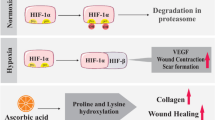
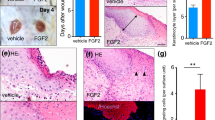
We studied the effect of bovine brain gangliosides, individual ganglioside GM1, and melatonin on the rate of wound closure under in vitro conditions and the effect of melatonin on the rate of wound healing under in vivo conditions. It was shown that bovine brain gangliosides and melatonin reliably increased cell migration in the experimental wound model. This effect was detected when the cell cultures were treated with the test preparations after wound infliction and when the cultures of human keratinocytes were pretreated before wounding. Analysis of the effect of melatonin on the rate of wound healing in vivo showed that melatonin accelerated this process, especially at the middle stages corresponding to the proliferation phase (days 3-6 after surgery). Histological analysis revealed intensification of epidermal cell proliferation at the edges of the wound starting from day 4 after surgery.
Similar content being viewed by others
References
Brodsky VY. Circahoralian (Ultradian) metabolic rhythms. Biochemistry (Moscow). 2014;79(6):483-495.
Brodsky VY, Vorotelyak EA, Terskikh VV, Vasil’ev AV, Mal’chenko LA, Zvezdina ND, Konchenko DS, Dubovaya TK. Dopamine disorganizes direct intercellular interactions in keratinocytes cultures: A comparison to hepatocytes. Russ. J. Dev. Biol. 2016;47(2):77-82.
Brodsky VY, Terskikh VV, Vasilyev AV, Zvezdina ND, Vorotelyak EA, Fateeva VI, Mal’chenko LA. Self-synchronization of the protein synthesis rhythm in HaCaT cultures of human keratinocytes. Russ. J. Dev. Biol. 2011;42(4):272-279.
Vorotelyak EA, Samarova AV, Vasil’ev AV, Terskikh VV. The effect of epidermal growth factor on migration of A-431 cells. Biol. Bull. 1997;24(6):600-602.
Zvezdina ND, Malchenko LA, Konchenko DS, Dubovaya TK, Brodskii VY. Effect of protein synthesis rhythm-organizing signal persists for a day after single administration of melatonin to rat. Bull. Exp. Biol. Med. 2014;156(3):323-326. doi: https://doi.org/10.1007/s10517-014-2340-4
Anisimov VN, Popovich IG, Zabezhinski MA, Anisimov SV, Vesnushkin GM, Vinogradova IA. Melatonin as antioxidant, genoprotector and anticarcinogen. Biochim. Biophys. Acta. 2006;1757(5-6):573-589.
Brodsky VY, Zvezdina ND. Melatonin as the most effective organizer of the rhythm of protein synthesis in hepatocytes in vitro and in vivo. Cell Biol. Int. 2010;34(12):1199-1204.
Bulbuller N, Dogru O, Yekeler H, Cetinkaya Z, Ilhan N, Kirkil C. Effect of melatonin on wound healing in normal and pinealectomized rats. J. Surg. Res. 2005;123(1):3-7.
Drobnik J. Wound healing and the effect of pineal gland and melatonin. J. Exp. Integr. Med. 2012;2(1):3-14.
Karasek M. Melatonin, human aging, and age-related diseases. Exp. Gerontol. 2004;39(11-12):1723-1729.
Kvetnoy IM, Ingel IE, Kvetnaia TV, Malinovskaya NK, Rapoport SI, Raikhlin NT, Trofimov AV, Yuzhakov VV. Gasrointestinal melatonin. Cellular identification and biological role. Neuro Endocrinol. Lett. 2002;23(2):121-132.
Maharaj DS, Glass BD, Daya S. Melatonin: new places in therapy. Biosci. Rep. 2007;27(6):299-320.
Pugazhenthi K, Kapoor M, Clarkson AN, Hall I, Appleton I. Melatonin accelerates the process of wound repair in full-thickness incisional wounds. J. Pineal Res. 2008;44(4):387-396.
Wang XQ, Lee S, Wilson H, Seeger M, Iordanov H, Gatla N, Whittington A, Bach D, Lu JY, Paller AS. Ganglioside GM3 depletion reverses impaired wound healing in diabetic mice by activating IGF-1 and insulin receptors. J. Invest. Dermatol. 2014;134(5):1446-1455.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Byulleten’ Eksperimental’noi Biologii i Meditsiny, Vol. 168, No. 8, pp. 195-200, August, 2019
Rights and permissions
About this article
Cite this article
Vorotelyak, E.A., Malchenko, L.A., Rogovaya, O.S. et al. Melatonin Stimulates Epithelium Migration in Wound Models In Vitro and In Vivo. Bull Exp Biol Med 168, 242–246 (2019). https://doi.org/10.1007/s10517-019-04683-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10517-019-04683-x