“The end of life has its own nature, also worth our attention.”
Mary Oliver (2009)
Abstract
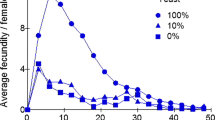
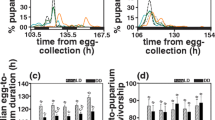
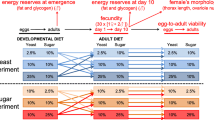
There are two life history landmarks that can be used to define the terminal period in individual Drosophila melanogaster females: the cessation of daily oviposition, which defines the start of the retired stage, and final oviposition, which defines the start of post-ovipository survival. The terminal period is a substantial component of D. melanogaster life history. Analysis of published data on the daily fecundity and survival of 3971 individually maintained, mated female flies reveals that the terminal period is far more variable within populations than other life history components, including total adult life span. It has been reported that there is a negative correlation between fecundity and duration of the terminal state in recently collected wild stocks. Here I show that the negative correlation occurs in multiple inbred and outbred lab-adapted populations as well. In terms of proportion of adult life, lower fecundity flies spend on average twice as much time in the terminal stage as higher fecundity flies from the same population. Both high and low fecundity flies experience end-of-life plateaus in mortality, with the former exhibiting higher plateau levels. The negative correlation between fecundity and terminal survival is of sufficient magnitude to create heterogeneity among the oldest old: the final 10% of survivors are predominately flies with a history of high fecundity, but about one in five is a low fecundity fly with long terminal stage.





Similar content being viewed by others
References
Arking R (2015) Independent chemical regulation of health and senescent spans in Drosophila. Invertebr Rep Dev 59:28–32
Bitner K, Shahrestani P, Pardue E, Mueller LD (2020) Predicting death by the loss of intestinal function. PLoS ONE 15(4):e0230970. https://doi.org/10.1371/journal.pone.0230970
Charlesworth B (1994) Evolution in age-structured populations. Cambridge University Press, Cambridge
Clark RI, Salazar A, Yamada R, Fitz-Gibbon S, Morselli M, Alcaraz J, Rana A, Rera M, Pellegrini M, William WJ, Walker DW (2015) Distinct shifts in microbiota composition during Drosophila aging impair intestinal function and drive mortality. Cell Reports 12:1656–1667
Curtsinger JW (2015) The retired fly: detecting life history transition in individual Drosophila melanogaster females. J Gerontol A-Biol 70:1455–1460
Curtsinger JW (2016) Retired flies, hidden plateaus, and the evolution of senescence in Drosophila melanogaster. Evolution 70:1297–1306
Curtsinger JW (2018) Reproductive homeostasis and senescence in Drosophila melanogaster. J Gerontol A Biol 74:1533–1538
Curtsinger JW, Khazaeli AA (2002) Lifespan, QTLs, age-specificity, and pleiotropy in Drosophila. Mech Ageing Dev 123:81–93
Curtsinger JW, Fukui H, Townsend D, Vaupel JW (1992) Demography of genotypes: failure of the limited life-span paradigm in Drosophila melanogaster. Science 258:461–463
Curtsinger JW, Fukui HH, Khazaeli AA, Kirscher A, Pletcher SD, Promislow DEL, Tatar M (1995) Genetic variation and aging. Annu Rev Genet 29:553–575
Curtsinger JW, Gavrilova NS, Gavrilov LA (2006) Biodemography of aging and age-specific mortality in Drosophila melanogaster. In: Masoro E, Austad S (eds) Handbook of the biology of aging, 6th edn. Academic Press, San Diego, pp 267–281
Drapeau MD, Gass EK, Simison MD, Mueller LD, Rose MR (2000) Testing the heterogeneity theory of late-life mortality plateaus by using cohorts of Drosophila melanogaster. Exp Gerontol 35:71–84
Finch CE, Kirkwood TBL (2000) Chance, Development, and Aging. Oxford University Press, New York
Flatt T (2020) Life-history evolution and the genetics of fitness components in Drosophila melanogaster. Genetics 214:3–48
Fukui HH, Ackert L, Curtsinger JW (1996) Deceleration of age-specific mortality rates in chromosomal homozygotes and heterozygotes of Drosophila melanogaster. Exp Gerontol 31:517–531
Fukui HH, Xiu L, Curtsinger JW (1993) Slowing of age-specific mortality rates in Drosophila melanogaster. Exp Gerontol 28:585–599
Hughes KA, Charlesworth B (1994) A genetic analysis of senescence in Drosophila. Nature 367:64–66
Khazaeli AA, Curtsinger JW (2014) Heterogeneity's Ruses: how hidden variation affects population trajectories of age-specific fecundity in Drosophila melanogaster. Demogr Res 30:313–332
Khazaeli AA, Pletcher SD, Curtsinger JW (1998) The fractionation experiment: reducing heterogeneity to investigate age-specific mortality in Drosophila. Mech Ageing Dev 105:301–317
Kirkwood TBL, Feder M, Finch CE, Franceschi C, Globerson A, Klingenberg CP, LaMarco K, Omholt S, Westendorp RGJ (2005) What accounts for the wide variation in life span of genetically identical organisms reared in a constant environment? Mech Ageing Dev 126:439–443
Klepsatel P, Galikova M, De Maio N, Ricci S, Schlotterer C, Flatt T (2013a) Reproductive and post-reproductive life history of wild-caught Drosophila melanogaster under laboratory conditions. J Evol Biol 26:1508–1520
Klepsatel P, Galikova M, De Maio N, Ricci S, Schlotterer C, Flatt T (2013b) Data from reproductive and post-reproductive life history of wild-caught Drosophila melanogaster under laboratory conditions. Dryad Digital Repository. https://doi.org/10.5061/dryad.3q332
Koliada A, Gavrilyuk K, Burdylyuk N, Strilbytska O, Storey KB, Kuharskii V, Lushchak O, Vaiserman A (2020) Mating status affects Drosophila lifespan, metabolism and antioxidant system. Comp Biochem Phys A 246:110716
Le Bourg E, Moreau M (2014) Individual late-life fecundity plateaus do exist in Drosophila melanogaster and are very common at old age. Exp Gerontol 55:102–106
Leips J, Gilligan P, Mackay TCF (2006) Quantitative trait loci with age-specific effects on fecundity in Drosophila melanogaster. Genetics 172(3):1595–1605
Medawar PB (1952) An unsolved problem of biology. Lewis, London
Miller PB, Obrik-Uloho OT, Phan MH, Medrano CL, Renier JS, Thayer JL, Wiessner G, Bloch-Qazi MC (2014) The song of the old mother: reproductive senescence in female Drosophila. Fly 8:127–139. https://doi.org/10.4161/19336934.2014.969144
Mueller LD, Rauser CL, Rose MR (2007) An evolutionary heterogeneity model of late-life fecundity in Drosophila. Biogerontology 8:147–161
Mueller LD, Shahrestani P, Rauser CL (2009) Predicting death in female Drosophila. Exp Gerontol 44:766–772
Oliver M (2009) Our world. Beacon Press, Boston, MA
Pletcher SD, Houle D, Curtsinger JW (1998) Age-specific properties of spontaneous mutations affecting mortality in Drosophila melanogaster. Genetics 148(1):287–303
Pletcher SD (1999) Model fitting and hypothesis testing for age-specific mortality data. J Evol Biol 12:430–439
Promislow DEL, Tatar M, Khazaeli AA, Curtsinger JW (1996) Age-specific patterns of genetic variance in Drosophila melanogaster. I. Mortality. Genetics 143:839–848
Rauser CL, Abdel-Aal Y, Shieh JA, Suen CW, Mueller LD, Rose MR (2005) Lifelong heterogeneity in fecundity is insufficient to explain late-life fecundity plateaus in Drosophila melanogaster. Exp Gerontol 40:660–670
Rera M, Clark RI, Walker DW (2012) Intestinal barrier dysfunction links metabolic and inflammatory markers of aging to death in Drosophila. Proc Natl Acad Sci USA 109:21528–21533
Reznick D, BryantHolmes MD (2005) The evolution of senescence and post-reproductive lifespan in guppies (Poecilia reticulata). PLoS Biol 4:e7
Rogina B, Wolverton T, Bross TG, Chen K, Müller H-G, Carey JR (2007) Distinct biological epochs in the reproductive life of female Drosophila melanogaster. Mech Ageing Dev 128:477–485
Shaw FH, Promislow DE, Tatar M, Hughes KA, Geyer CJ (1999) Toward reconciling inferences concerning genetic variation in senescence in Drosophila melanogaster. Genetics 152:553–566
Tower J, Agrawal S, Alagappan MP, Bell HS, Demeter M, Havanoor N, Hegde VS, Jia Y, Kothawade S, Lin X, Nadig C (2019) Behavioral and molecular markers of death in Drosophila melanogaster. Exp Gerontol 126:110707
Vaupel JW, Carey JR, Christiansen K, Johnson TE, Yashin AI, Holm NV, Iachine A, Khazaeli AA, Liedo P, Longo VD, Yi ZY, Manton KD, Curtsinger JW (1998) Biodemographic trajectories of longevity. Science 280:855–860
Williams GC (1957) Pleiotropy, natural selection, and the evolution of senescence. Evolution 11:398–411
Zajitschek F, Jin T, Colchero F, Maklakov AA (2014) Aging differently: diet-and sex-dependent late-life mortality patterns in Drosophila melanogaster. J Gerontol A-Biol 69:666–674
Acknowledgements
I am grateful to investigators in the Flatt, Le Bourg, Rose & Mueller, and Curtsinger & Khazaeli laboratories who painstakingly collected large datasets on individual life history, and to the principal investigators who made the data available for the scientific community. I thank A. A. Khazaeli (Minnesota) and T. Flatt (Fribourg) for comments on an early draft.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author claims no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Curtsinger, J.W. Terminal life history: late-life fecundity and survival in experimental populations of Drosophila melanogaster. Biogerontology 21, 721–730 (2020). https://doi.org/10.1007/s10522-020-09889-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10522-020-09889-5