Nano Sight 300, Malvern Panalytical, UK). Finally, the EVs marker CD-63 (anti-CD63, Abcam, UK) and membrane vesicle protein marker LMP-1 (anti-LMP-1, Developmental Studies Hybridoma Bank, USA) were detected by western blotting (Huynh et al. 2016; Kostich et al. 2000) to confirm that the final collected precipitate contained dauer EVs.
Lifespan assay
All lifespan tests were performed at 20 °C. NGM Petri dishes were prepared according to the instructions provided by the reagent company. NGM medium was sterilized at 120 °C, and additives containing cholesterol and streptomycin were added after the medium was cooled to 55 °C. NGM dishes were coated with 30 µl of E. coli OP50 and incubated for 8 h at room temperature before starting the assays. Synchronized L4 larvae (3 days following hatching) were transferred together with various concentrations of dauer EVs to 35-mm Petri dishes at 20 °C and then transferred to NGM dishes 2 h later (Fueser et al. 2021). The day of nematodes treatment was defined as day 0. These nematodes were observed daily and were considered dead if they did not respond to physical stimuli. Nematodes that were missing or hatching inside the eggs were not included in the statistics (Maures et al. 2014). The experiment was repeated three times independently with at least 30 worms in each group.
Motility
Body bending is defined as a shift of one wavelength relative to the long axis of the body. To measure the rate of body bending, the number of body bends in nematodes was recorded over 10 s after transferring 20 µl of S-basal droplets on days 2, 5, and 8 with and without dauer EVs treatment (Nourse et al. 2021). The experiment was repeated three times independently with 10 nematodes in each group.
Body length change experiment
To measure changes in nematode body length, nematodes treated with or without 1 × 107 particles/ml dauer EVs were photographed on 2% agar after anesthesia on days 5, 8, and 11, and their body length was quantified using ImageJ (Hong et al. 2022). The experiment was repeated three times independently with at least 20 nematodes in each group.
Lipofuscin accumulation assay
To study lipofuscin accumulation in nematodes treated with 1 × 107 particles/ml dauer EVs, treated or untreated nematodes were cultured for 5, 10, or 15 days; anesthetized with levamisole ((S)-2,3,5,6-tetrahydro-6-phenylimidazo(2,1-b) thiazole, Aladdin, China); and observed under a fluorescence microscope (Leica Microsystems, Germany) (Lin et al. 2019). The lipofuscin accumulation experiment was repeated three times. The images were analyzed using ImageJ software, and the total number of nematodes was counted. At least 20 nematodes were used in each group.
Reproduction assay
To determine the effect of 1 × 107 particles/ml dauer EVs on nematode oviposition, at least 10 nematodes were cultured and treated as described in the lifespan experiment. Ten nematodes (in triplicate) were randomly selected and transferred to fresh NGM dishes on the third day of culture (oviposition period) and allowed to lay eggs for 72 h (Yang et al. 2018).
Similarly, to determine the effect of dauer EVs (1 × 107 particles/ml) on age-associated vulval integrity defects (AVID), treatments were performed as described in the lifespan experiment. The AVID phenotype was observed and determined during aging until all nematodes died. The experiment was independently repeated three times with at least 20 nematodes in each group.
Effects of Dauer EVs on ROS accumulation of C. elegans
ROS levels were measured using 2′,7′-dichlorofluorescein diacetate (H2DCF-DA, Meilunbio, China). The nematodes were collected on day 8 after 1 × 107 particles/ml dauer EVs treatment with the same pre-treatment utilized in the lifespan experiment (Liu et al. 2022). The nematodes were collected, treated with H2DCF-DA, and observed under a fluorescent microscope. The experiment was repeated three times independently. Images were analyzed using ImageJ software with at least 20 nematodes per group.
Fat accumulation assay
To study lipid accumulation after 1 × 107 particles/ml dauer EVs treatment, nematodes were fixed in 1% paraformaldehyde after washing on the second and eighth days after EVs treatment and then stained at room temperature using a mixture of 2% oil red O (Bio-sharp, China) and 2% Triton X-100 in equal proportions, and the staining was observed under a fluorescence microscope and photographed (Shen et al. 2019). The experiment was repeated three times independently. The pictures were analyzed using ImageJ software with at least 20 nematodes in each group.
Statistical analysis
We used GraphPad Prism 8.0 to conduct statistical analyses. For lifespan experiments, the Kaplan–Meier method was utilized, and P values were calculated using the log-rank test. To compare two groups, Student’s t-test was performed. P < 0.05 was considered statistically significant.
Results
Dauer larvae secrete EVs carrying CD-63 and LMP-1 proteins
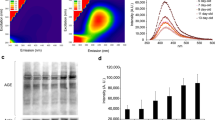
We examined cross-sections of nematodes using transmission electron microscopy (TEM) and found closed mouths, thickened cuticles, and dauer alae (Fig. 1A). To determine the number of obtained particles and their size distribution, nanoparticle tracking analysis (NTA) was performed. To maintain the concentration of particles within the operating range of Nano Sight 300, we diluted the samples at a ratio of 1:100 using S-basal before analysis and detection. Three replicates were examined, and they were found to have a dominant monodisperse particle population with an average size of 160 nm (Fig. 1B). Then, the separated particles were detected by scanning electron microscopy (SEM) and TEM. An obvious spherical structure was observed by SEM, and an obvious bilayer structure was observed by TEM (Fig. 1C and D). CD63 is a membrane protein recognized as a marker of eukaryotic outer membrane vesicles, and LMP-1 is a nematode homolog of the common mammalian outer membrane vesicle membrane protein LAMP-1 (Kowal et al. 2016; Vonk et al. 2018). Therefore, we performed western blotting of the isolated pellets using monoclonal antibodies against CD63 and LMP-1 and found strong immunoreactivity in the isolated pellets (Fig. 1E). Combined with the electron microscopy, NTA, and western blotting data, our isolated pellet was confirmed to contain dauer EVs.
Characterization of the EVs of dauer larvae. A Representative cross-sectional transmission electron microscopy images of dauer larvae, in which a distinctly thickened cuticle with a closed oral structure was visible. Scale bar = 5 µm; local magnification, × 5. B Nanoparticle tracking analysis of dauer EVs with a peak particle size of approximately 160 nm. C Scanning electron microscopy of dauer EVs. The nanoparticles were visible under the microscope, exhibiting a distinct spherical structure and a size range of 100–200 nm. Scale bar = 2 µm. D Transmission electron microscopy analysis of dauer EVs. The particles were subjected to uranyl acetate staining, and the lipid bilayer structure was clearly visible under the microscope. Scale bar = 100 nm. E Western blotting of dauer EVs and supernatant from wash. Anti-CD-63 and anti-LMP-1 antibodies showed strong immunogenicity at the expected sizes in the EVs group, while no strong immunogenicity was observed in the control group, the wash supernatant group
EVs extend the lifespan of C. elegans
To fully investigate the effect of dauer EVs on the longevity of C. elegans, we first explored whether dauer EVs could enter nematodes using the fluorescent membrane dye DIO (Supplementary Methods, Dauer EVs intake experiment), and green fluorescence-labeled dauer EVs were detected in the nematode oral cavity and anterior part of the intestine 2 h after exposure, indicating that dauer EVs entered the nematode oral cavity upon feeding and the nematode intestine upon pharyngeal pumping activity (Fig. 2A). We then treated type N2 C. elegans at the L4 period using three different doses of vesicles (1 × 106, 1 × 107, and 1 × 108 particles/ml; Supplementary Methods, Dauer EVs toxicity test). The results illustrated that these EV doses were not lethal to the nematodes within 6 h, indicating that short-term treatment with dauer EVs was not significantly toxic to N2 C. elegans (Fig. 2B).
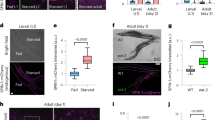
Toxicity assessment and longevity analysis of different doses of dauer EVs in wild-type N2 worms. A Intake study of dauer EVs. DIO-labeled dauer EVs were introduced into the nematode intestine through the oral cavity via the pharyngeal activity of the nematodes. The red arrow is the head of the nematode and the blue arrow is the tail of the nematode. B Toxicity analysis of dauer EVs. The survival rates of nematodes following exposure to dauer EVs (1 × 106, 1 × 107, and 1 × 108 particles/ml) for 6 h were not significantly different from the control, illustrating the absence of a significant toxic effect. C Lifespan analysis of nematodes in the presence of dauer EVs (1 × 106, 1 × 107, and 1 × 108 particles/ml). D Lifespan test of nematodes at the optimal dose (1 × 107 particles/ml). For panel (A), comparisons were performed using one-way ANOVA followed by the post-hoc Bonferroni test. Lifespan analysis was performed using the Kaplan–Meier method. Data are displayed as the mean ± SEM. Data were analyzed using an independent-samples t-test (ns = non-significant)
Immediately afterwards, we examined the effect of the three EVs doses on the lifespan of nematodes after 2 h. Treatment with dauer EVs (1 × 107 particles/ml) increased the mean lifespan of nematodes by 15.74% versus the control (17.747 ± 5.259 days vs. 15.333 ± 4.808 days, P = 0.001). Conversely, dauer EVs treatment at doses of 1 × 106 and 1 × 108 particles/ml had no significant effect on lifespan versus the control (Table S1 and Fig. 2C).
EVs improve the healthy lifespan of C. elegans
The aging process affects the longevity and healthy lifespan of nematodes, and healthy lifespan can be assessed by functional declines in performance tests (Bansal et al. 2015). We studied behavioral and functional phenotypes closely related to aging, including motility and reproductive capacity (Son et al. 2019). The mean lifespan of nematodes at the optimal EVs dose was increased by 14.441% versus the control (17.901 ± 9.567 days vs. 15.642 ± 8.6692 days, P = 0.002, Fig. 2D), in general agreement with the previous results. The first phenotype that we tested was the nematode bending rate, which reflects the nematode’s motility and gradually decreases with the aging process (Li et al. 2021). We measured the number of nematode bends per 10 s on days 2, 5, and 8. The numbers of body bends per 10 s on days 2, 5, and 8 in control nematodes were 22.2 ± 2.280 freq, 18.9 ± 1.807 freq, and 18.068 ± 1.437 freq, respectively, versus 22.567 ± 1.736 freq, 21.267 ± 1.741 freq, and 20.267 ± 1.660 freq, respectively, after dauer EVs treatment (all P < 0.0001). The results demonstrated that dauer EVs improved this phenotype (Table S2 and Fig. 3A). The second tested phenotype was the nematode reproductive capacity, which is believed to be closely related to longevity. The assessment of nematode oviposition and AVID phenotypes is an important basis for determining whether dauer EVs impair nematode reproductive capacity (Leiser et al. 2016; Lenz, et al. 2019). As presented in Fig. 3B and C, there was no significant difference in egg production between dauer EVs-treated and untreated nematodes at 72 h (P = 0.228) and no prevention of the senescence-induced AVID phenotype (P = 0.177), indicating that dauer EVs-induced longevity is not dependent on reproductive signaling pathways.
Effects of dauer EVs (1 × 107 particles/ml) on nematode physiological functions and biomarkers of aging (lipofuscin). A Effect of dauer EVs on nematode motility. The locomotor ability of nematodes, which gradually decreased with age, was better in the EVs group than in the control group. B Egg production was not significant difference within 72 h after dauer EVs treatment compared to that in the control group, and the AVID phenotype did not improve throughout the life span following dauer EVs treatment. C Dauer EVs increased the lifespan of nematodes without reducing reproductive capacity. D Body length did not differ between the EVs and control groups in the early stage (day 5), but in the middle and late stages (days 8 and 11, respectively), dauer EVs-treated nematodes had a significantly longer body length than control nematodes. E Representative fluorescence images of lipofuscin. Scale bar = 100 µm. F Intestinal lipofuscin levels were significantly lower in the middle and late stages (days 10 and 15, respectively) after dauer EVs treatment (1 × 107 particles/ml) compared to the control findings. Lipofuscin levels were quantified using ImageJ software. All analyses were performed using an independent-samples t-test. (*P < 0.05, **P < 0.005, ***P < 0.0005, ns = non-significant)
A longer lifespan is often associated with nematode growth capacity (Liao et al. 2011; Hong et al. 2022). To understand whether dauer EVs affect nematode growth, we measured the change in nematode body length as an indicator of the adult lifespan (Hong et al. 2022). On day 5 after treating the nematodes with EVs, body length was not significantly different between the control and EVs groups (P = 0.681). However, treated nematodes were significantly longer than untreated nematodes on days 8 (1.404 ± 0.081 mm vs. 1.389 ± 0.071 mm, P = 0.009) and 11 (1.438 ± 0.074 mm vs. 1.382 ± 0.063 mm, P = 0.003, Fig. 3D).
Lipofuscin is an autofluorescent substance that accumulates gradually with the age of C. elegans (Yu et al. 2021). We measured the in vivo autofluorescence intensity of lipofuscin on days 5, 10, and 15 of nematode growth, and the results indicated that lipofuscin accumulated gradually over time. However, lipofuscin accumulation in dauer EVs-treated nematodes was decreased relative to the control by 6.167% on day 5 (P = 0.117), 14.782% on day 10 (P = 0.010), and 22.620% on day 15 (P = 0.003, Fig. 3E and F).
EVs reduce in vivo ROS levels in C. elegans
To investigate whether the mechanism of lifespan extension in dauer EVs is related to ROS levels in C. elegans, we compared ROS levels in treated and untreated C. elegans using the ROS probe H2DCF-DA. ROS accumulation was reduced in worms treated with 1 × 107 particles/ml dauer EVs (P = 0.001, Fig. 4A). This suggests that the increased lifespan of dauer EVs-treated C. elegans is partly attributable to decreased ROS accumulation.
Effect of dauer EVs (1 × 107 particles/ml) on reactive oxygen species (ROS) and fat accumulation in nematodes. A ROS levels in nematodes were measured using 2′,7′-dichlorofluorescein diacetate staining on day 8, and significantly reduced ROS accumulation was observed in the EVs group compared to the control level. B Representative microscopic images of oil red O staining. Scale bar = 100 µm. C A significant decrease in nematode fat levels on day 8 was observed in the EVs group compared to the findings in the control group. ROS and fat levels were quantified using ImageJ software. Data are presented as the mean ± SEM. Data were analyzed using an independent-samples t-test (*P < 0.05, **P < 0.005, ***P < 0.0005)
EVs reduce lipid accumulation in nematodes
In wild-type C. elegans, increased ROS production might lead to excessive lipid accumulation (Hughes et al. 2007; Wang et al. 2018, 2022). The results revealed no significant difference in body fat levels in nematodes on day 2 (P = 0.172), whereas on day 8, dauer EVs-treated nematodes exhibited significantly lower body fat levels than control nematodes (P = 0.0004, Fig. 4B and C).
Discussion
The aging process is accompanied by a decline in the physiological functions of the organism, and it is the most important factor for the development of many diseases in later life (Benito-Cuesta et al. 2021). A number of structures involved in intercellular communication (including EVs) have been identified as effective anti-aging factors in studies of anti-aging programs (Yin et al. 2021).
This is the first study on the effects dauer EVs on longevity in C. elegans. Therefore, we first identified the obtained larvae to ensure that the extracted EVs were derived from dauer larvae, and based on their unique physiology (closed oral cavity, thickened cuticle), electron microscopic observation revealed that the obtained nematodes were dauer larvae. We extracted EVs from nematode tissues and liquid medium and identified membrane vesicles at three levels: size, morphology, and molecular characterization.
When EVs enter the organism and act on target cells, it is first necessary to determine whether they are biotoxic. We first investigated nematode mortality following exposure to EVs at doses of 1 × 106, 1 × 107, and 1 × 108 particles/ml. The results demonstrated that no significant biological toxicity occurred when nematodes were exposed to dauer EVs for a short period (6 h). Immediately afterward, we performed lifespan analysis at the aforementioned doses. Dauer EVs at 1 × 107 particles/ml most effectively prolonged the nematode lifespan, whereas the other doses had no significant effect. Previous reports of lifespan extension by plant molecules suggest a dose dependence of nematode lifespan extension, with nematode lifespan showing a bell shape at different doses our results are generally consistent (Powolny et al. 2011). Life extension is not significant at lower doses (1 × 106 particles/ml), possibly when the concentration is lower than the effective dose. On the other hand, under high concentration conditions(1 × 108 particles/ml), a non-significant shortening of nematode lifespan was observed, and high concentrations of dauer EVs may have reduced the involvement of key factors involved in lifespan extension thus inducing feedback regulation (Srivastava et al. 2017; Pandey et al. 2019). However, these findings need to be further explored.
The advantages of the C. elegans model in the field of aging as well as anti-aging research have been well documented (Shen et al. 2018). As biological individuals, aging-related indicators respond to the lifespan as well as the rate of aging of the organism and the length of the healthy lifespan. Aging is accompanied by changes in various physiological indicators. Therefore, C. elegans provides a good opportunity to identify biological and pharmacological interventions for anti-aging. Based on the C. elegans model, we systematically evaluated the effect of dauer EVs on the healthy lifespan. We found that these EVs prolonged the nematode lifespan without affecting nematode reproduction and grow. Instead, dauer EVs significantly enhanced nematode motility. We also found that age-related pigmentation changes were effectively alleviated by dauer EVs treatment, especially in the middle and late stages. These findings suggest that dauer EVs have the potential to improve the healthy lifespan, a hypothesis that was further confirmed by an in-depth study of ROS accumulation.
ROS are highly reactive oxygen molecules that accumulate with age, and they can cause genotoxicity and physiological damage, which are important factors that contribute to aging (Back et al. 2010). Excessive ROS levels can lead to DNA damage, alter gene expression, disrupt cell signaling, affect normal mitochondrial function, and ultimately lead to the disruption of normal cellular physiological functions and death (Labuschagne & Brenkman 2013; Zhang et al. 2022). We measured ROS levels in both groups of nematodes on day 8 and observed a decrease in ROS levels in nematodes after dauer EVs treatment, suggesting that dauer EVs are extend the nematode lifespan by reducing ROS accumulation.
According to existing studies, the redistribution and abnormal accumulation of fat in C. elegans during the aging process might lead to the toxic effects of adiposity and a decline in tissue function associated with aging (Savini et al. 2013). At the same time, excessive ROS accumulation can also lead to excessive fat accumulation, which is one of the causes of obesity (Wang et al. 2018). Therefore, after observing that dauer EVs mediated the reduction of ROS levels, we measured adiposity levels in nematodes. We measured the fat level in nematodes on days 2 and 8, and the nematode fat level was significantly lower in the dauer EVs group than in the control group on day 8, whereas no difference was noted between the groups in the early stage.
Our results suggest that dauer EVs prolong the nematode lifespan by reducing ROS levels without compromising normal nematode physiological functions, especially nematode reproductive capacity, but to some extent, dauer EVs alleviated the reduction of motility, accumulation of lipofuscin, and changes of body length associated with aging, thereby increasing the healthy nematode lifespan. Further studies will aim to trace the pathways by which dauer EVs affect ROS levels and determine whether their anti-aging mechanism can be replicated in other mammalian models. Our work provides an idea for studying EVs for expanding the individual lifespan as well as in vivo models of healthy lifespan extension.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Back P, Matthijssens F, Vlaeminck C, Braeckman BP, Vanfleteren JR (2010) Effects of sod gene overexpression and deletion mutation on the expression profiles of reporter genes of major detoxification pathways in Caenorhabditis elegans. Exp Gerontol 45(7–8):603–610
Bansal A, Zhu LJ, Yen K, Tissenbaum HA (2015) Uncoupling lifespan and healthspan in Caenorhabditis elegans longevity mutants. Proc Natl Acad Sci USA 112(3):E277–E286
Benito-Cuesta I, Ordóñez-Gutiérrez L, Wandosell F (2021) AMPK activation does not enhance autophagy in neurons in contrast to MTORC1 inhibition: different impact on β-amyloid clearance. Autophagy 17(3):656–671
Chen C-Y, Rao S-S, Tan Y-J, Luo M-J, Hu X-K, Yin H, Xie H (2019) Extracellular vesicles from human urine-derived stem cells prevent osteoporosis by transferring CTHRC1 and OPG. Bone Res 7:18
Choi JS, Cho WL, Choi YJ, Kim JD, Park H-A, Kim SY, Cho YW (2019) Functional recovery in photo-damaged human dermal fibroblasts by human adipose-derived stem cell extracellular vesicles. J Extracellular Vesicles 8(1):1565885
Crescitelli R, Lässer C, Lötvall J (2021) Isolation and characterization of extracellular vesicle subpopulations from tissues. Nat Protoc 16(3):1548–1580
Deng M, Yu TZ, Li D, Wang X, Zhou G, Liu W, Zhang WJ (2020) Human umbilical cord mesenchymal stem cell-derived and dermal fibroblast-derived extracellular vesicles protect dermal fibroblasts from ultraviolet radiation-induced photoaging in vitro. Photochem Photobiol Sci 19(3):406–414
Ewald CY, Castillo-Quan JI, Blackwell TK (2018) Untangling longevity, dauer, and healthspan in Caenorhabditis elegans insulin/IGF-1-signalling. Gerontology 64(1):96–104
Fafián-Labora JA, Rodríguez-Navarro JA, O’Loghlen A (2020) Small extracellular vesicles have GST activity and ameliorate senescence-related tissue damage. Cell Metabol 32(1):71
Fueser H, Rauchschwalbe M-T, Höss S, Traunspurger W (2021) Food bacteria and synthetic microparticles of similar size influence pharyngeal pumping of Caenorhabditis elegans. Aqua Toxicol (Amsterdam, Netherlands) 235:105827
Hibshman JD, Webster AK, Baugh LR (2021) Liquid-culture protocols for synchronous starvation, growth, dauer formation, and dietary restriction of. STAR Protocols 2(1):100276
Hong J, Song Y, Xie J, Xie J, Chen Y, Li P, Yu Q (2022) Acrolein promotes aging and oxidative stress via the stress response factor DAF-16/FOXO in. Foods (Basel, Switzerland) 11(11):1590
Hughes SE, Evason K, Xiong C, Kornfeld K (2007) Genetic and pharmacological factors that influence reproductive aging in nematodes. PLoS Genet 3(2):e25-265
Huynh JM, Dang H, Munoz-Tucker IA, O’Ketch M, Liu IT, Perno S et al (2016) ESCRT-dependent cell death in a Caenorhabditis elegans model of the lysosomal storage disorder mucolipidosis type IV. Genetics 202(2):619–638
Karp X, Greenwald I (2013) Control of cell-fate plasticity and maintenance of multipotency by DAF-16/FoxO in quiescent Caenorhabditis elegans. Proc Natl Acad Sci USA 110(6):2181–2186
Karp, X. (2018). Working with dauer larvae. WormBook: the online review of C. Elegans biology
Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R (1993) A C. elegans mutant that lives twice as long as wild type. Nature 366(6454):461–464
Kostich M, Fire A, Fambrough DM (2000) Identification and molecular-genetic characterization of a LAMP/CD68-like protein from Caenorhabditis elegans. J Cell Sci 113(Pt 14):2595–2606
Kowal J, Arras G, Colombo M, Jouve M, Morath JP, Primdal-Bengtson B, Théry C (2016) Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci USA 113(8):E968–E977
Labuschagne CF, Brenkman AB (2013) Current methods in quantifying ROS and oxidative damage in Caenorhabditis elegans and other model organism of aging. Ageing Res Rev 12(4):918–930
Leiser SF, Jafari G, Primitivo M, Sutphin GL, Dong J, Leonard A, Kaeberlein M (2016) Age-associated vulval integrity is an important marker of nematode healthspan. Age (dordr) 38(5–6):419–431
Lenz KA, Miller TR, Ma H (2019) Anabaenopeptins and cyanopeptolins induce systemic toxicity effects in a model organism the nematode Caenorhabditis elegans. Chemosphere 214:60–69
Li H, Yu X, Meng F, Zhao Z, Guan S, Wang L (2021) Ferulic acid supplementation increases lifespan and stress resistance via insulin/IGF-1 signaling pathway in C. elegans. Int J Mol Sci 22(8):4279
Liao VH-C, Yu C-W, Chu Y-J, Li W-H, Hsieh Y-C, Wang T-T (2011) Curcumin-mediated lifespan extension in Caenorhabditis elegans. Mech Ageing Dev 132(10):480–487
Lin C, Zhang X, Xiao J, Zhong Q, Kuang Y, Cao Y, Chen Y (2019) Effects on longevity extension and mechanism of action of carnosic acid in Caenorhabditis elegans. Food Funct 10(3):1398–1410
Liu J-Y, Zheng R-Q, Wang Y, Liu Y-H, Jiang S, Wang X-Z, Zhang W-N (2022) The endogenous metabolite glycerophosphocholine promotes longevity and fitness in Caenorhabditis elegans. Metabolites 12(2):177
Maures TJ, Booth LN, Benayoun BA, Izrayelit Y, Schroeder FC, Brunet A (2014) Males shorten the life span of C. elegans hermaphrodites via secreted compounds. Science 343(6170):541–544
Nourse JB, Harshefi G, Marom A, Karmi A, Cohen Ben-Ami H, Caldwell KA, Treinin M (2021) Conserved nicotine-activated neuroprotective pathways involve mitochondrial stress. Iscience 24(3):102140
Oh M, Lee J, Kim YJ, Rhee WJ, Park JH (2018) Exosomes derived from human induced pluripotent stem cells ameliorate the aging of skin fibroblasts. Int J Mol Sci 19(6):1715
Pandey T, Sammi SR, Nooreen Z et al (2019) Anti-ageing and anti-Parkinsonian effects of natural flavonol, tambulin from Zanthoxyllum aramatum promotes longevity in Caenorhabditis elegans. Exp Gerontol 120:50–61
Powolny AA, Singh SV, Melov S, Hubbard A, Fisher AL (2011) The garlic constituent diallyl trisulfide increases the lifespan of C. elegans via skn-1 activation. Exp Gerontol. 46(6):441–452
Roefs MT, Sluijter JPG, Vader P (2020) Extracellular vesicle-associated proteins in tissue repair. Trends Cell Biol 30(12):990–1013
Russell JC, Kim T-K, Noori A, Merrihew GE, Robbins JE, Golubeva A, Kaeberlein M (2020a) Composition of Caenorhabditis elegans extracellular vesicles suggests roles in metabolism, immunity, and aging. GeroScience 42(4):1133–1145
Russell JC, Postupna N, Golubeva A, Keene CD, Kaeberlein M (2020) Purification and analysis of Caenorhabditis elegans extracellular vesicles. J Visual Exp. https://doi.org/10.3791/60596
Savini I, Catani MV, Evangelista D, Gasperi V, Avigliano L (2013) Obesity-associated oxidative stress: strategies finalized to improve redox state. Int J Mol Sci 14(5):10497–10538
Shen P, Yue Y, Park Y (2018) A living model for obesity and aging research: Caenorhabditis elegans. Crit Rev Food Sci Nutr 58(5):741–754
Shen P, Zhang R, McClements DJ, Park Y (2019) Nanoemulsion-based delivery systems for testing nutraceutical efficacy using Caenorhabditis elegans: demonstration of curcumin bioaccumulation and body-fat reduction. Food Res Int 120:157–166
Shetty AK, Kodali M, Upadhya R, Madhu LN (2018) Emerging anti-aging strategies—scientific basis and efficacy. Aging Dis 9(6):1165–1184
Son HG, Altintas O, Kim EJE, Kwon S, Lee S-JV (2019) Age-dependent changes and biomarkers of aging in Caenorhabditis elegans. Aging Cell 18(2):e12853
Srivastava S, Sammi SR, Laxman TS, Pant A, Nagar A, Trivedi S, Bhatta RS, Tandon S, Pandey R (2017) Silymarin promotes longevity and alleviates Parkinson’s associated pathologies in Caenorhabditis elegans. J Funct Foods 31:32–43
Storci G, De Carolis S, Papi A, Bacalini MG, Gensous N, Marasco E, Bonafè M (2019) Genomic stability, anti-inflammatory phenotype, and up-regulation of the RNAseH2 in cells from centenarians. Cell Death Differ 26(9):1845–1858
Vonk LA, van Dooremalen SFJ, Liv N, Klumperman J, Coffer PJ, Saris DBF, Lorenowicz MJ (2018) Mesenchymal stromal/stem cell-derived extracellular vesicles promote human cartilage regeneration. Theranostics 8(4):906–920
Wang K, Chen S, Zhang C, Huang J, Wu J, Zhou H, Lyu J (2018) Enhanced ROS production leads to excessive fat accumulation through DAF-16 in Caenorhabditis elegans. Exp Gerontol 112:20–29
Wang YZ, Guo SY, Kong RL, Sui AR, Wang ZH, Guan RX, Supratik K, Zhao J, Li S (2022) Scorpion venom heat-resistant synthesized peptide increases stress resistance and extends the lifespan of Caenorhabditis elegans via the insulin/IGF-1-like signal pathway. Front Pharmacol 13:919269
Wirick MJ, Cale AR, Smith IT, Alessi AF, Starostik MR, Cuko L, Karp X (2021) daf-16/FOXO blocks adult cell fate in Caenorhabditis elegans dauer larvae via lin-41/TRIM71. PLoS Genet 17(11):e1009881
Yang Z-Z, Yu Y-T, Lin H-R, Liao D-C, Cui X-H, Wang H-B (2018) Lonicera japonica extends lifespan and healthspan in Caenorhabditis elegans. Free Radical Biol Med 129:310–322
Yin Y, Chen H, Wang Y, Zhang L, Wang X (2021) Roles of extracellular vesicles in the aging microenvironment and age-related diseases. J Extracell Vesicles 10(12):e12154
Yoshida M, Satoh A, Lin JB, Mills KF, Sasaki Y, Rensing N, Imai S-I (2019) Extracellular vesicle-contained eNAMPT delays aging and extends lifespan in mice. Cell Metabol 30(2):329
Yu X, Li H, Lin D, Guo W, Xu Z, Wang L, Guan S (2021) Ginsenoside prolongs the lifespan of via lipid metabolism and activating the stress response signaling pathway. Int J Mol Sci 22(18):9668
Zeng J, Zhang X, Wang J, Cheng X, Zhang Y, Zhou W (2020) Comparison of donepezil, memantine, melatonin, and liuwei dihuang decoction on behavioral and immune endocrine responses of aged senescence-accelerated mouse resistant 1 mice. Front Pharmacol 11:350
Zhang B, Pan C, Feng C, Yan C, Yu Y, Chen Z, Wang X (2022) Role of mitochondrial reactive oxygen species in homeostasis regulation. Redox Rep 27(1):45–52
Acknowledgements
We thank all the staff members for their contributions and efforts in conducting the survey.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
LF and ZW conceived the study; LF and JM completed the experimental design for the study; YW, BY and YJ provided technical support for the research; JM, YW and LC performed experiment; LF directed the manuscript; JM wrote the manuscript; LW, ZC and GM examined the data and format. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ma, J., Wang, Yt., Chen, Lh. et al. Dauer larva-derived extracellular vesicles extend the life of Caenorhabditis elegans. Biogerontology 24, 581–592 (2023). https://doi.org/10.1007/s10522-023-10030-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10522-023-10030-5