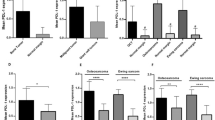
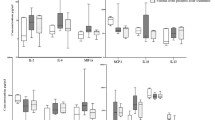
The levels of sPD-1 and sPD-L1 were analyzed in blood serum of 132 patients (age 14-70 years) with primary bone tumors: osteosarcoma (N=39), chondrosarcoma (N=42), Ewing sarcoma (N=9), chordoma (N=12), giant-cell bone tumor (GCBT) (N=16), benign neoplasms (N=14) and in and practically healthy subjects (age 19-58 years; N=27). sPD-L1 levels in all studied bone neoplasms were significantly higher than in the control. Serum sPD-1 level in GCBT patients was significantly higher than in the control, benign neoplasms, chondrosarcoma, and chordoma patients, but did not differ from osteosarcoma group. sPD-1 concentration in Ewing sarcoma was significantly higher than in chordoma and chondrosarcoma, but did not differ from the control. sPD-1 level in chondrosarcoma patients was also lower than in osteosarcoma, Ewing sarcoma, and in the control. Both sPD-1 and sPD-L1 concentrations were not significantly associated with the type of affected bone, process localization, disease stage, tumor histological grade, patients’ age and sex. These results suggest the possibility of using these biological markers for preliminary assessment of the character of the process in the bone.
Similar content being viewed by others
References
Gershtein ES, Utkin DO, Goryacheva IO, Khulamkhanova MM, Petrikova NA, Vinogradov II, Alferov AA, Stilidi IS, Kushlinskii NE. Soluble forms of immune checkpoint receptor PD-1 and its ligand PD-L1 in plasma of patients with ovarian neoplasms. Al’manakh Klin. Med. 2018;46(7):690-698. Russian.
Ding Y, Sun C, Li J, Hu L, Li M, Liu J, Pu L, Xiong S. The Prognostic significance of soluble programmed death ligand 1 expression in cancers: a systematic review and meta-analysis. Scand. J. Immunol. 2017;86(5):361-367.
Guo X, Wang J, Jin J, Chen H, Zhen Z, Jiang W, Lin T, Huang H, Xia Z, Sun X. High serum level of soluble programmed death ligand 1 is associated with a poor prognosis in Hodgkin lymphoma. Transl. Oncol. 2018;11(3):779-785.
Huang HF, Zhu H, Yang XT, Guo XY, Li SS, Xie Q, Tian XB, Yang Z. Progress in research on tumor immune PD-1/PD-L1 signaling pathway in malignant bone tumors. Zhonghua Zhong Liu Za Zhi. 2019;41(6):410-414.
Kabir TF, Chauhan A, Anthony L, Hildebrandt GC. Immune Checkpoint Inhibitors in Pediatric Solid Tumors: Status in 2018. Ochsner J. 2018;18(4):370-376.
Kim HJ, Park S, Kim KJ, Seong J. Clinical significance of soluble programmed cell death ligand-1 (sPD-L1) in hepatocellular carcinoma patients treated with radiotherapy. Radiother. Oncol. 2018;129(1):130-135.
Kushlinskii NE, Gershtein ES, Morozov AA, Goryacheva IO, Filipenko ML, Alferov AA, Bezhanova SD, Bazaev VV, Kazantseva IA. Checkpoint Receptor (sPD-L1) in Blood Serum of Patients with Renal Cell Carcinoma. Bull. Exp. Biol. Med. 2019;166(3):353-357.
Kyi C, Postow MA. Checkpoint blocking antibodies in cancer immunotherapy. FEBS Lett. 2014;588(2):368-376.
Nagato T, Ohkuri T, Ohara K, Hirata Y, Kishibe K, Komabayashi Y, Ueda S, Takahara M, Kumai T, Ishibashi K, Kosaka A, Aoki N, Oikawa K, Uno Y, Akiyama N, Sado M, Takei H, Celis E, Harabuchi Y, Kobayashi H. Programmed death-ligand 1 and its soluble form are highly expressed in nasal natural killer/T-cell lymphoma: a potential rationale for immunotherapy. Cancer Immunol. Immunother. 2017;66(7): 877-890.
Sunshine J, Taube JM. PD-1/PD-L1 inhibitors. Curr. Opin. Pharmacol. 2015;23:32-38.
Theodoraki MN, Yerneni SS, Hoffmann TK, Gooding WE, Whiteside TL. Clinical significance of PD-L1(+) exosomes in plasma of head and neck cancer patients. Clin. Cancer Res. 2018;24(4):896-905.
Tsukahara T, Emori M, Murata K, Mizushima E, Shibayama Y, Kubo T, Kanaseki T, Hirohashi Y, Yamashita T, Sato N, Torigoe T. The future of immunotherapy for sarcoma. Expert. Opin. Biol. Ther. 2016;16(8):1049-1057.
Unni KK, Inwards CY. Dahlin’s Bone Tumors: General Aspects and Data on 10,165 Cases. Philadelphia, 2006.
Wei W, Xu B, Wang Y, Wu C, Jiang J, Wu C. Prognostic significance of circulating soluble programmed death ligand-1 in patients with solid tumors: A meta-analysis. Medicine (Baltimore). 2018;97(3):e9617. doi: https://doi.org/10.1097/MD.0000000000009617
Zhu X, Lang J. Soluble PD-1 and PD-L1: predictive and prognostic significance in cancer. Oncotarget. 2017;8(57):97,671-97,682.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Byulleten’ Eksperimental’noi Biologii i Meditsiny, Vol. 170, No. 7, pp. 79-83, July, 2020
Rights and permissions
About this article
Cite this article
Kushlinskii, N.E., Alferov, A.A., Timofeev, Y.S. et al. Key Immune Checkpoint PD-1/PD-L1 Signaling Pathway Components in the Blood Serum from Patients with Bone Tumors. Bull Exp Biol Med 170, 64–68 (2020). https://doi.org/10.1007/s10517-020-05005-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10517-020-05005-2