Abstract
Ginseng volatile oil (GVO) is one of the main components of ginseng and has antibacterial and anti-inflammatory properties. In this study, gas chromatography–mass spectrometry (GC–MS) was applied to characterize GVO chemical composition, and 73 volatile components were detected from GVO. Caenorhabditis elegans was used as animal model to further elucidate the antioxidant and anti-aging effects of GVO in vivo. The results suggested that GVO significantly prolonged the lifespan of C. elegans and promoted its health without damaging its reproductive capacity. In addition, GVO increased the antioxidant capacity and survival rate of nematodes after heat shock. Transcriptional sequencing showed that autophagy-related genes atg-4.2, atg-7, lgg-2, and cyd-1 were up-regulated, and superoxide dismutase 1 (sod-1) expression was increased after GVO pretreatment. Considering the role of autophagy and antioxidant in aging, the expression of autophagy substrate P62 protein in BC12921 strain was analyzed and found to decrease by more than 50.00% after treatment with GVO. In addition, the lifespan of SOD-1 mutant nematodes was not significantly different from that of the control group. SOD activity and autophagy were activated, which is a clear expression of hormesis. All these results suggest that GVO prolongs the lifespan and healthspan of C. elegans, and its biological functions may be related to hormesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aging is a natural biological process characterized by a decline in the body’s physiological and psychological adaptability to the environment. The incidence of cancer, neurodegenerative diseases, cardiovascular diseases, diabetes, and other diseases annually increases with the degree of aging (2016), which is therefore considered to be the main cause of these chronic diseases (Niccoli and Partridge 2012). Population aging poses a great threat to the international health care system and economic development. Drug therapy can effectively alleviate aging, but long-term anti-aging drugs such as aspirin and metformin can cause adverse reactions such as anti-platelet aggregation, diarrhea, nausea, and abdominal pain (de Vries et al. 2020; Johnston et al. 2019). Therefore, safe and effective anti-aging strategies must be urgently developed.
Traditional Chinese medicine (TCM) has been widely applied in clinical practice, such as COVID-19 and cancer treatment (Xiao et al. 2020; Liu et al. 2019), and delays aging. In particular, Lonicera japonica, Polygonum multiflorum, glycyrrhizae radix, Lycium barbarum polysaccharides, and astragaloside IV have significant anti-aging activity (Yang et al. 2018; Saier et al. 2018; Ruan et al. 2016; Zhang et al. 2019, 2021). Ginseng (Panax ginseng C. A. Meyer) is a kind of TCM with high medicinal value and has the effects of reducing oxidative stress, anti-aging, and preventing senile dementia (Wang et al. 2009, 2011a). As one of the main components of ginseng (0.1–0.5%), the essential oil of ginseng also has a wide range of medicinal value. A small amount of ginseng volatile oil (GVO) has the excitatory effect, and its appropriate use has a sedative function and can paralyze nerves at large doses (Han et al. 2013). GVO also has antibacterial, anti-tumor, and myocardial ischemia effects (Zhang et al. 2013; Jiang et al. 2008; Bae et al. 2001). Whether this substance can delay aging is unclear.
Caenorhabditis elegans is an organism with a short natural lifespan, strong reproductive ability, small body size, and easy maintenance in the laboratory environment. Almost all of the gene families involved in mammalian neuron function and the signaling pathways associated with aging are found in nematodes (Guarente and Kenyon 2000; Gershon and Gershon 2002). Therefore, C. elegans was used as an in vivo experimental model to investigate the effect and possible mechanism of GVO on life extension.
In this study, GVO significantly prolonged the lifespan of nematodes, delayed their senescence-related physiological functions, and improved their anti-stress ability, and its life-prolonging activity is related to autophagy and antioxidant pathways. The antioxidant capacity and autophagy activity of C. elegans increased by GVO may be attributed to the activation of hormesis pathway by it.
Materials and methods
CO2-based supercritical fluid extraction (SFE)
GVO was extracted from ginseng through SFE with CO2 as solvent. The extraction conditions were set as follows: (1) extraction pressure, 25 MPa; extraction temperature, 40 °C; (3) extraction time, 1.5 h; and (4) CO2 flow rate, 10 mL/min. The obtained GVO was stored at low temperature to avoid light for later use.
Gas chromatography–mass spectrometry (GC–MS)
The structure and purity of the compounds were characterized by GC–MS using Agilent ChemStation data system. The chromatographic conditions were as follows: db-1 capillary column; helium as carrier gas, constant flow 1.0 mL/min; and injector temperature, 280 °C. For the heating procedure, the initial temperature of 60 °C was kept for 3 min and then increased to 280 °C at 10 °C/min. Afterward, 1 μL was injected for 5 min. The relative contents of GVO can be obtained through area normalization.
Caenorhabditis elegans strains and maintenance
Caenorhabditis elegans strains including N2, Bristol (wild type), BC12921 (rCesT12G3.1::GFP + pCeh361) and sod-1(FX776) and Escherichia coli OP50 (E. coli OP50) were reserved in the laboratory. All C. elegans specimens were cultured on standard nematode culture medium (NGM) and maintained in accordance with the standard rules (Brenner 1974). Unless otherwise stated, all nematodes were cultured at 20 °C and fed on OP50. Eggs were collected within 4 h to obtain age-synchronized nematodes through oviposition synchronization.
GVO administration
First, dimethyl sulfoxide (DMSO) was used to dilute GVO, and the mixed solution was then added to E. coli OP50 at specified concentrations (0, 12.5, 25, and 50 μg/mL). The final DMSO content of 0.1% was used in each dose. NGM with GVO solution was dried and used to culture C. elegans for biological assays.
Lifespan assay
Age-synchronized L4-stage hermaphrodites were selected and treated with different concentrations of GVO (0, 12.5, 25, and 50 μg/mL). The first day of treatment with GVO was recorded as Day 0, and the nematodes were transferred to the newly prepared plate on each subsequent day. Survival, death, and loss were recorded. Deaths due to eggs hatching inside the body were not included in the data. Living status was determined by the reaction of the worm picker.
Adversity resistance assay
Caenorhabditis elegans specimens at L4 stage were randomly placed in NGM plates containing different concentrations of GVO. After three consecutive days of administration, the animals were heat-stimulated at 35 °C for 4 h and then placed back at 20 °C for recovery for 12 h. The survival rate was recorded.
DPPH free radical scavenging
The in vitro antioxidant capacity of GVO was determined by examining its absorption of stable free radical DPPH (Sigma-Aldrich GmbH, Steinheim, Germany) (Blois 1958). In brief, 100 μL of different concentrations of GVO were mixed with 100 μL of 200 μM DPPH (diluted with methanol) and reacted in the dark for 30 min. The absorption value of the mixture was measured at 517 nm. The free radical scavenging ability of methyl xanthine was calculated by the following formula:
where A0 represents the light absorption value in the presence of only DPPH, and A1 represents the light absorption value in the presence of GVO.
Measurement of reactive oxygen species (ROS)
The synchronized L4-stage N2 worms exposed to different concentrations of GVO for 8 days were collected to measure endogenous ROS levels by using the ROS kit (Beyotime, Shanghai China) with H2DCF-DA as the molecular probe (Wolfe and Liu 2007). M9 buffer (NaCl 5 g, Na2HPO4·12H2O 15.12 g, MgSO4 0.25 g, and KH2PO4 3 g in 1 L of H2O) was used to collect and wash the nematodes at least three times. Each group was then added with 10 μL of 10 mM H2DCF-DA, and the mixture was shaken and incubated at 37 °C for 1 h. The nematode was repeatedly cleaned with M9 buffer to remove excess probes, and then neatly placed on 2% agarose pads. Fluorescence was quantified at excitation/emission wavelengths of 488 nm/525 nm with FV3000 confocal laser microscope (Olympus, Japan).
Fluorescence quantification of age pigment
As the worm ages, its gut produces an autofluorescence pigment called lipofuscin (Zhou et al. 2018; Lin et al. 2019). Adult nematodes treated with or without GVO for 8 days were collected and anaesthetized with levamisole (Aladdin, Shanghai, China) and immobilized on 2% agarose pads for determination its lipofuscin levels. Fluorescence intensity was observed using BX53 fluorescence microscope (Olympus, Japan).
Motility and body size assay
The nematodes were treated with different concentrations of GVO according to the above methods. The locomotor capacity and body length of at least 10 nematodes in each dish were observed on the Day 8 of adults. The locomotor capacity of the nematode is determined by the frequency of its head moving from left to right within 10 s. UOPVIEW software was used to measure the relative length of worms in different GVO treatment groups and control group.
Spawning assay
For the spawning assay, this study was slightly improved according to the previous method (Yang et al. 2018). The synchronized L4-staged N2 worms was selected and pretreatment with different concentrations of GVO, with one in each plate and 10 plates in each group. Worms were transferred to fresh plates every 24 h, and the total number of eggs per nematode in each group during the first 3 days of peak spawning was recorded.
Determination of SOD and MDA levels
Age-synchronized nematodes treated or not treated with GVO (12.5, 25, and 50 μg/mL) for 5 days were collected to measure SOD and MDA levels. Total protein contents were determined by the bicinchoninic acid (BCA) assay kit (Beyotime, Shanghai, China). SOD and MDA contents were measured following the instructions of the total superoxide dismutase kit and malondialdehyde determination kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). Results were normalized by protein contents represented as U/mg protein, and nmol/mg protein, respectively.
Transcriptome sequencing
Age-synchronized nematodes were collected after growing for 10 days in the presence or absence of 50 μg/ mL GVO. The nematode transcriptome was sequenced by Novo Genomics (Beijing, China). Up- or down-regulated genes were identified by filtering the RNA-Seq data with the following cut-off: twofold change in expression level and a false discovery rate analogue of q-value less than 0.05.
Gene expression analysis by quantitative PCR
Total RNA was extracted from about 500 worms using Trizol reagent. Nucleic acid/protein analyzer (Du730, Beckman Coulter, Inc., Fullerton, California, USA) and gel electrophoresis were used to measure the quality and quantity of RNA. Complementary DNA (cDNA) was obtained using the Thermo Scientific kit (Thermo Fisher Scientific, MA, USA) under the following conditions: 25 °C for 5 min, 42 °C for 60 s, and 70 °C for 5 min. Bio-Rad Minioption real-time PCR detection system (Bio-Rad, Hercules, CA, USA) and SYBR Green Super Mix (Takara Biotechnology, Dalian, China) were used for real-time quantitative PCR analysis. The gene expression level was analyzed by 2−∆∆Ct method. Primer sequences for real-time PCR were shown in Table 1.
Determination of P62 protein accumulation in BC12921 strain
The accumulation of P62 protein in BC12921 strain was determined as described above (Wei et al. 2016). The worms were pretreated with or without GVO for 8 days. M9 buffer was used to collect and rinse the nematodes three times. The nematodes were anesthetized with levamisole and neatly placed on 2% agarose spacers. Fluorescence intensity was measured at the excitation wavelength and emission wavelength of 485 and 535 nm by using FV3000 confocal laser microscope (Olympus, Japan).
Statistical analysis
All fluorescence images were taken at × 10 magnification and Image J software (NIH, Bethesda, MD, USA) was used to measure the relative fluorescence intensity of the worms. Comparisons between two groups were calculated using Student’s t test. All statistical analyses were performed with GraphPad Prism8.0 software. Data were presented mean ± SD of three independent experiments. P < 0.05 indicates that the difference is statistically significant.
Results
Identification and quantification of the active compounds in GVO
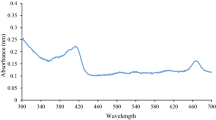
Seventy-three components were detected from GVO (Fig. 1A; Table 2). The main components in GVO are panaxynol (17.51%), panaxydol (17.15%), ethyl linoleate (16.52%), palmitic acid (4.57%), ethyl palmitate (4.11%), (−) -α-neoclovene (3.61), β-ginseng ene (3.05), α-humulene (2.84%), ethyl oleate (2.72%), and trans-β-farnesene (2.51%).
Ginseng volatile oil (GVO) chemical components and its effect on the lifespan and survival rate of N2 wild-type C. elegans after heat shock. A Chemical components in GVO analyzed by GC–MS. In chromatogram, 1 represents panaxynol, and 2 represents panaxydol, B GVO prolongs nematode lifespan in a dose-dependent manner, and C GVO supplementation significantly increased the survival rate of nematodes after heat shock compared with that of the control group. Statistical analysis was performed using Prism8.0, and p values were calculated using the Student’s t test. p < 0.05 was considered statistically significant. Data were presented mean ± SD of three independent experiments
GVO extended the lifespan and thermal stress resistance of wild-type C. elegans
Different concentrations of GVO (12.5, 25, and 50 μg/mL) were added to C. elegans diet to evaluate its life-extending activity. The results showed that different concentrations of GVO could prolong the lifespan of C. elegans (Fig. 1B). The median lifespan of the control group in the suitable growing environment was 13.67 days (Table 3), and that of the worms in 50 μg/mL group was up to 17.67 days, which was 29.26% higher than that for the control group. In addition, the median lifespan of C. elegans in the 25 μg/mL group was extended to 16.67 days, which was 21.94% higher than that of the control group. The median lifespan of nematodes in 12.5 μg/mL group was 15.00 days, which was increased by 9.73% compared with that of the control group. These results indicated that GVO could significantly prolong the lifespan of nematode N2 in a concentration-dependent manner.
Aging is characterized by a reduced ability to withstand environmental pressures. In this study, the resistance to thermal stress of GVO-treated nematodes was investigated (Fig. 1C). The survival rate of C. elegans in the control group was 59.33%, and that of nematodes in 50 and 25 μg/mL GVO groups were significantly higher than those in the control group (81.3% and 72.67%, respectively). The survival rate of nematodes in 12.5 μg/mL GVO group was slightly higher than that in control group, but the difference was not statistically significant. Thus, treatment with GVO significantly improved the thermal tolerance of nematodes.
Effects of GVO on the physiological responses of wild-type C. elegans
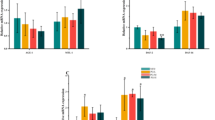
The spawning function of nematodes was first evaluated to determine whether GVO extends their healthy lifespan. N2 nematodes treated with GVO for 3 days showed no differences in spawning compared with the control group (Fig. 2A). The body swing frequency of N2 nematodes for 8 days in 10 s were then examined. In the control group, the average head swing frequency was 14.43 within 10 s. In the 12.5, 25, and 50 μg/mL GVO groups, the head swing frequency was 17.6, 22.33, and 26.1 times higher than that of the control, respectively. The nematodes treated with GVO showed higher body swing frequency than those in the control group (Fig. 2B).
Effects of ginseng volatile oil on the physiological indices and antioxidant capacity of C. elegans. A GVO had no effect on the reproductive capacity of nematodes, B GVO improved the head swinging ability of nematodes, C GVO improved the body size of nematodes, and D GVO reduced lipofuscin levels in nematodes. Statistical analysis was performed using Prism8.0, and p values were calculated using the Student’s t test. p < 0.05 was considered statistically significant. Data were presented mean ± SD of three independent experiments
Aging is often accompanied by a decrease in other physiological indicators. Hence, the body size of nematodes treated with different methods was evaluated at Day 8. The body size of N2 worms treated with GVO at 12.5, 25, and 50 μg/mL was 3.62%, 8.89%, and 13.25% higher than that of the control group, respectively (Fig. 2C). The level of lipofuscin in nematodes accumulates with age can reflect the senescence level. The lipofuscin content in N2 worms treated with GVO at 12.5, 25, and 50 μg/mL was 15%, 31%, and 50% lower than that in control group, respectively (Fig. 2D).
GVO increased the antioxidant capacity of wild-type C. elegans
The free radical scavenging ability of GVO was first evaluated in vitro to investigate whether GVO enhances the antioxidant capacity of nematodes. The DPPH free radical scavenging rates of the control samples was 48.25%, and the free radical scavenging rates of 12.5, 25, and 50 μg/mL GVO were 74.67%, 76.3%, and 71.39%, respectively. These values are indicative of its strong free radical scavenging ability (Fig. 3A).
GVO increased the antioxidant capacity of C. elegans. A In vitro evaluation of antioxidant activity using DPPH assay, B, C effect of GVO on ROS fluorescence in C. elegans, D SOD activity, and E MDA content. All fluorescence images were taken at × 10 magnification and Image J software (NIH, Bethesda, MD, USA) was used to measure the relative fluorescence intensity of the worms. Statistical analysis was performed using Prism8.0, and p values were calculated using the Student’s t test. p < 0.05 was considered statistically significant. Data were presented mean ± SD of three independent experiments
In another set of experiments, the effect of GVO on intracellular ROS levels in wild type worms was explored. Reactive oxygen indicator dichlorofluorescein diacetate (H2DCF-DA) was used to determine the reactive oxygen levels of C. elegans N2. H2DCF-DA without fluorescence can be oxidized to 2′,7′-dichlorofluorescein (DCF) only in the presence of intracellular ROS to determine the intracellular ROS level. The 8-day pre-treatment with GVO significantly reduced ROS at all concentrations. Compared with those of the control nematodes, the ROS levels were reduced by 13.69%, 20.57%, and 34.49% for the GVO-treated groups at 12.5, 25, and 50 μg/mL, respectively (Fig. 2B, C).
The effects of GVO on SOD activity and MDA content in nematode are shown in Fig. 3D, E. After 8 days of treatment, the SOD activity in 12.5, 25, and 50 μg/mL GVO groups was increased by 7.44%, 15.45%, and 27.37%, respectively, compared with that of the control group. Therefore, GVO can promote the clearance of superoxide anion free radical by up-regulating the expression of SOD in nematodes to protect the body from injury. The amount of MDA often reflects the degree of lipid peroxidation in the body and indirectly reflects the degree of cell damage. Given that GVO can improve the antioxidant capacity of N2 nematodes, their MDA levels were measured. Compared with those of the control, the MDA contents of 12.5, 25, and 50 μg/mL GVO treat groups were decreased by 5%, 10.34%, and 15.34%, respectively.
Genome-wide transcriptional profiling of N2 worms
RNA sequencing analysis was performed on the 10th to compare the transcriptional profiles of N2 worms in the 50 μg/mL GVO-treated and control groups to broadly identify genetic contributions to GVO-induced longevity extension. After GVO treatment for 10 days, 3139 upregulated and 3190 downregulated genes were found in the treated worms (Fig. 4A, Supplementary Material, Table 1).
Potential mechanism of GVO extending lifespan of C. elegans. A A total of 3139 genes upregulated and 3190 genes downregulated were found in GVO-treated nematodes compared with those of untreated worms at Day 10, B validation of the differentially expressed genes screened by RNA-Seq, C 50 μg/mL GVO did not extend lifespan in SOD-1 mutant worms; and D, E expression of P62 protein in BC12921 strain treated with 50 μg/mL GVO was > 50% lower than that in the control group. All fluorescence images were taken at 10 × magnification and Image J software (NIH, Bethesda, MD, USA) was used to measure the relative fluorescence intensity of the worms. Statistical analysis was performed using Prism8.0, and p values were calculated using the Student’s t test. p < 0.05 was considered statistically significant. Data were presented mean ± SD of three independent experiments
Six differentially expressed genes, namely, atg-4.2, atg-7, sod-1, lgg-2, cyd-1 and tax-4, were selected to determine their expression changes by qPCR to verify the RNA-Seq results. The expression of all these genes was consistent with the results of RNA-Seq assay (Fig. 4B).
Lifespan extension of C. elegans by GVO is related to SOD-1
GVO can improve the antioxidant capacity of nematodes and increase their SOD enzyme activity. The level of sod-1 gene, a member of superoxide dismutase family, was increased as indicated by transcriptome sequencing. GVO may prolong the lifespan of nematodes by influencing the antioxidant pathway. For hypothesis testing, SOD-1 mutant nematodes were treated with or without 50 μg/mL GVO to observe changes in their lifespan. The results showed that after 50 μg/mL GVO treatment, the longevity of SOD-1 mutants had no significant difference compared with that of the control group (Fig. 4C). The life-prolonging activity of GVO may be affected by SOD-1.
Autophagy is involved in the lifespan extension of C. elegans by GVO
Autophagy is the main degradation system in cells. Through this process, the metabolic needs of cells and the renewal of organelles can be completed. Genetic and pharmacological experiments on C. elegans showed that all the life-extension mechanisms ranging from reduced insulin/IGF-1 signaling to spermidine supplementation, required the assistance of autophagy genes. In this study, BC12921 nematode was used to evaluate the effect of GVO on autophagy activity. The fluorescence intensity of BC12921 was decreased by 57.95% after 50 μg/mL GVO treatment (Fig. 4D, E). These results indicated that GVO treatment increased the autophagy activity of nematodes.
Discussion
Aging is the loss and degeneration of body from tissue structure to physiological function, however, this process is not fully understood. Studies on humans and model organisms revealed that GVO has various health benefits, including antioxidant, anti-tumor, and liver protection functions (Jiang et al. 2014; Wang et al. 1992; Reyes et al. 2017; Bak et al. 2012). However, its effect on biological lifespan has not been reported.
GC–MS showed that the main components of GVO are panaxynol and panaxydol. Panaxynol, the most abundant substance in the GVO, is a compound of polyacetylenes with main carbon chain of C that protects from liver injury (Lee et al. 2019), alleviates anxiety and depression-based behaviors (Sun et al. 2020), and inhibits cardiac hypertrophy (Qu et al. 2015). Panaxydol is the second most abundant substance in GVO and has significant properties of anti-inflammatory, anti-platelet agglutination, and inhibition of cell oxidase, leukemia cell growth (Yan et al. 2011) and thrombosis. In addition, panaxydol has potential anticancer activity, especially against EGFR cancers (Wang et al. 2011b; Kim et al. 2016). The synergistic action between compounds maximizes the anti-aging effect of the whole substance (Wang et al. 2018). Treatment with 75% ethanol extract of L. japonica extends the average lifespan of nematodes to 21.87%, and its life-prolonging activity is greater than that of main compounds, such as chlorogenic acid, 1,5-dicaffeoylquinic acid, and 1,3-dicaffeoylquinic acid (Yang et al. 2018). Panaxynol and panaxydol may also play a synergistic role in the anti-aging activity of GVO.
The effect of GVO on longevity was studied using C. elegans. The results showed that GVO extended the lifespan of nematodes in a dose-dependent manner. In the preliminary experiment, all the GVO concentrations used in this study did not inhibit the growth of E. coli OP50. Therefore, GVO did not prolong the lifespan of nematodes by restricting their diet. This needs to be further studied.
Aging is a decline in the body’s ability to adapt to the environment. In addition to genetic makeup that plays a dominant role, the environment is crucial in determining longevity (Chen et al. 2019; Wang et al. 2008). Here, different pretreated worms were exposed to heat shock. The survival rate of C. elegans treated with GVO was significantly increased under heat stress. This result indicated that GVO could improve the resistance of nematodes to heat and other harsh environments. Coix seed oil (Chen et al. 2020), L. japonica (Yang et al. 2018), and glycyrrhizae radix (Ruan et al. 2016) can prolong the lifespan and survival rate of nematodes after heat shock. These results are consistent with the finding that GVO can prolong the lifespan and improve the survival rate of nematodes after heat shock.
In the reproductive capacity test, GVO did not affect the total progeny yield of nematodes, indicating that this substance did not prolong the lifespan of nematodes at the cost of damaging their reproductive capacity (Meng et al. 2018). Motor ability and muscle structure will gradually decline with age (Glenn et al. 2004). Hence, the head swing frequency and body length of the worms were measured. The results showed that the head swing frequency and body length of C. elegans increased significantly after treatment with GVO compared with those of the control group. Lipofuscin is a fluorescent compound that accumulates with age and can be found in everything from nematodes to humans and even their retinal pigment epithelium (Wolf 1993; Wang et al. 2020). In line with other studies, GVO has the same effect as orange and apple extracts in reducing lipofuscin levels in nematodes (Wang et al. 2020; Vayndorf et al. 2013). The inhibiting effect of GVO on age pigment accumulation was dose-dependent and was most significant at 50 μg/mL (50% lower than that of the control). These results indicate that GVO can promote the health of C. elegans.
Free radicals are chemical entities that contain unpaired electrons and are usually highly reactive (Ionita 2021). High concentrations of free radicals can damage cellular structures and cause harm to organisms (Dröge 2002). DPPH is a stable free radical in nitrogen center and is often used for antioxidant evaluation in vitro (Sirivibulkovit et al. 2018). This study showed that GVO at different concentrations had significant scavenging ability for DPPH free radical in vitro. Variations among different concentrations were not statistically significant. The body generates oxygen free radicals through enzymatic and non-enzymatic systems that attack polyunsaturated fatty acids in biofilms and trigger lipid peroxidation, thus forming lipid peroxides, such as MDA (Doroshow 2020; Gaweł et al. 2004). Lipid peroxidation converts ROS into active chemicals and amplifies the effects of ROS through chain or chain branched-chain reactions. Therefore, an initial ROS can lead to the formation of many lipidolytic products, some of which are harmless, and some can cause cell metabolism, dysfunction, or even death (Prie et al. 2016). Oxygen free radicals cause cell damage by peroxidizing polyunsaturated fatty acids in biofilms and decomposing the products of adihydroperoxides. Therefore, the amount of MDA can often reflect the degree of lipid peroxidation in the body and indirectly reflect the degree of oxidative damage. MDA and SOD are often simultaneously determined. The level of SOD activity indirectly reflects the ability of the organism to remove oxygen free radicals. In this study, the SOD activity of nematodes significantly increased after treatment with GVO, and the intracellular ROS level and MDA content significantly decreased. This may be due to the phenolic substances contained in GVO, which are absorbed by nematodes and thus exhibit strong antioxidant activity.
Transcriptomic results showed that atg-4.2, atg-7 and lgg-2 genes were up-regulated. These genes play important roles in the autophagy pathway, and their expression level is proportional to the autophagy activity (Manil-Ségalen et al. 2014; Yang and Hekimi 2010; Hars et al. 2007). The up-regulated expression of these genes suggests that GVO may affect autophagy activity in C. elegans. Given the important role of autophagy in aging and protein homeostasis, we speculate that the beneficial effects of GVO might involve autophagy (Wong et al. 2020). For hypothesis testing, the effect of GVO on P62 protein expression in BC12921 nematodes was evaluated. BC12921 nematode was labeled with SQST-1-GFP, a homologue of P62 in mammals. With the increase in autophagy activity, the degradation degree of SQST-1-GFP also increased, and the fluorescence intensity of BC12921 decreased (Wei et al. 2016). Therefore, the expression of P62 in BC12921 strain significantly decreased by 56.54% after GVO treatment, suggesting that autophagy is a necessary pathway for GVO to prolong the lifespan of C. elegans. In addition, the expression of sod-1, a member of the superoxide dismutase family, was up-regulated. This finding further verified that GVO could improve the antioxidant capacity of nematodes and might prolong the lifespan of nematodes through the antioxidant pathway. Furthermore, GVO had no effect on the lifespan of SOD-1 mutant nematodes. Indicated that life-prolonging activity of GVO may be related to autophagy and antioxidant pathways.
Hormesis in aging is defined as the beneficial life-sustaining effects of cells in response to one or more rounds of mild stress (Rattan 2001, 2004). Studies have shown that dietary components such as vitamins, antioxidants, micronutrients and minerals, as well as ethanol and even herbicides and pesticides, show classic hormesis responses (Calabrese and Blain 2005). The essence of hormetins is that these compounds have biologically beneficial effects through one or more maintenance and repair as well as stress responses (Ali and Rattan 2006). Many TCMs have been reported to have anti-aging effects, which may be achieved through hormesis (Rattan and Ali 2007). In our study, all the nematodes treated with GVO showed high autophagy activity and antioxidant activity. SOD activity and autophagy were activated, this is a clear expression of mild stress-induced phenomenon of hormesis by the activation of one or more stress responses. It is speculated that GVO may activate the hormesis pathway to exert its life-prolonging activity, which needs further research verification.
In summary, the main components of GVO are panaxynol and panaxydol. Adding GVO to the nematode diet may increase the health, lifespan, and antioxidant capacity of N2 wild-type C. elegans in a dose-dependent manner. GVO may exert its anti-aging function through hormesis pathway. Enhanced antioxidant activity and autophagy activation as expression of hormesis may be involved in GVO-induced longevity and health life extension in nematode worms. GVO has the potential to be developed as an anti-aging drug.
References
Alzheimer’s Association (2016) 2016 Alzheimer’s disease facts and figures. Alzheimers Dement 12:459–509
Ali RE, Rattan SI (2006) Curcumin’s biphasic hormetic response on proteasome activity and heat-shock protein synthesis in human keratinocytes. Ann N Y Acad Sci 1067:394–399
Bae EA, Han MJ, Baek NI, Kim DH (2001) In vitro anti-Helicobacter pylori activity of panaxytriol isolated from ginseng. Arch Pharmacal Res 24:297–299
Bak MJ, Jun M, Jeong WS (2012) Antioxidant and hepatoprotective effects of the red ginseng essential oil in H(2)O(2)-treated hepG2 cells and CCl(4)-treated mice. Int J Mol Sci 13:2314–2330
Blois MS (1958) Antioxidant determinations by the use of a stable free radical. Nature 181:1199–1200
Brenner S (1974) The genetics of Caenorhabditis elegans. Genetics 77:71–94
Calabrese EJ, Blain R (2005) The occurrence of hormetic dose responses in the toxicological literature, the hormesis database: an overview. Toxicol Appl Pharmacol 202:289–301
Chen H, Wang C, Li H, Ma R, Yu Z, Li L, Xiang M, Chen X, Hua X, Yu Y (2019) A review of toxicity induced by persistent organic pollutants (POPs) and endocrine-disrupting chemicals (EDCs) in the nematode Caenorhabditis elegans. J Environ Manag 237:519–525
Chen XY, Liao DC, Yu YT, Wei CM, Xuan LY, Li S, Wang HB (2020) Coix seed oil prolongs lifespan and enhances stress resistance in Caenorhabditis elegans. Biogerontology 21:245–256
De Vries ST, Denig P, Ekhart C, Mol PGM, Van Puijenbroek EP (2020) Sex differences in adverse drug reactions of metformin: a longitudinal survey study. Drug Saf 43:489–495
Doroshow JH (2020) Effect of anticancer quinones on reactive oxygen production by adult rat heart myocytes. Oxid Med Cell Longev 2020:8877100
Dröge W (2002) Free radicals in the physiological control of cell function. Physiol Rev 82:47–95
Gaweł S, Wardas M, Niedworok E, Wardas P (2004) Malondialdehyde (MDA) as a lipid peroxidation marker. Wiad Lek 57:453–455
Gershon H, Gershon D (2002) Caenorhabditis elegans—a paradigm for aging research: advantages and limitations. Mech Ageing Dev 123:261–274
Glenn CF, Chow DK, David L, Cooke CA, Gami MS, Iser WB, Hanselman KB, Goldberg IG, Wolkow CA (2004) Behavioral deficits during early stages of aging in Caenorhabditis elegans result from locomotory deficits possibly linked to muscle frailty. J Gerontol A 59:1251–1260
Guarente L, Kenyon C (2000) Genetic pathways that regulate ageing in model organisms. Nature 408:255–262
Han HJ, Kim HY, Choi JJ, Ahn SY, Lee SH, Oh KW, Kim SY (2013) Effects of red ginseng extract on sleeping behaviors in human volunteers. J Ethnopharmacol 149:597–599
Hars ES, Qi H, Ryazanov AG, Jin S, Cai L, Hu C, Liu LF (2007) Autophagy regulates ageing in C. elegans. Autophagy 3:93–95
Ionita P (2021) The chemistry of DPPH(·) free radical and congeners. Int J Mol Sci 22(4):1545
Jiang LP, Lu Y, Nie BM, Chen HZ (2008) Antiproliferative effect of panaxynol on RASMCs via inhibition of ERK1/2 and CREB. Chem Biol Interact 171:348–354
Jiang R, Sun L, Wang Y, Liu J, Liu X, Feng H, Zhao D (2014) Chemical composition, and cytotoxic, antioxidant and antibacterial activities of the essential oil from ginseng leaves. Nat Prod Commun 9:865–868
Johnston SC, Elm JJ, Easton JD, Farrant M, Barsan WG, Kim AS, Lindblad AS, Palesch YY, Zurita KG, Albers GW, Cucchiara BL, Kleindorfer DO, Lutsep HL, Pearson C, Sethi P, Vora N (2019) Time course for benefit and risk of clopidogrel and aspirin after acute transient ischemic attack and minor ischemic stroke. Circulation 140:658–664
Kim HS, Lim JM, Kim JY, Kim Y, Park S, Sohn J (2016) Panaxydol, a component of Panax ginseng, induces apoptosis in cancer cells through EGFR activation and ER stress and inhibits tumor growth in mouse models. Int J Cancer 138:1432–1441
Lee D, Lee J, Vu-Huynh KL, Van Le T, H., Tuoi Do, T. H., Hwang, G. S., Park, J. H., Kang, K. S., Nguyen, M. D. & Yamabe, N. (2019) Protective effect of panaxynol isolated from Panax vietnamensis against cisplatin-induced renal damage: in vitro and in vivo studies. Biomolecules 9(12):890
Lin C, Zhang X, Xiao J, Zhong Q, Kuang Y, Cao Y, Chen Y (2019) Effects on longevity extension and mechanism of action of carnosic acid in Caenorhabditis elegans. Food Funct 10:1398–1410
Liu X, Li M, Wang X, Dang Z, Yu L, Wang X, Jiang Y, Yang Z (2019) Effects of adjuvant traditional Chinese medicine therapy on long-term survival in patients with hepatocellular carcinoma. Phytomedicine 62:152930
Manil-Ségalen M, Lefebvre C, Jenzer C, Trichet M, Boulogne C, Satiat-Jeunemaitre B, Legouis R (2014) The C. elegans LC3 acts downstream of GABARAP to degrade autophagosomes by interacting with the HOPS subunit VPS39. Dev Cell 28:43–55
Meng F, Li J, Rao Y, Wang W, Fu Y (2018) Gengnianchun extends the lifespan of Caenorhabditis elegans via the insulin/IGF-1 signalling pathway. Oxid Med Cell Longev 2018:4740739
Niccoli T, Partridge L (2012) Ageing as a risk factor for disease. Curr Biol 22:R741–R752
Prie BE, Iosif L, Tivig I, Stoian I, Giurcaneanu C (2016) Oxidative stress in androgenetic alopecia. J Med Life 9:79–83
Qu C, Li B, Lai Y, Li H, Windust A, Hofseth LJ, Nagarkatti M, Nagarkatti P, Wang XL, Tang D, Janicki JS, Tian X, Cui T (2015) Identifying panaxynol, a natural activator of nuclear factor erythroid-2 related factor 2 (Nrf2) from American ginseng as a suppressor of inflamed macrophage-induced cardiomyocyte hypertrophy. J Ethnopharmacol 168:326–336
Rattan SI (2001) Applying hormesis in aging research and therapy. Hum Exp Toxicol 20:281–285; discussion 293–294
Rattan SI (2004) Aging intervention, prevention, and therapy through hormesis. J Gerontol A 59:705–709
Rattan SI, Ali RE (2007) Hormetic prevention of molecular damage during cellular aging of human skin fibroblasts and keratinocytes. Ann N Y Acad Sci 1100:424–430
Reyes AWB, Hop HT, Arayan LT, Huy TXN, Park SJ, Kim KD, Min W, Lee HJ, Rhee MH, Kwak YS, Kim S (2017) The host immune enhancing agent Korean red ginseng oil successfully attenuates Brucella abortus infection in a murine model. J Ethnopharmacol 198:5–14
Ruan Q, Qiao Y, Zhao Y, Xu Y, Wang M, Duan J, Wang D (2016) Beneficial effects of Glycyrrhizae radix extract in preventing oxidative damage and extending the lifespan of Caenorhabditis elegans. J Ethnopharmacol 177:101–110
Saier C, Büchter C, Koch K, Wätjen W (2018) Polygonum multiflorum extract exerts antioxidative effects and increases life span and stress resistance in the model organism Caenorhabditis elegans via DAF-16 and SIR-2.1. Plants (basel) 7(3):60
Sirivibulkovit K, Nouanthavong S, Sameenoi Y (2018) Paper-based DPPH assay for antioxidant activity analysis. Anal Sci 34:795–800
Sun X, Zhang T, Zhao Y, Cai E, Zhu H, Liu S (2020) Panaxynol attenuates CUMS-induced anxiety and depressive-like behaviors via regulating neurotransmitters, synapses and the HPA axis in mice. Food Funct 11:1235–1244
Vayndorf EM, Lee SS, Liu RH (2013) Whole apple extracts increase lifespan, healthspan and resistance to stress in Caenorhabditis elegans. J Funct Foods 5:1236–1243
Wang H, Liu J, Li T, Liu RH (2018) Blueberry extract promotes longevity and stress tolerance via DAF-16 in Caenorhabditis elegans. Food Funct 9:5273–5282
Wang J, Deng N, Wang H, Li T, Chen L, Zheng B, Liu RH (2020) Effects of orange extracts on longevity, healthspan, and stress resistance in Caenorhabditis elegans. Molecules 25(2):351
Wang M, Li F, Li X, Zhang L, E, Z. (1992) Effects of ginseng volatile oil on cytochemical components of SGC-823 gastric carcinoma in cell culture. Zhongguo Zhong Yao Za Zhi 17(110–2):128
Wang X, Shen L, Yu H, Wang D (2008) Toxicity evaluation in a paper recycling mill effluent by coupling bioindicator of aging with the toxicity identification evaluation method in nematode Caenorhabditis elegans. J Environ Sci (china) 20:1373–1380
Wang Y, Liu J, Zhang Z, Bi P, Qi Z, Zhang C (2011a) Anti-neuroinflammation effect of ginsenoside Rbl in a rat model of Alzheimer disease. Neurosci Lett 487:70–72
Wang YZ, Chen J, Chu SF, Wang YS, Wang XY, Chen NH, Zhang JT (2009) Improvement of memory in mice and increase of hippocampal excitability in rats by ginsenoside Rg1’s metabolites ginsenoside Rh1 and protopanaxatriol. J Pharmacol Sci 109:504–510
Wang ZJ, Song L, Guo LC, Yin M, Sun YN (2011b) Induction of differentiation by panaxydol in human hepatocarcinoma SMMC-7721 cells via cAMP and MAP kinase dependent mechanism. Yakugaku Zasshi 131:993–1000
Wei S, Chen W, Qin J, Huangli Y, Wang L, Shen Y, Tang H (2016) Multitarget-directed oxoisoaporphine derivatives: anti-acetylcholinesterase, anti-β-amyloid aggregation and enhanced autophagy activity against Alzheimer’s disease. Bioorg Med Chem 24:6031–6039
Wolf G (1993) Lipofuscin, the age pigment. Nutr Rev 51:205–206
Wolfe KL, Liu RH (2007) Cellular antioxidant activity (CAA) assay for assessing antioxidants, foods, and dietary supplements. J Agric Food Chem 55:8896–8907
Wong SQ, Kumar AV, Mills J, Lapierre LR (2020) Autophagy in aging and longevity. Hum Genet 139:277–290
Xiao M, Tian J, Zhou Y, Xu X, Min X, Lv Y, Peng M, Zhang Y, Yan D, Lang S, Zhang Q, Fan A, Ke J, Li X, Liu B, Jiang M, Liu Q, Zhu J, Yang L, Zhu Z, Zeng K, Li C, Zheng Y, Wu H, Lin J, Lian F, Li X, Tong X (2020) Efficacy of Huoxiang Zhengqi dropping pills and Lianhua Qingwen granules in treatment of COVID-19: a randomized controlled trial. Pharmacol Res 161:105126
Yan Z, Yang R, Jiang Y, Yang Z, Yang J, Zhao Q, Lu Y (2011) Induction of apoptosis in human promyelocytic leukemia HL60 cells by panaxynol and panaxydol. Molecules 16:5561–5573
Yang W, Hekimi S (2010) Two modes of mitochondrial dysfunction lead independently to lifespan extension in Caenorhabditis elegans. Aging Cell 9:433–447
Yang ZZ, Yu YT, Lin HR, Liao DC, Cui XH, Wang HB (2018) Lonicera japonica extends lifespan and healthspan in Caenorhabditis elegans. Free Radic Biol Med 129:310–322
Zhang J, Xue X, Qiao Y, Li D, Wei Q, Zhang F, Qin X (2021) Astragaloside IV extends lifespan of Caenorhabditis elegans by improving age-related functional declines and triggering antioxidant responses. Rejuvenation Res 24:120–130
Zhang L, Ravipati AS, Koyyalamudi SR, Jeong SC, Reddy N, Bartlett J, Smith PT, de la Cruz M, Monteiro MC, Melguizo A, Jiménez E, Vicente F (2013) Anti-fungal and anti-bacterial activities of ethanol extracts of selected traditional Chinese medicinal herbs. Asian Pac J Trop Med 6:673–681
Zhang Z, Zhou Y, Fan H, Billy KJ, Zhao Y, Zhan X, Yang L, Jia Y (2019) Effects of Lycium barbarum polysaccharides on health and aging of C. elegans depend on daf-12/daf-16. Oxid Med Cell Longev 2019:6379493
Zhou Y, Xu Q, Zhou X, Song S, Zhu B (2018) Stress resistance and lifespan extension of Caenorhabditis elegans enhanced by peptides from mussel (Mytilus edulis) protein hydrolyzate. Food Funct 9:3313–3320
Acknowledgements
All authors have read and approved the manuscript for publication. And claimed they had no interest in each other.
Funding
This work was supported by the Department of Science and Technology of Jilin Province (JJKH20201026KJ, [20210204033YY); Key Laboratory of Precision Infectious Diseases of Jilin Province (20200601011JC); Engineering Laboratory for Precision Prevention and Control of Common Diseases in Jilin Province.
Author information
Authors and Affiliations
Contributions
LW was responsible for the experiment operation and manuscript writing. PQ was responsible for experimental design. ZO and DL were in charge of data processing. GW, JZ, and FW were responsible for editing the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing financial interests or personal relationships that could have influenced the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, L., Qiao, P., Ouyang, Z. et al. Ginseng volatile oil prolongs the lifespan and healthspan of Caenorhabditis elegans. Biogerontology 23, 485–497 (2022). https://doi.org/10.1007/s10522-022-09956-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10522-022-09956-z