Abstract
Evidence and observations favoring the hypothesis that Venus is habitable, and the celestial mechanisms promoting the interplanetary transfer of life, are reviewed. Venus may have been contaminated with Earthly life early in its history via interplanetary transfer of microbe-laden bolide ejecta; and this seeding with life may have continued into the present via spacecraft and due to radiation pressure and galactic winds blowing microbial-laden dust ejected from the stratosphere via powerful solar winds, into the orbit and atmosphere of Venus. Venus may have had oceans and rivers early in its history until 750 mya, and, hypothetically, some of those species which, theoretically, colonized the planet during that time, may have adapted and evolved when those oceans evaporated and temperatures rose. Venus may be inhabited by a variety of extremophiles which could flourish within the lower cloud layers, whereas others may dwell 10 m below the surface where temperature may be as low as 200 ∘C—which is within the tolerance level of some hyperthermophiles. Speculation as to the identity of mushroom-shaped specimens photographed on the surface of Venus by the Russian probe, Venera 13 support these hypotheses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Overview: hypotheses, observations, evidence
Observations and evidence for the possibility of life on the subsurface and clouds of Venus, and celestial mechanics promoting the transfer of life from Earth, are reviewed, and images of specimens resembling fungal mushroom-shaped organisms photographed by the Russian Space Probe Venera 13, on the surface of Venus are presented (Figs. 1–5) with the caveat that similarities in morphology are not proof of life.
A number of scientists have hypothesized that Earth may be seeding this solar system, including Venus, Mars, and the Moons of Jupiter and Saturn, with microbes buried within ejecta following impact by comets and meteors (Cockell 1999; Gladman et al. 1996; Melosh 2003; Mileikowsky et al. 2000a,b; Gao et al. 2014; Schulze-Makuch et al. 2004), and via microbes attached to space craft (Joseph 2018). Extremophiles attached to dust particles which are lofted into Earth’s upper atmosphere and via powerful solar winds blown into space (Joseph and Schild 2010) may also be transported by radiation pressure and galactic winds into the atmosphere of Venus (Arrhenius 1908, 1918). Atmospheric contamination by Earthly life would account for data indicating microbes may be dwelling within and attached to particles in the lower cloud layers of Venus (Bullock 2018; Limaye et al. 2018; Grinspoon and Bullock 2007).
In support of the solar-galactic-wind microbe-laden-dust hypothesis is evidence of an interplanetary (Misconi and Weinberg 1978) circumsolar ring of dust (Krasnopolsky and Krysko 1979) which appears to follow (Leinert and Moster 2007) and to have possibly originated external to the orbit of Venus (Altobelli et al. 2003; Jones et al. 2013; Leinert and Moster 2007; Russell and Vaisberg 1983) and which penetrates the planet’s atmosphere (Carrillo-Sánchez et al. 2020). As to dust originating from Earth, it is probable that microbes may be adhering to some of these particles (Arrhenius 1908, 1918).
Cockell (1999) contends that atmospheric conditions at higher altitudes would freeze but not necessarily kill microorganisms. As these organisms fall toward the surface they would encounter environments between the middle and lower cloud layers that are conducive to biological activity (Limaye et al. 2018). Moreover, the turbulence experienced may enhance the viability of some species (Gibson 2000; Gibson and Thomas 1995) including plankton which have been hypothesized to be dwelling in the clouds of Venus (Konesky 2009; Schulze-Makuch et al. 2004).
The Venusian subsurface may also provide a livable habitat for photoautotrophic, lithobiontic, poikilohydric, and fungal hyperthermophiles which have evolved and adapted to these conditions. For example, temperatures at 10 m below the surface may range from 305 ∘C to 200 ∘C–which is within the tolerance level of some thermophiles (Baross and Deming 1983; Kato and Qureshi 1999). It is well established that extremophiles are “capable of living in extreme environments such as highly acidic or alkaline conditions, at high salt concentration, with no oxygen, extreme temperatures (as low as −20 ∘C and as high as 300 ∘C), at high concentrations of heavy metals and in high pressure environments” (Kato and Takai 2000).
Organisms deep beneath the surface may also have access to moisture drawn from subterranean sources before completely evaporating. In support of this hypothesis, when the Russian probe Venera 13 landed on Venus and in so doing blew away surrounding top soil, specimens resembling fungi were later photographed by Venera, as reported here (Figs. 1, 2, 3, 4, 5).
2 Terrestrial analogs: life in the clouds
Over 1800 different types of bacteria and other species thrive and flourish within the troposphere, the first layer of the Earth’s atmosphere (Brodie et al. 2007). These denizens of the air include fungi and fungal spores, lichens, algae, protozoa, diatoms, plants, pollen, seeds, and invertebrates including insects, spiders, mites, and nematodes (Adhikari et al. 2006; Diehl 2013; Fröhlich-Nowoisky et al. 2009; Griffin 2004; Polymenakou 2012); many of which become attached to dust and other particles (Möhler et al. 2007; Sattler et al. 2001).
Due to tropical storms, monsoons, and seasonal upwellings of columns of air (Dehel et al. 2008; Deleon-Rodriguez et al. 2013; Randel et al. 1998, 2010), microbes, spores, fungi, along with dust, dirt, water, methane, and other gases may be transported to the stratosphere (Griffin 2004; Imshenetsky et al. 1978). Many organisms eventually fall back to the surface where they may negatively impact (Griffin et al. 2007; Polymenakou 2012) or promote the growth and metabolic activity of other species (Jones et al. 2008).
Microorganisms and spores have been recovered at heights of 40 km (Soffen 1965), 61 km (Wainwright et al. 2010) and up to 77-km (Imshenetsky et al. 1978). These include Mycobacterium and Micrococcus, and fungi Penicillium notatum Circinella muscae, and Aspergillus niger, 77-km above Earth’s surface (Imshenetsky et al. 1978). These atmospheric fungi and microorganisms play a major role in organic compound degradation and the alteration and chemical composition of the atmosphere (Amato et al. 2007a,b; Ariya et al. 2002; Côté et al. 2008).
Clouds and wind are an effective transport mechanism for the dispersal of bacteria, virus particles, algae, protozoa, lichens, and fungi including those dwelling in soil and water and which may be transported by air thousands of miles across land and oceans (Prospero et al. 2005; Polymenakou 2012) or lofted into the stratosphere (Griffin 2004; Imshenetsky et al. 1978) where they may continue to thrive.
3 Atmospheric transfer of biota from Earth
How often or how much of this atmospheric biota is lofted into space, is unknown. However, under extreme conditions, and if solar winds strike with sufficient force, then water molecules, surface dust (Schroder and Smith 2008), along with air-borne bacteria, spores, fungi, lichens, algae, and other microbes may be ejected into space (Joseph and Schild 2010), where, as demonstrated experimentally, this biota may easily survive (Novikova et al. 2016; Setlow 2006; Horneck et al. 1994, 2002; Nicholson et al. 2000; Onofri et al. 2012; McLean and McLean 2010; Kawaguchi et al. 2013).
For example, between September 22–25, 1998, NASA’s Ultraviolet Imager aboard the Polar spacecraft, detected and measured a series of coronal mass ejections and a powerful solar wind which created a shock wave that struck the magnetosphere with sufficient force to cause helium, hydrogen, oxygen, and other gases to gush from Earth’s upper atmosphere into space (Moore and Horwitz 1998). When the CME struck on Sept. 24, the pressure jumped to 10 nanopascals, whereas normally the pressure is around 2 or 3 nanopascals. Thus it could be predicted that airborne microbes attached to dust particles were also swept from the stratosphere into space; and those survivors which were eventually deposited in the atmosphere and clouds of Venus may have begun to flourish and multiply.
As determined by Leinert and Moster (2007) interplanetary dust accumulates outside the orbit of Venus, and the dust ring is similar to that found along Earth’s orbit. Krasnopolsky and Krysko (1979) based on data from the Venera 9 and 10 orbiters, observed a dust layer at heights of 100–700 km above the surface of Venus, and which had a ring formation. According to the data provided by Carrillo-Sánchez et al. (2020) these interplanetary dust particles are mass contributors to the atmosphere of Venus.
The Ulysses space probe was equipped with dust detectors and reported 374 impacts, beginning within the orbit of Venus at 0.7 AU (Grün et al. 1992). At 2 AU Ulysses detected fluxes in the dust density “compatible with a population of interplanetary dust particles moving on low to moderately eccentric (\(e = 0.1\) to 0.5) and low inclination (\(i = 0~\mbox{deg}\) to 30 deg) orbits” thereby indicating at least two dust particle populations in different orbital trajectories (Grün et al. 1992). It is not likely that those dust particles, with a radii smaller than 0.3 mm, detected between and within the orbits of Venus and Mars, originated from the outer solar system beyond Mars (Altobelli et al. 2005; Landgraf et al. 2000). Competition between gravitation and radiation pressure determines how deep dust can penetrate such that the smallest are believed to be filtered by radiation pressure before entering the inner solar system (Altobelli et al. 2005). Therefore, the smallest dust particles following or ringing Venus (Leinert and Moster 2007; Krasnopolsky and Krysko 1979), most likely originated within the orbits of Mars, Earth and Venus.
As determined by Leinert and Moster (2007) these inner planetary dust particles range in size from 0.1 mm to 0.001 mm. Most bacteria range from 0.002 to 0.0002 mm in diameter (Evans 2016), whereas fungal spores (e.g. Penicillium, Cladosporium, Aspergillis) range from 0.016 to 0.0036 in diameter (Reponen et al. 2001). And microbes in Earth’s upper atmosphere are often attached to particles of dust (Möhler et al. 2007; Sattler et al. 2001) which may range in size from 0.077 to 0.002 mm (Arimoto et al. 1997; Beltzer et al. 1988; Maring et al. 2000; Tegen and Lacis 2012).
Galactic winds and radiation pressure can propel dust particles at speeds ranging from 300 and 3,000 km/sec (Chandrasekhar 1989; Heckman and Thompson 2017; Ishibashi and Fabian 2015; Murray et al. 2011; Wibking et al. 2018). According to the calculations of Nobel laureate (Arrhenius 1908, 1918) if microbes ranging in size from 0.002–0.0002 mm in diameter are attached to particles ranging from 0.02–0.002 mm in diameter, or if fungal spores ranging in size from 0.0036 to 0.0016 mm are attached to particles ranging from 0.036 to 0.016 mm in diameter, and “if we assume that the spore starts with zero velocity” and originate from Earth, then due to the sun’s gravity, galactic winds and radiation pressure, it would take “forty days” to travel from Earth to Venus and would then fall into the atmosphere of Venus with a velocity of approximately two kilometers per second.
As to organisms which may survive the descent to the surface of Venus—especially during the planet’s early history—they may have also remained viable and may have evolved over time (Cockell 1999; Schulze-Makuch et al. 2004). In this regard, and as reported here, there are mushroom-shaped specimens on the surface of Venus; the source of which may be the lower clouds (Limaye et al. 2018) which in turn may have been seeded from microbe-laden interplanetary dust (Arrhenius 1908, 1918; Joseph and Schild 2010), bolide particles originating on Earth (Cockell 1999; Gladman et al. 1996; Grinspoon and Bullock 2007; Melosh 1988; Schulze-Makuch et al. 2004) or contamination by space craft (Joseph 2018). However, due to the poor quality of the Venera 13 photos, it is unknown if these are in fact Venusian fungi or mushroom-shaped anomalies. One can only hypothesize and speculate.
In support of the spacecraft-Earth-to-Venus-transfer-of-life scenario is evidence that viable fungi and bacteria not only survived heat-treatment sterilization of spacecraft (La Duc et al. 2014; Venkateswaran et al. 2012; Puleo et al. 1977) but may have survived the journey from Earth to Mars, as successive photos taken months apart depict what appears to be masses of fungal-bacterial organisms growing on the NASA’s Mars rovers Curiosity and Opportunity (Joseph 2018; Joseph et al. 2019). There is also evidence (but no proof) that fungi, lichens, algae and microbes may have, at different times in that planet’s history, colonized the surface of Mars (Dass 2017; Joseph 2014, 2016; Joseph et al. 2019; Krupa 2017; Levin and Straat 2016; Noffke 2015; Rabb 2018; Rizzo and Cantasano 2009, 2016; Roffman 2019; Ruffi and Farmer 2016; Small 2015). These putative Martian organisms include mushroom- and lichen-shaped specimens similar to those reported here (Figs. 1–4). In fact, 15 Martian specimens resembling “puff balls” emerged from beneath the surface and dramatically increased in size over a period of three days (Joseph et al. 2019); observations which may indicate the rovers are stimulating the growth of these specimens, or they are the source. Hence, probes from Earth may have also transported innumerable microbes to Venus.
Similarities in morphology are not proof of life, and there is no definitive proof of life on Mars or Venus. Moreover, several scientists have taken issue with the findings of Joseph et al. (2019) and questioned if fungi and lichens could flourish on the Red Planet (Armstrong 2019a,b; Kidron 2019). On the other hand, simulation studies have shown that these and other species can survive in a Mars-like environment (De Vera et al. 2014, 2019; De la Torre Noetzel et al. 2017; Onofri et al. 2012, 2019; Pacelli et al. 2016; Sanchez et al. 2012; Schuerger et al. 2017; Selbman et al. 2015; Zakharova et al. 2014).
Life-simulation studies conducted with Venus-like environments also supports the possibility of life (Seckbach et al. 1970). Moreover, many scientists have agued that a variety of terrestrial life forms could survive on or below the surface and especially within the cloud layers (Cockell 1999; Grinspoon and Bullock 2007; Ksanfomality 2013; Limaye et al. 2018; Schulze-Makuch et al. 2004). Thus there is reason to suspect that biota already adapted to life in the clouds of Earth, and which survived a journey to Venus, may flourish and multiply.
4 Transfer of life by spacecraft
Several space craft and balloons have passed through the atmosphere and crashed or landed on Venus (Marov et al. 1998). Since all attempts to completely sterilize space craft have failed (La Duc et al. 2014; Venkateswaran et al. 2012; Puleo et al. 1977), these vehicles were likely transporting billions of microorganisms to Venus—beginning with the Venera 3 Soviet probe on March 1, 1966, followed by the Venera 5 and 6 in May of 1969 and Venera 7 (12/15/70), Venera 8 (7/22/1972), Venera 9 (10/22/1975), and two Pioneer Space craft in 1978, as well as probes from the European Space Agency and Japan (Marov et al. 1998).
As based on published studies on survival following heat treatment sterilization of spacecraft and equipment, at a minimum, billions of organisms, or their spores, per spacecraft, were likely transported to Venus (as well as Mars). For example, immediately after sterilization, 350 to 500 distinct colonies (on average) consisting of millions of organisms, including fungi, were found per square meter on the outer surfaces of the Viking Landers, rovers, and other spacecraft (La Duc et al. 2014; Venkateswaran et al. 2012; Puleo et al. 1977). As to species which were not or could not be cultured, and those masses of fungi and bacteria growing within the equipment’s interior, the number of survivors is unknown. Many of the survivors of these failed attempts at sterilization, including fungi, can also survive long duration exposure to space (Novikova et al. 2016; Setlow 2006; Horneck et al. 1994, 2002; Nicholson et al. 2000; Onofri et al. 2012; McLean and McLean 2010; Kawaguchi et al. 2013) well in excess of the four months it takes a space craft to reach Venus (Marov et al. 1998). Moreover, after clean-room sterilization, and prior to launch, these crafts and much of this equipment was then exposed to the biota-laden external environment.
Following the explosion of the space shuttle Columbia, during its reentry from space, and subsequent crash in 2003, viable organisms, including the nematode, Caenorhabditis elegans (Szewczyk et al. 2005) and Microbispora sp (McLean et al. 2006) survived. Likewise, it can be predicted that spaceships crashing on Venus (or Mars) would still be laden with viable microorganisms.
5 Bolide ejection and spores: transfer of life from Earth to Venus
Microbe-laden debris ejected into space following terrestrial impact by asteroids, comets, and meteors, may have repeatedly transferred life from Earth to Venus, Mars and the moons of Saturn and Jupiter. Likewise, life-bearing debris from other worlds, in the form of dust, meteors, asteroids or comets, including those originating from outside this solar system, may have delivered and may continue to transport viable microorganisms to Earth and other planets and their moons (Arrhenius 1908; Joseph and Schild 2010; Wickramasinghe et al. 2018). In addition to meteors, ejecta in the form of comets may be playing a significant role in the wider context of the interstellar transport of life (Hoyle and Wickramasinghe 1978; Joseph and Wickramasinghe 2010; Wickramasinghe et al. 2018).
Many species of microbe have evolved the ability to survive a violent hypervelocity impact, shock pressures of 100 GPa, and extreme acceleration and ejection into space including the vacuum and frigid temperatures of an interstellar environment; the cosmic rays, gamma rays, UV rays, and ionizing radiation they would encounter; and the descent through the atmosphere and the crash landing onto the surface of a planet (Burchell et al. 2004; Horneck et al. 2001a, 2001b; Horneck et al. 1994; Mastrapaa et al. 2001; Nicholson et al. 2000, 2012; Mitchell and Ellis 1971).
It is reasonable to assume that Venus and Mars, beginning in the early asteroid-comet-meteor bombardment phase 3.8 bya (Cockell 1999; Gladman et al. 1996) may have been repeatedly and continually seeded with life from Earth, attached to dust, ejecta, and more recently, via space craft. In the early history of Mars, Earth and Venus, these planets may have repeatedly exchanged life—the ultimate source of which is as yet unknown.
When a meteor, asteroid, or comet, strikes this planet, the surface area of ejecta may be heated to temperatures in excess of 100 C upon impact (Artemieva and Ivanov 2004; Fritz et al. 2005), which is well within the tolerance range of thermophiles (Baross and Deming 1983; Kato and Qureshi 1999; Stetter 2006). Moreover, because the ejecta-surface is acting as a heat shield, the interior may never be heated above 100 ∘C (Burchell et al. 2004; Horneck et al. 2002). Spores can survive post shock temperatures of over 250 ∘C (Horneck et al. 2002). Thus, innumerable microbes may remain viable despite violent impact-induced ejection into space.
Meteors at least ten kilometers across will also punch a hole in the atmosphere before striking the planet (Van Den Bergh 1989) and will eject tons of dust, rocks, and boulders (Beech et al. 2018; Gladman et al. 1996; Melosh 2003) through that hole into space (Van Den Bergh 1989), along with any adhering microbes, fungi, algae, and lichens (Gladman et al. 1996; Melosh 2003; Mileikowsky et al. 2000a,b) before air can rush back in to completely fill the gap (Van Den Bergh 1989). Thus, following the initial impact, ejected debris and adhering organisms would not be subject to extreme heating as they pass through the atmosphere. There are currently 200 known terrestrial impact craters (Earth Impact Database 2019), and this planet may have been struck thousands of times (Melosh 1989), resulting in the ejection of millions of rocks, boulders and tons of debris into space (Beech et al. 2018; Gladman et al. 1996; Melosh 1989, 2003; Van Den Bergh 1989). Many scientists have argued that some of this debris and any adhering microbes likely landed on Mars and Venus (Beech et al. 2018; Gladman et al. 1996; Melosh 2003; Davies 2007; Fajardo-Cavazosa et al. 2007; Hara et al. 2010; Schulze-Makuch et al. 2005). Given that microbes can survive the shock of a violent impact and hyper velocity launch ejecting them into space (Mastrapaa et al. 2001; Burchell et al. 2001, 2004; Nicholson et al. 2000, 2012; Hazael et al. 2017; Horneck et al. 2008), as well as the descent to the surface of a planet (Burchell et al. 2001; Horneck et al. 2002; McLean and McLean 2010), the interplanetary transfer of viable microorganisms, via bolides, within our Solar System, is overwhelmingly likely (Mileikowsky et al. 2000a,b; Beech et al. 2018), beginning, possibly, soon after life appeared on Earth over 3.8 bya. Over the course of the last 550 million years there have been a total of 97 major impacts, leaving craters at least 5 kilometers across (Earth Impact Database 2019), and it’s been estimated that in consequence, approximately “1013 kg of potentially life-bearing matter has been ejected from Earth’s surface into the inner solar system” (Beech et al. 2018) during this time frame. Consider, for example, the Chicxulub crater, formed approximately 66 Mya, which has a 150 km diameter (Alvarez et al. 1980). It’s been estimated, given an 25 km/s impactor velocity, that up to \(5.5 \times 10^{12}~\mbox{kg}\) of debris may have been ejected into space (Beech et al. 2018), along with unknown volumes of water, and perhaps millions of trillions of organisms buried within this ejecta. According to calculations by Beech et al. (2018), given an impact velocity greater than 23 km/s, this microbial-laden ejecta could have entered the orbits of and intercepted Venus, Mars and other planets and moons within a few weeks or months.
Studies have demonstrated that bolide ejecta provides nutrients that can sustain trillions of microorganisms, including algae and fungi, perhaps for thousands of years Mautner (1997, 2002). However, ejecta may orbit in space for millions of years before impacting another planet (Gladman et al. 1996; Melosh 2003). Microbes and fungi, however, are well adapted to survive life in space for even tens of millions of years; accomplished via the formation of spores. Cano and Borucki (1995) have reported that spores, embedded in amber, may remain viable for 25- to 40-million-years, whereas Vreeland et al. (2000) have reanimated 250 million-year-old halotolerant bacterium from a primary salt crystal.
Specifically in the absence of water, nutrients, or under extreme life-neutralizing conditions, microbes and fungi may instantly react by forming a highly mineralized enclosure consisting of heat or cold shock proteins which wrap around their DNA and which alters the chemical and enzymatic reactivity of its genome making it nearly impermeable to harm (Setlow and Setlow 1995; Marquis and Shin 2006; Sunde et al. 2009). “In the dormant stage a spore has no metabolism and resists cycles of extreme heat and cold, extreme desiccation including vacuum, UV and ionizing radiation, oxidizing agents and corrosive chemicals” (Nicholson et al. 2000). Space experiments and the Long Duration Exposure Facility Mission have shown that bacteria and fungal spores can easily survive the vacuum of space and constant exposure to solar, UV, and cosmic radiation with just minimal protection (Horneck 1993; Horneck et al. 1995; Mitchell and Ellis 1971). Moreover, survival rates increase significantly from 30% to 70% if coated with dust, or embedded in salt or sugar crystals (Horneck et al. 1994). Spores buried beneath the surface of ejecta could easily survive in space for millions of years. Although the full spectrum of UV rays are deadly against spores, a direct hit is unlikely, even if unprotected while traveling through space. A few meters of surface material provides a significant degree of protection for those buried deep inside (Horneck et al. 2002). Although high-energy radiation or particles which strike a meteor from Earth may create secondary radiation, studies have shown that as the thickness of surrounding material increases beyond 30 cm, the dose rate and lethal effects of heavy ions, including secondary radiation, depreciates significantly (Horneck et al. 2002).
It’s been estimated that because microbes and spores are so small that even when bombarded with photons and deadly gamma and UV radiation, they can drift in space for up to a million years before being struck (Horneck et al. 2002). If shielded by 2 meters of meteorite, even after 25 million years in space, a substantial number of spores would survive (Horneck et al. 2002). B. subtilis spores can even survive a direct hit (Horneck et al. 2002).
Further, many species form colonies with those in the outer layers creating a protective crust, which blocks out radiation and protects those in the inner layers from the hazards of space (Nicholson et al. 2000). Therefore, even if dwelling just beneath the surface of ejecta, colonies of living microbes provide their own protection.
Of the 60,556 meteorites so far documented, 227 originated on Mars, and 360 are from the Moon (Meteoritical Bulletin Database 2019). Meteors from Venus have not yet been identified. However, given the estimated 1000 impact craters detected by the Magellan spacecraft (Schaber et al. 1992) clearly Venus has been repeatedly impacted by meteors which survived descent through the atmosphere without vaporization.
It can be predicted, depending on size, that the surface of ejecta from Earth, would be subject to extremely high temperatures for only a few seconds upon striking the atmosphere of Venus. And if the bolide fragments or explodes, it could be predicted that it may shed spores into the Venusian cloud layers. A variety of organisms remain viable even following atmospheric explosions (Szewczyk et al. 2005), and despite reentry speeds of up 9700 km h−1 (McLean et al. 2006), and high velocity impact onto a planet’s surface (Burchell et al. 2001; Horneck et al. 2002; McLean and McLean 2010). Therefore, microbial-infested bolides from Earth could have repeatedly contaminated Venus throughout its history. And those survivors which could adapt, would likely go forth, multiply, and then evolve.
6 Can life survive on Venus? Terrestrial analogs
It has been argued that a variety of terrestrial organisms could tolerate the high temperatures and dwell in the atmosphere of Venus (Clarke et al. 2013; Cockell 1999; Grinspoon and Bullock 2007; Konesky 2009). These include thermophilic photothrophs (Arrhenius 1918; Cockell 1999), algae (Seckbach and Libby 1970) and acidophilic microbes (Schulze-Makuch et al. 2004). Sagan and Morowtz (1967) have hypothesized that even complex multi-cellular organisms swim between the thick layers of clouds.
Venus, at ground level has a temperature of 465 ∘C (Avduevsky et al. 1971), with a surface atmospheric pressure of 9.5 MPa (Bougher et al. 1997; Donahue and Russell 1997). Although there are trace amount of H2O and water vapors, the atmosphere is 96.5% CO2, 3.5% nitrogen, and subject to a high sulfur cycle due to active volcanism (Donahue and Hodges 1992; Barstow et al. 2012).
Several terrestrial species, such as algae and Cyanidium caldarium can survive environments which are 100% CO2 (Seckbach and Libby 1970; Seckbach et al. 1970). Chlorella will continue to grow at 0.6 atm and can survive CO2 of 1 atm, (Pirt and Pirt 1980). The biological activity of some species of fungi and archaea is inhibited by the presence of oxygen and increases with increased levels of carbon dioxide (Lenhart et al. 2012). Therefore, the high levels of CO2 in the atmosphere of Venus would not preclude colonization by a variety of microorganisms and fungi.
Numerous species of prokaryotes and eukaryotes easily survive pressures of 9.5 MPa at a depth of 950 m beneath the sea, including elephant seals and sperm whales; whereas microbes, such as obligate barophiles and shewanella and Moritella (Deming et al. 1992; Kato 1999; Kato et al. 1998a,b, 2010; Robb et al. 2007) flourish within the Mariana trench at a depth of 10,898 m (108 Mpa).
Terrestrial microbes and communities of hyperthermophiles have also been discovered up to 3.5 km below ground (Biddle et al. 2008; Chivian et al. 2008; Doerfert 2009; Moser et al. 2005; Gohn et al. 2008). Baccilus infernus, have been discovering thriving at depths of 2700 meters where the weight and pressure is 300 x that of the surface (Boone et al. 1995). Genomic analysis of the bacterium, Desulforudis audaxviator—discovered 2.8 km beneath the surface—indicates this species metabolizes minerals in place of sunlight and “is capable of an independent life-style well suited to long-term isolation from the photosphere deep within Earth’s crust” (Chivian et al. 2008). Species similar to these may also thrive at great depths or just beneath the surface of Venus, despite the high temperatures.
Some species of hyperthermophiles continue to grow at a temperature of 122 ∘C (Robb et al. 2007), and have been discovered thriving adjacent to 400 ∘C thermal vents (Stetter 2006)–the most resilient of which include Pyrolobus fumarii (Baross and Deming 1983) and Methanopyrus kandleri (Kurr et al. 1991). However, there are no known terrestrial species which can survive direct exposure to temperatures above 300 ∘C (Kato and Qureshi 1999; Kato and Takai 2000).
Based on data from Earth, unless any hypothetical Venusian organisms have evolved heat-related adaptive features enabling them to survive surface temperatures of 465 ∘C, then the most likely habitats are the clouds or beneath the ground where subsurface temperatures may be significantly cooler—as is the case on Earth (Davis and Schubert 1981; Smerdon et al. 2004; Al-Temeemi and Harris 2001).
7 Venusian water vapors and habitability
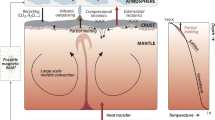
In addition to atmospheric water vapors (Donahue and Hodges 1992; Barstow et al. 2012)—which could sustain life dwelling in the clouds of Venus—the atmosphere contains high levels of deuterium with a deuterium-to-hydrogen ratio which is approximately 150 times that of terrestrial water (Donahue and Russell 1997). The D/H ratio indicates that Venus at one time had rivers and shallow oceans (Donahue et al. 1997), thereby making early Venus habitable (Way et al. 2016; Way and Del Genio 2019). What became of this water is unknown.
The D/H ratio leaves open the possibility there are tremendous reservoirs of water buried deep beneath the surface, and which, like terrestrial underground water under high temperature, arid surface conditions (Al-Sanad and Ismael 1992), may seep upward only to evaporate. Hence, subsurface water evaporation may account for the trace amounts of cloud-water and contribute to the D/H ratio.
Therefore, Venus may have had lakes and oceans for billions of years—making it more conducive to life (Kasting 1988; Colin and Kasting 1992; Donahue and Russell 1997; Way et al. 2016; Way and Del Genio 2019)—which then may have slowly evaporated over the following years, the remainder possibly remaining deep beneath the soil. Hence, early in its history Venus could have become home to Earthly archae, bacteria, and fungi which may have evolved, or migrated to the clouds or beneath the soil. As summed up by Cockell (1999), the possible “existence of hot surface oceans on Venus may have favored the successful evolution or transfer of early life.”
On the surface of Venus there is no evidence of water vapor. Given that sea water will completely evaporate at 420 ∘C it is not likely there is any surface water. The only source of moisture would be deep beneath the surface or within the clouds. Any species which colonized the surface of Venus early in its history, and in order to survive under present day conditions, would have had to evolve as temperatures rose and surface water was lost (Schulze-Makuch et al. 2004); or they would have had to migrate to the clouds or beneath the surface.
However, even present day organisms transferred from Earth may remain viable. Cockell (1999) has argued: “Neither the pressure (9.5 MPa) nor the high carbon dioxide concentrations (97%) represent a critical constraint to the evolution of life on the surface or in the atmosphere. The most significant constraints to life on the surface are the lack of liquid water and the temperature (464 ∘C). In the lower and middle cloud layers of Venus, temperatures drop and water availability increases, generating a more biologically favorable environment. However, acidity and the problem of osmoregulation in hygroscopic sulfuric acid clouds become extreme and probably life-limiting. If it is assumed that these constraints can be overcome, considerations on the survival of acidophilic sulfate-reducing chemoautotrophs suspended as aerosols in such an environment show that Venus does come close to possessing a habitable niche.”
In support of Cockell’s (1999) argument is evidence indicating that microbes may have colonized the cloud layers of Venus (Limaye et al. 2018), and the observations, presented in this report, of mushroom-shaped surface features, the latter of which are similar to those observed on Mars (Dass 2017; Joseph 2016; Joseph et al. 2019). Moreover, Grinspoon (1993, 1997) and Grinspoon and Bullock (2007), have argued that bright radar surface signatures may be evidence that living organisms have colonized portions of the Venusian surface.
8 Life in the clouds of Venus
In 1967, Sagan and Morowtz (1967) presented a scenario where multi-cellular organisms, up to 4 cm in diameter may dwell in the upper Venusian atmosphere and metabolize and generate hydrogen as propellants and a means of floatation. At this writing, the only evidence remotely suggestive of multi-cellular organisms have been photographed at ground level (Ksanfomality 2013 and as reported here).
Venus has three cloud layers which circle the planet at an altitude of 48–68 km s and which are composed of H2SO4 aerosols, with an acid concentration ranging from 98% in the lower atmosphere to 81% in the upper atmosphere (Bougher et al. 1997; Donahue and Russell 1997). The clouds of Venus also contain high levels of deuterium and trace amounts of water (Donahue and Hodges 1992; Barstow et al. 2012) which could sustain life.
According to Limaye et al. (2018): “The lower cloud layer of Venus (47.5–50.5 km)” provides “favorable conditions for microbial life, including moderate temperatures and pressures (∼60 ∘C and 1 atm), and the presence of micron-sized sulfuric acid aerosols,” which could sustain sulfate-reducing microbes.
Konesky (2009) argues in favor of the life-cloud hypothesis, and states “there exists a habitable region in the atmosphere, centered at approximately 50 km, where the temperature ranges from 30 to 80 ∘C and the pressure is one bar.” He’s suggested that organisms similar to plankton may dwell in the upper atmosphere. Likewise, Schulze-Makuch et al. (2004) hypothesized that Venusian clouds, 48 to 65 km above the surface, could harbor aeroplankton (which metabolize sulphur), and acidophilic microbes who employ sulfur allotropes as photo-protective pigments which enable them to engage in photosynthesis.
The upper cloud layers of Venus are subject to wind speeds of up to 355 km/h (100 meters a second) and then increase to more than 700 km/h in the lower layers (Blamont et al. 1986). Although increasing turbulence can have an inhibitory influence on the growth and metabolism of a variety of species, the opposite is true of phytopklanton and diatoms. Turbulence can increase photosynthetic activity (Gibson 2000; Gibson and Thomas 1995; Thomas et al. 1995), thus supporting the plankton hypotheses of Konesky (2009) and Schulze-Makuch et al. (2004).
9 Radiation and UV absorption
Schulze-Makuch et al. (2004) have argued “that the cloud deck of the Venusian atmosphere may provide a plausible refuge for microbial life.... Here we make the argument that such an organism may utilize sulfur allotropes present in the Venusian atmosphere, particularly \(\text{S}_{8}\), as a UV sunscreen, as an energy-converting pigment, or as a means for converting UV light to lower frequencies that can be used for photosynthesis. Thus, life could exist today in the clouds of Venus.”
Fungi, lichens, and prokaryotes can survive long-term exposure to gamma, and solar UV radiation and remain viable (Horneck et al. 2002; McLean and McLean 2010; Nicholson et al. 2000; Novikova et al. 2016; Onofri et al. 2012; Satoh et al. 2011; Tugay et al. 2006; Sancho et al. 2007; Raggio et al. 2011). A variety of terrestrial species thrive in and are attracted to highly radioactive environments (Beckett et al. 2008; Dadachova et al. 2007; Tugay et al. 2006; Wember and Zhdanova 2001), including fungi, lichens and numerous species of microbe. UV rays would have no detrimental effects on these species.
Vesper et al. (2008) and Novikova et al. (2016), Novikova (2009) reported that fungi are invigorated and grow rapidly within the International Space Station as a consequence of the heightened radiation levels. Fungi also flourish on the outskirts and along the walls of the highly radioactive Chernobyl nuclear power plant (Dighton et al. 2008; Zhdanova et al. 2004). Fungi (Wember and Zhdanova 2001; Zhdanova et al. 2004) and radiation-tolerant bacteria (Moseley and Mattingly 1971; Ito et al. 1983) will seek out and grow towards sources of radiation which serve as an energy source for metabolism (Dighton et al. 2008; Tugay et al. 2006). These organisms can easily repair radiation-damaged DNA due to a redundancy of genes with repair functions (White et al. 1999).
Ground and low cloud level radiation may not be nearly as intense as first believed as an unknown UV absorber has been detected in the cloud layers (Krasnopolsky 2017; Markiewicz et al. 2014; Rossi et al. 2015); thus making Venus more hospitable to life (Limaye et al. 2018). Although it’s possible that sulfur dioxide and iron chloride are acting as UV absorbers, the albedo, between 330 and 500 nm, and the refraction index between 1.07 and 1.7 (Laven 2008), and the spatial and temporal changes in contrasts (Markiewicz et al. 2014; Rossi et al. 2015), may be evidence that microbial populations, attached to particles in the lower cloud layers of Venus (Limaye et al. 2018), are waxing and waning in biomass.
Specifically, as based on observations from spacecraft and terrestrial telescopes, it is likely that the clouds of Venus’ have a high density of micron-sized particles (Hansen and Hovenier 1974; Knollenberg and Hunten 1980). Although a variety of absorbing mechanisms have been proposed (reviewed by Limaye et al. 2018), if these particles originated in space and are from Earth, of if they are comprised of sulfuric acid or other substances elevated from the surface, is unknown (Pérez-Hoyos et al. 2017). Regardless of their source or composition, it is not likely that these particles, alone, are acting as UV absorbers since their numbers are insufficient and incompatible with Venera 14 observations (Krasnopolsky 2017; Limaye et al. 2018). The refraction indices also exceed the values expected from sulfuric acid cloud particles except in the presence of FeCl3 (Markiewicz et al. 2014), the latter of which may be contributing to cloud condensation nuclei.
Grinspoon (1993, 1997) proposed that the “unknown ultraviolet absorber” may be a photosynthetic pigment and that “terrestrial” photosynthetic, acid-resistant organisms are metabolizing and engaging in phototrophic and chemotrophic activities involving sulfur and iron within the Venusian clouds. Grinspoon and Bullock (2007), have also argued that irregularly shaped cloud particles may be living organisms. Another possibility is that living organisms are attached to and feeding on these particles.
To adequately account for the amount of UV rays absorbed, the biota dwelling in the clouds of Venus would have to be massive in numbers. According to the calculations of Bullock (2018): “If microbes grow to represent one tenth of the aerosol mass, the total biomass in Venus’ clouds would be 3.6 billion tonnes, or about the biomass of the Earth’s oceans.”
Certainly a biomass of this magnitude would absorb a significant amount of UV rays; and this position, and Bullock’s calculations, are supported by Limaye et al. (2018) who have argued: “for Venus’ lower clouds, the mass loading estimates (∼0.1–100 mg m−3) are comparable to the upper biomass value for terrestrial biological aerosols (∼44 mg m−3), while the particle size regime (≤8 μm) opens the possibility that the clouds may similarly harbor suspensions of single cells or aggregated microbial communities. In theory, the 2- and 8-μm-sized particles (modes 2′ and 3) could harbor a maximum of ∼108 and 1010 cells.”
The possibility of iron- and sulfur-centered metabolism in these clouds, coupled with particle and biomass density as estimated by Bullock (2018) and Limaye et al. (2018), may account for the spectral refraction anomalies observed in polarized and unpolarized light. UV rays are being absorbed and metabolized by Venusian organisms.
If the cloud layers of Venus harbor biology, then we would expect they would exhibit spectral signatures similar to terrestrial clouds. As pointed out by Limaye et al. (2018) data from the Galileo, Pioneer, Messenger and Akatsuki space probes “are tantalizingly similar to the absorption properties of terrestrial biological molecules.”
10 Speculations: Venusian atmospheric organisms
Limaye et al. (2018) also propose that microorganisms similar to A. ferrooxidans may dwell in the clouds of Venus as “this bacterium thrives at extremely low pH values (pH 1 to 2), fixes both CO2 and nitrogen gas from the atmosphere (Valdes et al. 2008), and obtains its energy for growth from the oxidation of hydrogen, ferrous iron, elemental sulfur, or partially oxidized sulfur compounds.” Other candidates include chemolithoautotrophic and acidophilic \(\gamma \)-proteobacterium, both of which thrive under low pH and anaerobic conditions and at temperatures of 50–60 ∘C, and oxidize elemental sulfur and using \(\text{Fe}_{3}^{+}\) as a terminal electron acceptor. Likewise, green sulfur bacteria and archaeal Stygiolobus oxidize elemental sulfur to yield sulfuric acid under acidic and anaerobic conditions.
Therefore, if the analyses of Bullock (2018) and Limaye et al. (2018) are correct, then species similar to Stygiolobus, green sulfur bacteria, sulfate-reducing bacteria, and A. ferrooxidans, and a biomass equal to that found in the oceans of Earth, may be thriving within the clouds of Venus. Bullock (2018) and Limaye and colleagues (2018) also point out that cloud-based microbial populations would have to be replenished on relatively fast timescales. According to the calculations of Bullock (2018): “The residence time of mode 3 (\(r=3.6~\upmu \mbox{m}\)) aerosols in Venus’ lower cloud is about 2 days. The rate equation shows that if the microbial lifetime is shorter than this, a steady state population can exist within the aerosols.” That is, as microbes reproduce they replace those which fall to the surface and can transfer between aerosol, otherwise they must be replenished from the surface or from space.
Because of the very strong downdrafts and updrafts (e.g. Blamont et al. 1986), the powerful winds of Venus may be continually transferring and recycling biomass to and from the surface to the atmosphere, and from the upper cloud layers to the lower layers. Conversely, if via galactic winds microbes and microbe-laden dust are expelled from the upper atmosphere of Earth (Joseph and Schild 2010) into the path of Venus, they would continually contaminate the upper atmosphere and descend from the upper to lower cloud layers where they may flourish, reproduce, and perhaps evolve, only to be eventually deposited on the surface. Conversely, those dwelling on or just beneath the surface would be lofted back into the Venusian atmosphere via strong updrafts. Life would continually be recycled from clouds to the surface and from the surface (or subsurface) back to the clouds, and replenished by organisms from Earth.
11 Life beneath the subsurface
Venera 13 landed in the Beta-Phoebe region at median elevations in the upland rolling plains province, an area described as a “stony desert” with lose soil, compacted sand, pebbles, and rocks similar to terrestrial tholeiitic basalts which make up much of Earth’s ocean crust (Surkov et al. 1983). The Venusian basalts were found to contain high levels of potassium and covered with soil and soil patches (Florensky et al. 1983; Surkov et al. 1983). Garvin et al. (1984) likened the area to the bedrock at Snake River Plain, in Idaho, USA, and noted that many large and small rocks were partly buried beneath the sandy soil.
On Earth, endolithic microorganisms flourish in hyper-arid rocky deserts and extreme environmental conditions by colonizing the interior or the undersides of rocks including those just beneath the surface (Weirzchos 2012; Pointing and Belnap 2012); conditions which allow photosynthesis, and within which water molecules may be trapped. However, in the absence of water these organisms are able to “reversibly activate metabolism” until moisture becomes available (Harel et al. 2004). Generally, these hot desert micro-habitats are dominated by fungi, lichens, cyanobacteria, mosses and heterotrophic bacteria (Pointing and Belnap 2012).
The surface temperature of Venus, as determined by Venera 7, was 747 K/473.85 ∘C for the first 50 seconds after landing and to have a constant temperature of 739 K ∘/465.85 ∘C (Avduesvesky et al. 1971). Basalt has high thermal insulating properties (Eppelbaum et al. 2014; Mostafa et al. 2004) such that temperatures beneath these rocks would be much cooler. Temperatures beneath the Venusian soil are unknown.
In hot and arid climates, temperatures 1 m to 10 m beneath the surface are significantly cooler than surface temperatures (Davis and Schubert 1981; Smerdon et al. 2004; Al-Temeemi and Harris 2001). At these shallow depths, and due to these more temperate subsurface vs higher surface temperatures, microorganisms are able to engage in considerable metabolic activity (Blume et al. 2002; Buchanan and King 1992; Kaiser and Heinemeyer 1993). Investigators have found a considerable biomass of microorganisms and fungi up to 15 cm beneath the subsurface (Dobbins et al. 1992; Fierer et al. 2012; Crocker et al. 2000; Krumholz 2000) even in arid high temperature environments (Weirzchos 2012; Pointing and Belnap 2012; Steven et al. 2013; Mueller et al. 2015).
Because of its density, soil is an excellent modulator of heat such that soil temperatures decrease at a rate exponential to soil depth until reaching 10 m; below which thermal conductivity is fairly uniform (Davis and Schubert 1981; Smerdon et al. 2004; Al-Temeemi and Harris 2001). In high temperature environments, heat transfer reduction from the surface to the subsurface can be as much as 57% (Al-Temeemi and Harris 2001); i.e. 43% of surface temperature.
Kuwait is located in a dry desert region and is characterized by high temperature extremes and intense solar radiation with a mean air temperature ranging from 37 ∘C to 45 ∘C during the summer months of June through August (Al-Temeemi and Harris 2001). There is little precipitation and soil water content is 2% (Al-Sanad and Ismael 1992; Al-Temeemi and Harris 2001). However, it is much cooler beneath the surface and temperatures steadily decrease with depth. For example, Al-Temeemi and Harris (2001) established that if the average surface air temperature is 41 ∘C, the subsurface temperature, at a depth of 1 m, reaches a maximum of 36 ∘C, whereas at a depth of 10 m the subsurface temperature is a constant 27 ∘C; i.e. a reduction of 12.2% and 34.2% respectively.
Al-Temeemi and Harris (2001), also determined that when compared to atmospheric temperatures the comparative decline is even more pronounced due to subterranean cooling. They concluded there are “significant temperature drops below-ground when air temperatures are at their highest.”
These findings support the likelihood that subsurface temperatures on Venus are much cooler than the surface and may be habitable for a variety of microorganisms. Hypothetically these may include photoautotrophic, lithobiontic, poikilohydric, and fungal hyperthermophiles which have evolved and adapted to the Venusian environment. Specifically, by applying to Venus the 12.2% and 34.2% reductions in subsurface temperatures as determined by Al-Temeemi and Harris (2001) then, at a depth of 1 m temperatures will have fallen by 56.6 ∘C yielding a temperature of 407.4 ∘C, followed by a reduction of 158.7 ∘C and thus a subsurface temperature of 305.3 ∘C at 10 m. However, given the reduction value of 57% (Al-Temeemi and Harris 2001), temperatures 10 m beneath the surface might fall to 200 ∘C—well within the limit for the hardiest hyper-thermophiles on Earth (Kato and Takei 2000). However, although some species may survive at this temperature, there would likely be little or no biological activity—according to terrestrial standards. On the other hand, because basalt has high thermal insulating properties (Eppelbaum et al. 2014; Mostafa et al. 2004), temperatures beneath these rocks could be much cooler.
The hyper-arid, waterless surface of Venus may also draw moisture and water up from the subterranean depths, just as occurs on Earth and the deserts of Kuwait (Al-Sanad and Ismael 1992). If so, then any organisms living 10 m below ground may be continually supplied with water as it rises to the surface and completely evaporates.
12 Speculations: fungi and mushroom-shaped specimens on the subsurface of Venus?
Specimens resembling fungi have been observed on the surface of Venus as reported here (Figs. 1, 2, 3, 4, 5). Unfortunately, because of the poor quality of the Venera 13 photos, positive identification is impossible, and one can only speculate and propose hypotheticals based on morphological similarities to terrestrial mushrooms.
Specifically, upon examining panoramic color images from the 1982 Soviet Venera 13 mission—reprocessed by NASA—five well defined specimens with caps and stalks which resemble the classic terrestrial mushroom were observed (Figs. 1, 4). These specimens protrude approximately 2 to 3 cm from the surface, and the caps are approximately 3 to 4 cm in diameter. Several smaller, less defined specimens with a cap and what may be stalks appear to the far left of center and which are bordered by additional specimens which form a crescent of mushroom-shapes (Figs. 2, 5).
Examination of the original black and white Venera 13 photos–which provides a 180 degree view—reveals a third well defined specimen at the bottom left (three in total, Fig. 4). Clusters of additional poorly defined specimens are observed to the upper left (Figs. 2, 5).
Therefore, five well defined specimens and numerous smaller surface structures which resemble classic terrestrial mushrooms have been observed on the surface of Venus. There is no known geological or weathering process on Earth which can sculpt mushroom-like shapes from rocks. However, it must be stressed that similarities in morphology are not proof of life.
These Venusian specimens may have been uncovered from beneath the surface when Venera 13 landed and blew away top soil, rocks, and dust surrounding the landing site. Garvin (1981) reports that due to surface winds and turbulence, clouds of dust were recorded by the Venera crafts for up to 30 seconds after impact and which persisted for up to 7 minutes.
It is unknown if these are mushroom-shaped rocks, fossils, or living organisms that had evolved and had been growing beneath the surface where temperatures would be cooler and which may provide access to any underground moisture drawn toward the surface. One can only speculate and propose hypotheticals. Based on terrestrial standards, if alive, they would have been obliterated almost instantly upon exposure to surface conditions. However, the number and density of these specimens also increase with increasing distance from the lander (Figs. 2, 4), suggesting those closer in proximity were destroyed by the sudden turbulence of the craft’s landing. This leaves three explanations: they are mushroom-shaped rocks, they are fossilized organisms, or they have evolved into hyper-extremophiles perfectly adapted to the Venusian surface/subsurface environment.
It must be emphasized this author does not claim to have discovered fossilized or current life on Venus but is speculating and proposing hypotheticals. However, several of these specimens are similar to what may be fungal-lichen organisms growing on Mars (see Joseph et al. 2019) as well as on Earth, which supports the interplanetary transfer hypothesis. Hypothetically, if the specimens observed on Venus are fossils, or organisms which evolved and adapted, this supports the possibility that fungi, in general, may be hyper-extremophiles, capable of colonizing Mars, Venus, and the harshest of alien environments.
The author’s observations and interpretation of these mushroom-shaped specimens are supported by the findings of Ksanfomality (2013). Based on examination of NASA-enhanced panoramic images from the 1982 Soviet Venera 13 mission, Ksanfomality (2013) observed what he believed to be two fungal shaped objects at a distance of 15 to 20 cm from the buffer of the landing module. Ksanfomality (2013) has estimated these specimens to be elevated 3 cm above the surface and to have a diameter of approximately 8 cm. These do not have mushroom-shapes and are to the far (90 ∘) right of the specimens observed by this author and cannot be observed in Figs. 1–5.
13 Photosynthesis, water, hydrocarbons, anaerobic respiration
It is unknown if the fungal and mushroom-shaped specimens reported here are fossils, living organism, or mushroom-shaped rocks which may have been uncovered when the Venera-13 landed on the surface. One can only speculate. If fossils, then they may be relics from a time when Venus had oceans, rivers, and a less extreme environment.
It has been suggested that Venus may have had liquid water on the surface and provided a habitable environment for billions of years (Grinspoon and Bullock 2007; Way et al. 2016; Way and Del Genio 2019). Way and Del Genio (2019), based on the results from four simulations, determined that Venus “may have had enough condensable water on its surface for a shallow ocean…” and “would have been able to maintain stable temperatures—from a low of 20 ∘C (68 ∘F) to a high of 50 ∘C (122 ∘F)—for about three billion years” i.e. until about 750 mya. The finding of Donahue and colleagues (Donahue et al. 1982; Donahue and Hodges 1992)—based on comparisons of atmospheric deuterium/hydrogen ratios with Earth—support those contentions.
On Earth, in this general time frame, a variety of prokaryotes and eukaryotes had evolved. Between 600 to 800 mya, this biota included acritarchs, protozoa, ediacarans, amoebozoans, cercozoans, plankton, algae, coccoid and filamentous cyanobacteria, and fungi (Butterfield 2005a,b; Bottjer et al. 2006; Narbonne 2005; Narbonne and Gehling 2003; Shen et al. 2008). Hypothetically, if Venus was also habitable and wet at this time, then similar species may have also evolved. If this view is correct, then as surface water evaporated and temperatures rose, survivors may have adapted, evolved, migrated beneath the surface, and/or via the powerful winds of Venus (Blamont et al. 1986), were lofted to the clouds (Cockell 1999; Schulze-Makuch et al. 2004; Grinspoon and Bullock 2007; Limaye et al. 2018).
It is unknown if there is water beneath the Venusian surface. Budisa and Schulze-Makuch (2014) have hypothesized that in the absence of water and high concentration of carbon dioxide, that life on Venus may be sustained by “supercritical fluids” which “have different properties compared to regular fluids and could play a role as life-sustaining solvents on other worlds... One location where CO2 should occur in the supercritical state is in the near-subsurface of Venus.”
Although the amount of light reaching the surface is only approximately 70% of that of Earth (within 0.5–9 klx), Venusian organisms, dwelling in the clouds, or beneath the surface, could derive energy from photosynthesis. Some terrestrial prokaryotes, as a solvent, utilize hydrogen sulfide, H2S as the electron donor, whereas some autotrophic prokaryotes living beneath the surface engage in chemosynthesis instead of photosynthesis (Durvasula and Rao 2018; Gerday and Glansdorff 2007).
The atmosphere of Venus is 96.5% CO2, 3.5% nitrogen, and due to active volcanism, subject to a high sulfur cycle (Donahue and Hodges 1992; Barstow et al. 2012). Seckbach and Libby (1970) in an experiment designed to mimic the Venusian environment, grew algae in pure CO2, at elevated temperature and in an acidic medium, and reported these specimens continued to thrive.
A variety of anaerobic species survive and flourish in anoxic, high CO2, environments (Kelly et al. 2001; Holliger et al. 1998; Emerson et al. 2016) such as Shewanella—a gram-negative, proteobacteria (Fredrickson et al. 2008; Hunt et al. 2010). In the absence of oxygen, anaerobes utilize substances such as sulphate (\(\text{SO} _{4}^{2-}\)), nitrate (NO3), or sulphur (S), as terminal electron acceptors to convert energy into adenosine triphosphate (ATP) which is conserved and utilized when performing energy requiring processes (Grein et al. 2013; Kelly et al. 2001; Richter et al. 2012; Holliger et al. 1998). Some eukaryotic species which normally respire oxygen (e.g. sub-arctic dwarf shrubs), can survive high levels of CO2, under hypoxic conditions, with no indication of damage (Preece and Phoenix 2013).
The metabolic activity of some eukaryotic fungi and anaerobic archaea is inhibited by oxygen and amplified with increased levels of CO2 (Lenhart et al. 2012). Given the high sulfur cycle, a variety of sulfate reducing anaerobic organisms (e.g. from the sulfur molybdoenzyme family) could adapt to the Venusian environment as they utilize sulfur compounds for sulfur metabolism to obtain energy (Grein et al. 2013).
So far, hydrocarbons have not been detected in the atmosphere (Plummer 1969); and without this evidence any possibility of life on Venus must be viewed with caution.
On Earth, fungi and microorganisms dwelling in clouds, and on the surface/subsurface, have a major role in organic compound degradation (Amato et al. 2007a,b; Ariya et al. 2002; Côté et al. 2008); and they play the same role in regard to hydrocarbons (Aydin et al. 2017; Heider et al. 1998). It is possible that the lack of evidence for Venusian hydrocarbons is due to rapid mineralization and consumption, serving as an energy source for Venusian organisms; similar to what occurs on Earth. A variety of microorganisms, including ferric iron-reducing, sulfate-reducing, and anaerobic hydrocarbon degrading bacteria (Heider et al. 1998) as well as anaerobic fungi (Aydin et al. 2017), catabolize hydrocarbon compounds. These species might be well adapted for life beneath the surface or within the clouds of Venus.
14 Conclusions
Venus may have been contaminated with Earthly life early in its history, and that seeding with life may have continued into the present via microbial-laden debris, dust, and spacecraft. Venus may have had oceans and rivers until as recently as 750 mya, and, hypothetically, species which colonized the planet may have adapted and evolved when those oceans evaporated and temperatures rose. However, Venus may still provide a habitable environment for a variety of hyperextremophiles including fungi which might be continually arriving from Earth.
Change history
01 October 2020
Editor’s Note: This article has now been retracted https://doi.org/10.1007/s10509-020-03860-z
23 June 2020
Editor's Note: Readers are alerted that the conclusions of this article are subject to criticisms that are being considered by the editors. Editorial action will be taken as appropriate once the investigation of the concerns is complete and all parties have been given an opportunity to respond in full.
01 October 2020
This article has been retracted. Please see the retraction notice for more detail: https://doi.org/10.1007/s10509-019-3678-x
References
Adhikari, A., Reponen, T., Grinshpun, S.A., Martuzevicius, D., LeMasters, G.: Correlation of ambient inhalable bioaerosols with particulate matter and ozone: a two-year study. Environ. Pollut. 140, 16–28 (2006)
Al-Sanad, H., Ismael, N.F.: Thermal properties of desert sands in Kuwait. J. Univ. Kuwait 19, 207–215 (1992)
Al-Temeemi, A.A., Harris, D.J.: The generation of subsurface temperature profiles for Kuwait. Energy Build. 33, 837–841 (2001)
Altobelli, N., Kempf, S., Landgraf, M., Srama, R., Dikarev, V., Kru¨ger, H., Moragas-Klostermeyer, G., Grün, E.: Cassini between Venus and Earth: detection of interstellar dust. J. Geophys. Res. 108(A10), 8032 (2003). https://doi.org/10.1029/2003JA009874
Altobelli, N., Kempf, S., Kru¨ger, H., Landgraf, M., Roy, M., Gru¨n, E.: Interstellar dust flux measurements by the Galileo dust instrument between the orbits of Venus and Mars. J. Geophys. Res. 110, A07102 (2005). https://doi.org/10.1029/2004JA010772. 2005
Alvarez, L.W., Alvarez, W., Asaro, F., Michel, H.V.: Extraterritorial cause for the Cretaceous-Tertiary extinction. Science 208, 1095–1108 (1980)
Amato, P., Demeer, F., Melaouhi, A., Fontanella, S., Martin-Biesse, A.-S., Sancelme, M., Laj, P., Delort, A.-M.: A fate for organic acids, formaldehyde and methanol in cloud water: their biotransformation by microorganisms. Atmos. Chem. Phys. 7, 4159–4169 (2007a)
Amato, P., Parazols, M., Sancelme, M., Mailhot, G., Laj, P., Delort, A.-M.: An important oceanic source of microorganisms for cloud water at the Puy de Do^me (France). Atmos. Environ. 41, 8253–8263 (2007b)
Arimoto, B., Ray, B.J., Lewis, N.F., Tomza, U., Duce, R.A.: Mass-particle size distributions of atmospheric dust and the dry deposition of dust to the remote ocean. Atmospheres 102, 15867–15874 (1997)
Ariya, P.A., Nepotchatykh, O., Ignatova, O., Amyot, M.: Microbiological degradation of atmospheric organic compounds. Geophys. Res. Lett. 29, 2077–2080 (2002)
Armstrong, R.A.: Could Lichens survive on Mars? J. Astrobiol. Space Sci. Rev. 1, 235–241 (2019a). 2019
Armstrong, R.A.: The lichen symbiosis: lichen “extremophiles” and survival on Mars. J. Astrobiol. Space Sci. Rev. 1, 378–397 (2019b)
Arrhenius, S.: Worlds in the Making. Harper and Brothers, New York (1908)
Arrhenius, S.: The Destinies of the Stars. Putnam, New York (1918)
Artemieva, N., Ivanov, B.: Launch of Martian meteroties in oblique impacts. Icarus 171, 183 (2004)
Avduevsky, V.S., Marov, M.Y., Rozhdestvensky, M.K., Borodin, N.F., Kerzhanovich, V.V.: Soft landing of Venera 7 on the Venus surface and preliminary results of investigations of the Venus atmosphere. J. Atmos. Sci. 28, 263–269 (1971)
Aydin, S., Karaçay, H.A., Shahia, A., Gökçe, S., Ince, B., Incea, O.: Aerobic and anaerobic fungal metabolism and omics insights for increasing polycyclic aromatic hydrocarbons biodegradation. Fungal Biol. Rev. 31, 61–72 (2017)
Baross, J.A., Deming, J.W.: Growth of black smoke bacteria at temperature at least 250 Celsius. Nature 303, 423–426 (1983)
Barstow, J.K., Tsang, C.C.C., Wilson, C.F., Irwin, P.G.J., Taylor, F.W., McGouldrick, K., Drossart, P., Piccioni, G., Tellmann, S.: Models of the global cloud structure on Venus derived from Venus express observations. Icarus 217, 542–560 (2012)
Beckett, R., Kranner, I., Minibayeva, F.: Stress physiology and the symbiosis. In: Nash, T. III (ed.) Lichen Biology, pp. 134–151. Cambridge University Press, Cambridge (2008). https://doi.org/10.1017/CBO9780511790478.009
Beech, M., Comte, M., Coulson, I.: Lithopanspermia—the terrestrial input during the past 550 million years. Am. J. Astrophys. Astron. 7(1), 81–90 (2018)
Beltzer, P.R., Carder, K.L., Duce, R.A., Merrill, J.T., Tindale, N.W.: Long-range transport of giant mineral aerosol particles. Nature 336, 568–571 (1988)
Biddle, J.F., Fitz-Gibbon, S., Schuster, S.C., Brenchley, J.E., House, C.H.: Metagenomic signatures of the Peru Margin subseafloor biosphere show a genetically distinct environment. Proc. Natl. Acad. Sci. USA 105, 10583–10588 (2008)
Blamont, J.E., Young, R.E., Seiff, A., Ragent, B., Sagdeev, R., Linkin, V.M., Kerzhanovich, V.V., Ingersoll, A.P., Crisp, D., Elson, L.S., Preston, R.A., Golitsyn, G.S., Ivanov, V.N.: Implications of the VEGA balloon results for Venus atmospheric dynamics. Science 231, 1422–1425 (1986)
Blume, E., Bischoff, M., Reichert, J.M., Moorman, T., Konopkab, A., Turcod, R.F.: Surface and subsurface microbial biomass, community structure and metabolic activity as a function of soil depth and season. Appl. Soil Ecol. 20(3), 171–181 (2002).
Boone, D.R., Liu, Y., Zhao, Z.J., Balkwill, D.L., Drake, G.R., Stevens, T.O., Aldrich, H.C.: Bacillus infernus sp. nov., an Fe(III)- and Mn(IV)-reducing anaerobe from the deep terrestrial subsurface. Int. J. Syst. Bacteriol. 45(3), 441–448 (1995)
Bottjer, D.J., Clapham, M.E., Xiao, S., Kaufman, A.J.: Evolutionary paleoecology of ediacaran benthic marine animals. In: Xiao, S., Kaufman, A.J. (eds.) Neoproterozoic Geobiology and Paleobiology, pp. 91–114. Springer, Dordrecht (2006)
Bougher, S.W., Hunten, D.M., Phillips, R.J.: Venus II: Geology, Geophysics, Atmosphere, and Solar Wind Environment. University of Arizona Press, Tucson (1997)
Brodie, E.L., DeSantis, T.Z., Parker, J.P.M., Zubietta, I.X., Piceno, Y.M., Andersen, G.L.: Urban aerosols harbor diverse and dynamic bacterial populations. Proc. Natl. Acad. Sci. USA 104, 299–304 (2007)
Buchanan, M., King, L.D.: Seasonal fluctuations in soil microbial biomass carbon, phosphorus, and activity in no-till and reduced-chemical-input maize agroecosystems. Biol. Fertil. Soils 13, 211–217 (1992)
Budisa, N., Schulze-Makuch, D.: Supercritical carbon dioxide and its potential as a life-sustaining solvent in a planetary environment. Life 4, 331–340 (2014)
Bullock, M.: The Total Possible Biomass of Venus’ Clouds, American Astronomical Society, DPS Meeting #50 (2018), id.102-04
Burchell, M.J., Mann, J., Bunch, A.W., Brandob, P.F.B.: Survivability of bacteria in hypervelocity impact. Icarus 154, 545–547 (2001)
Burchell, M.J., Mann, J., Bunch, A.W.: Survival of bacteria and spores under extreme shock pressures. Mon. Not. R. Astron. Soc. 352, 1273–1278 (2004)
Butterfield, N.J.: Probable proterozoic fungi. Paleobiology 31, 165–182 (2005a)
Butterfield, N.J.: Reconstructing a complex early neoproterozoic eukaryote, Wynniatt formation Arctic Canada. Lethaia 38, 155–169 (2005b)
Cano, R.J., Borucki, M.K.: Revival and identification of bacterial spores in 25– to 40-million-year-old Dominican amber. Science 268, 1060–1064 (1995)
Carrillo-Sánchez, J.D., Gómez-Martín, J.C., Bones, D.L., Nesvorný, D., Pokornýde, P., Bennae, M., Flynng, G.J., Planea, J.M.C.: Cosmic dust fluxes in the atmospheres of Earth, Mars, and Venus. Icarus 335, 33–43 (2020)
Chandrasekhar, S.: Radiative Transfer and Negative Ion of Hydrogen, vol. 2. University of Chicago Press, Chicago (1989)
Chivian, D., Brodie, E.L., Alma, E.J., et al.: Environmental genomics reveals a single-species ecosystem deep within Earth. Science 322, 275–278 (2008)
Clarke, A., Morris, G.J., Fonseca, F., Murray, B.J., Acton, E., Price, H.C.: A low temperature limit for life on Earth. PLoS ONE 8, e66207 (2013)
Cockell, C.S.: Life on venus. Planet. Space Sci. 47, 1487–1501 (1999)
Colin, J., Kasting, J.F.: Venus. A search for clues to early biological possibilities. In: Exobiology in the Solar System. NASA Technical Publication 512 (1992)
Côté, V., Kos, G., Mortazavi, R., Ariya, P.A.: Microbial and “de novo” transformation of dicarboxylic acids by three airborne fungi. Sci. Total Environ. 390, 530–537 (2008)
Crocker, F.H., Fredrickson, J.K., White, D.C., Ringelberg, D.B., Balkwill, D.L.: Phylogenetic and physiological diversity of Athrobacter strains isolated from unconsolidated subsurface sediments. Microbiology 146, 1295–1310 (2000)
Dadachova, E., Bryan, R.A., Huang, X., Moadel, T., Schweitzer, A.D., Aisen, P., et al.: Ionizing radiation changes the electronic properties of melanin. PLoS ONE (2007). https://doi.org/10.1371/journal.pone.0000457
Dass, R.S.: The high probability of life on mars: a brief review of the evidence. Cosmology 27, 62–73 (2017)
Davies, P.C.W., Gregoy, R., Bock, J.A. (Eds.) The Transfer of Viable Microorganisms Between Planets. Novartis Foundation Symposia, (2007)
Davis, A.J., Schubert, R.P.: Alternative Natural Energy Sources in Building Design. Van Nostrand Reinhold, New York (1981)
De la Torre Noetzel, R., et al.: Survival of lichens on the ISS-II: ultrastructural and morphological changes of Circinaria gyrosa after space and Mars-like conditions. In: EANA2017: 17th European Astrobiology Conference, 14-17 August, 2017, Aarhus, Denmark, pp. 14–17 (2017)
De Vera, J.-P., et al.: Results on the survival of cryptobiotic cyanobacteria samples after exposure to Mars-like environmental conditions. Int. J. Astrobiol. 13, 35–44 (2014)
De Vera, J.-P., Alawi, M., Backhaus, T., et al.: Limits of life and the habitability of Mars: The ESA space experiment BIOMEX on the ISS. Astrobiology 18, 145–157 (2019)
Dehel, T., Lorge, F., Dickinson, M.: Uplift of microorganisms by electric fields above thunderstorms. J. Electrost. 66, 463–466 (2008)
Deleon-Rodriguez, N., Lathem, T.L., Rodriguez, R.L., Barazesh, J.M., Anderson, B.E., Beyersdorf, A.J., Ziemba, L.D., Bergin, M., Nenes, A., Konstantinidis, K.T.: Microbiome of the upper troposphere: species composition and prevalence, effects of tropical storms, and atmospheric implications. Proc. Natl. Acad. Sci. USA 110, 2575–2580 (2013)
Deming, J.W., Somers, L.K., Straube, W.L., Swartz, D.G., Colin., J.: Venus. A search for clues to early biological possibilities. Exobiology in the Solar System, NASA Technical Publication 512 (1992)
Diehl, R.H.: The airspace is habitat. Trends Ecol. Evol. 28, 377–379 (2013)
Dighton, J., Tugay, T., Zhdanova, N.: Fungi and ionizing radiation from radionuclides. FEMS Microbiol. Lett. 281, 109–120 (2008)
Dobbins, D.C., Aelion, C.M., Pfaender, F.K.: Subsurface, terrestrial microbial ecology and biodegradation of organic chemicals: a review. Crit. Rev. Environ. Control 22, 67–136 (1992)
Doerfert, S.N.: Methanolobus zinderi sp. nov., a methylotrophic methanogen isolated from a deep subsurface coal seam. Int. J. Syst. Evol. Microbiol. 59, 1064–1069 (2009)
Donahue, T.M., Hodges, R.R. Jr.: Past and present water budget of Venus. J. Geophys. Res. 97, 6083–6091 (1992)
Donahue, T.M., Russell, C.T.: The Venus atmosphere and ionosphere and their interaction with the solar wind; an overview. In: Bougher, S.W. (ed.) Venus II. Geology, pp. 3–31. University of Arizona Press, Tucson (1997)
Donahue, T.M., Hoffman, J.H., Hodges, R.R., Watson, A.J.: Venus was wet—a measurement of the ratio of deuterium to hydrogen. Science 216, 630–633 (1982)
Durvasula, R.V., Rao, D.V.S.: Extremophiles: From Biology to Biotechnology. CRC Press, Boca Raton (2018). Earth Impact Database 2019. http://www.passc.net/EarthImpactDatabase
Earth Impact Database: http://www.passc.net/EarthImpactDatabase (2019)
Emerson, J.B., Thomas Walter Alvarez, B.C., Banfield, J.F.: Metagenomic analysis of a high carbon dioxide subsurface microbial community populated by chemolithoautotrophs and bacteria and archaea from candidate phyla. Environ. Microbiol. 18, 1686–1703 (2016)
Eppelbaum, L., Kutasov, I., Pilchin, A.: Thermal properties of rocks and density of fluids. In: Applied Geothermics. Lecture Notes in Earth System Sciences Springer, Berlin (2014)
Evans, H.: Bacteria: Microbiology and Molecular Genetics. Syrawood, New York (2016)
Fajardo-Cavazosa, P., Schuerger, A.C., Nicholson, W.L.: Testing interplanetary transfer of bacteria between Earth and Mars as a result of natural impact phenomena and human spaceflight activities. Acta Astronaut. 60, 534–540 (2007)
Fierer, N., Leff, J.W., Adams, B.J., Nielsen, U.N., Bates, S.T., Lauber, C.L., et al.: Cross-biome metagenomic analyses of soil microbial communities and their functional attributes. Proc. Natl. Acad. Sci. USA 109, 21390–21395 (2012). https://doi.org/10.1073/pnas.1215210110
Florensky, C.P., Basilevsky, A.T., Kryuchov, V.P., Kusmin, R.O., Nikolaeva, O.V., Pronin, A.A., Chernaya, I.M., Tyuflin, Y.S., Selivanov, A.S., Naraeva, M.K., Ronca, L.B.: Venera 13 and 14: sedimentary rocks on Venus? Science 221, 57 (1983)
Fredrickson, J., Romine, M.F., Beliaev, A.S., et al.: Towards environmental systems biology of Shewanella. Nat. Rev. Microbiol. 6, 592–603 (2008)
Fritz, J., Artemieva, N.A., Greshake, A.: Ejection of Martian meteorites. Meteorit. Planet. Sci. 40, 1393 (2005)
Fröhlich-Nowoisky, J., Pickersgill, D.A., Després, V.R., Pöschl, U.: High diversity of fungi in air particulate matter. Proc. Natl. Acad. Sci. USA 106, 12814–12819 (2009)
Gao, P., Zhang, X., Crisp, D., Bardeen, C.G., Yung, Y.L.: Bimodal distribution of sulfuric acid aerosols in the upper haze of Venus. Icarus 231, 83–98 (2014)
Garvin, J.B.: Landing induced dust clouds on Venus and Mars. In: Proceedings of the Lunar and Planetary Society, 12B, pp. 1493–1505 (1981)
Garvin, J.B., Head, J.W., Zuber, M.T., Helfenstein, P.: Venus: the nature of the surface from Venera panoramas. J. Geophys. Res. 89, 3381–3399 (1984)
Gerday, C., Glansdorff, N.: Physiology and Biochemistry of Extremophiles. ASM Press, Washington (2007)
Gibson, C.H.: Laboratory and ocean studies of phytoplankton response to fossil turbulence. Dyn. Atmos. Ocean. 31, 295–306 (2000)
Gibson, C.H., Thomas, W.H.: Effects of turbulence intermittency on growth inhibition of a red tide dinoflagellate, Gonyaulax polyedra Stein. J. Geophys. Res. 100, 24841–24846 (1995)
Gladman, B.J., Burns, J.A., Duncan, M., Lee, P.C., Levison, H.F.: The exchange of impact ejecta between terrestrial planets. Science 271, 1387–1392 (1996)
Gohn, G.S., et al.: Deep drilling into the Chesapeake Bay impact structure. Science 320, 1740–1745 (2008)
Grein, F., Sofia, A.R.A., VenceslauInês, S., Pereira, A.C.: Unifying concepts in anaerobic respiration: insights from dissimilatory sulfur metabolism. Biochim. Biophys. Acta, Bioenerg. 187, 145–160 (2013)
Griffin, D.W.: Terrestrial microorganisms at an altitude of 20,000 m in Earth’s atmosphere. Aerobiologia 2004(20), 135–140 (2004)
Griffin, D.W., Kubilay, N., Kocak, M., Gray, M.A., Borden, T.C., Shinn, E.A.: Airborne desert dust and aeromicrobiology over the Turkish Mediterranean coastline. Atmos. Environ. 41, 4050–4062 (2007)
Grinspoon, D.H.: Probing Venus’s cloud structure with Galileo NIMS. Planet Space Sci. 41, 515–542 (1993)
Grinspoon, D.H.: Venus Revealed: A New Look Below the Clouds of Our Mysterious Twin Planet. Addison Wesley, Reading, MA (1997), 355 pp.
Grinspoon, D.H., Bullock, M.A.: Astrobiology and Venus exploration. In: Esposito, L.W., Stafan, E.R., Cravens, T.E. (eds.) Exploring Venus as a Terrestrial Planet, pp. 191–206. Am. Geophys. Union, Washington (2007)
Grün, E., Baguhl, M., Fechtig, H., Hanner, M.S., Kissel, J., Lindblad, B.-A., Linkert, D., Linkert, G., Mann, I., McDonnell, J.A.M., Morfill, G.E., Polanskey, C., Riemann, R., Schwehm, G., Siddique, N., Zook, H.A.: Galileo and Ulysses dust measurements: Fz Venus to Jupiter. Geophys. Res. Lett. 19, 1311–1314 (1992)
Hansen, J.E., Hovenier, J.W.: Interpretation of the polarization of Venus. J. Atmos. Sci. 31, 1137–1160 (1974)
Hara, T., Takagi, K.M., Kajiura, D.: Transfer of life-bearing meteorites from Earth to other planets. J. Cosmol. 7, 1731–1742 (2010)
Harel, Y., Ohad, I., Kaplan, A.: Activation of photosynthesis and resistance to photo inhibition in cyanobacteria within biological desert crust. Plant Physiol. 136, 3070–3079 (2004)
Hazael, R., Fitzmaurice, B.C., Fogilia, F., Appleby-Thomas, G., McMilan, P.F.: Bacterial survival following shock compression in the GigaPascal range. Icarus 293, 1–7 (2017)
Heckman, T.M., Thompson, T.A.: Galactic winds and the role played by massive stars. In: Handbook of Supernovae, pp. 2431–2454. Springer, Cham (2017)
Heider, J., Spormann, A.M., Beller, H.R., Widdel, F.: Anaerobic bacterial metabolism of hydrocarbons. FEMS Microbiol. Rev. 22, 459–473 (1998)
Holliger, C., Wohlfarth, G., Diekert, G.: Reductive dechlorination in the energy metabolism of anaerobic bacteria. FEMS Microbiol. Rev. 22(5), 383 (1998) (PDF)
Horneck, G.: Responses of Bacillus subtilis spores to space environment: results from experiments in space. Orig. Life Evol. Biosph. 23, 37–52 (1993)
Horneck, G., Bücker, H., Reitz, G.: Long-term survival of bacterial spores in space. Adv. Space Res. 14, 41–45 (1994)
Horneck, G., Eschweiler, U., Reitz, G., Wehner, J., Willimek, R., Strauch, G.: Biological responses to space: results of the experiment exobiological unit of ERA on EURECA I. Adv. Space Res. 16, 105–118 (1995)
Horneck, G., et al.: Bacterial spores survive simulated meteorite impact. Icarus 149, 285 (2001a)
Horneck, G., Rettberg, P., Reitz, G., Wehner, J., Eschweiler, U., Strauch, K., Panitz, C., Starke, V., Baumstark-Khan, C.: Orig. Life Evol. Biosph. 31, 527–547 (2001b).
Horneck, G., Mileikowsky, C., Melosh, H.J., Wilson, J.W., Cucinotta, F.A., Gladman, B.: Viable transfer of microorganisms in the solar system and beyond. In: Horneck, G., Baumstark-Khan, C. (eds.) Astrobiology. Springer, Berlin (2002)
Horneck, G., Stoffler, D., Ott, S., et al.: Microbial rock inhabitants survive hypervelocity impacts on Mars-like host planets: first phase of lithopanspermia experimentally tested. Astrobiology 8, 17–44 (2008)
Hoyle, F., Wickramasinghe, N.C.: Lifecloud: the Origin of Life in the Galaxy. Dent, London (1978)
Hunt, K.A., Flynn, J.M., Naranjo, B., Shikhare, I.D., Gralnick, J.A.: In: Substrate-Level Phosphorylation Is the Primary Source of Energy Conservation During Anaerobic Respiration of Shewanella Oneidensis Strain MR-1, vol. 192, pp. 3345–3351 (2010)
Imshenetsky, A.A., Lysenko, S.V., Kazakov, G.A.: Upper boundary of the biosphere. Appl. Environ. Microbiol. 35, 1–5 (1978)
Ishibashi, W., Fabian, A.C.: AGN feedback: galactic-scale outflows driven by radiation pressure on dust. Mon. Not. R. Astron. Soc. 451, 93–102 (2015)
Ito, H., Watanabe, H., Takeshia, M., Iizuka, H.: Isolation and identification of radiation-resistant cocci belonging to the genus deinococcus from sewage sludges and animal feeds. Agric. Biol. Chem. 47, 1239–1247 (1983). https://doi.org/10.1271/bbb1961.47.1239
Jones, S.E., Newton, R.J., McMahon, K.D.: Potential for atmospheric deposition of bacteria to influence bacterioplankton communities. FEMS Microbiol. Ecol. 64, 388–394 (2008)
Jones, M.H., Bewsher, D., Brown, D.S.: Imaging of a circumsolar dust ring near the orbit of Venus. Science 342, 960–963 (2013)
Joseph, R.: Life on Mars: lichens, fungi, algae. Cosmology 22, 40–62 (2014)
Joseph, R.: A high probability of life on Mars, the consensus of 70 experts. Cosmology 25, 1–25 (2016)
Joseph, R.: Sterilization failure and fungal contamination of Mars and NASA’s Mars rovers. J. Cosmol. 30, 51–97 (2018). 2018
Joseph, R., Schild, R.: Biological cosmology. J. Cosmol. 10, 40–75 (2010)
Joseph, R., Wickramasinghe, N.C.: Comets and contagion: evolution and diseases from space? J. Cosmol. 7, 1750–1770 (2010)
Joseph, R.G., Dass, R.S., Rizzo, V., Cantasano, N., Bianciardi, G.: Evidence of life on Mars? J. Astrobiol. Space Sci. Rev. 1, 40–81 (2019)
Kaiser, E.A., Heinemeyer, O.: Seasonal variations of soil microbial biomass carbon within the plough layer. Soil Biol. Biochem. 25, 1649–1655 (1993)
Kasting, J.F.: Runaway and moist greenhouse atmospheres and the evolution of Earth and Venus. Icarus 74, 195–212 (1988)
Kato, C.: Molecular analyses of the sediment and isolation of extreme barophiles from the deepest Mariana trench. In: Horikoshi, K., Tsujii, K. (eds.) Extremophiles in Deep-Sea Environments. Springer, Tokyo (1999)
Kato, C., Qureshi, M.H.: Pressure response in deep-sea piezophilic bacteria. J. Mol. Microbiol. Biotechnol. 1(1), 87–92 (1999)
Kato, C., Takai, K.: Microbial diversity of deep-sea extremophiles–piezophiles, hyperthermophiles, and subsurface microorganisms. Biol. Sci. Space 2000, 341–352 (2000)
Kato, C., Li, L., Nakamura, Y., Nogi, Y., Tamaoka, J., Horikoshi, K.: Extremely barophilic bacteria isolated from the Mariana trench, challenger deep, at a depth of 11,000 meters. Appl. Environ. Microbiol. 64, 1510–1513 (1998a)
Kato, C., Li, L., Nogi, Y., Nakamura, Y., Tamoako, H., Horiskoshi, K.: Extremely barophilic bateria isolated from Mariana trench, challenger deep, at a depth of 11,000 meters. Appl. Enviorn. Microbiol. 64, 1510–1513 (1998b)
Kato, S., Takano, Y., Kakegawa, T., Inoue, H.O.K., Kobayashi, C., Utsumi, M., Marumo, K., Kobayashi, K., Ito, Y., Ishibashi, J., Yamagishi, A.: Biogeography and biodiversity in sulfide structures of active and inactive vents at deep-sea hydrothermal fields of the Southern Mariana trough. Appl. Environ. Microbiol. 76, 2968–2979 (2010)
Kawaguchi, Y., Yang, Y., Kawashiri, N., et al.: Orig. Life Evol. Biosph. 43, 411 (2013). https://doi.org/10.1007/s11084-013-9346-1
Kelly, D.J., Poole, R.K., Hughes, P.N.J.: Microaerobic physiology: aerobic respiration, anaerobic respiration, and carbon dioxide metabolism. In: Mobley, H.L.T., Mendz, G.L., Hazell, S.L. (eds.) Helicobacter Pylori: Physiology and Genetics. ASM Press, Washington (2001)
Kidron, G.J.: Cyanobacteria and lichens may not survive on Mars. The negev desert analogue. J. Astrobiol. Space Sci. Rev. 1, 369–377 (2019). 2019
Knollenberg, R.G., Hunten, D.M.: The microphysics of the clouds of Venus—results of the Pioneer Venus particle size spectrometer experiment. J. Geophys. Res. 85, 8039–8058 (1980)
Konesky, G.: Can Venus shed microorganisms? In: Proc SPIE, vol. 7441 (2009). https://doi.org/10.1117/12.828643
Krasnopolsky, V.A.: On the iron chloride aerosol in the clouds of Venus. Icarus 286, 134–137 (2017)
Krasnopolsky, V.A., Krysko, A.A.: Venera 9, 10: Is there a dust ring around Venus? Planet. Space Sci. 27, 951–957 (1979)
Krumholz, L.R.: Microbial communities in the deep subsurface. Hydrogeol. J. 8, 4–10 (2000)
Krupa, T. A.: Flowing water with a photosynthetic life form in Gusav Crater on Mars. Lunar and Planetary Society, XLVIII (2017)
Ksanfomality, L.W.: An object of assumed Venusian flora. Dokl. Phys. 58(5), 204–206 (2013)
Kurr, M., Huber, R., Konig, H., Jannasch, H.W., et al.: Methanopyrus kandleri, gen. and sp. Nov. represents a novel group of hyperthermophilic methanogens, growing at 110 ∘C. Arch. Microbiol. 156(4), 239–247 (1991). https://doi.org/10.1007/BF00262992
La Duc, M.T., Venkateswaran, K., Conley, C.A.: A genetic inventory of spacecraft and associated surfaces. Astrobiology 2014(14), 15–23 (2014)
Landgraf, M., Baggaley, W.J., Grün, E., Krüger, H., Linkert, G.: Aspects of the mass distribution of interstellar dust grains in the solar system from in situ measurements. J. Geophys. Res. 105, 10,343–10,352 (2000)
Laven, P.: Effects of refractive index on glories. Appl. Opt. 47, H133 (2008)
Leinert, C., Moster, B.: Evidence for dust accumulation just outside the orbit of Venus. Astron. Astrophys. 472, 335–340 (2007)
Lenhart, K., et al.: Evidence for methane production by saprotrophic fungi. Nat. Commun. 2012(3), 1046 (2012). https://doi.org/10.1038/ncomms2049
Levin, G.V., Straat, P.A.: The case for extant life on mars and its possible detection by the Viking labeled release experiment. Astrobiology 16(10), 798–810 (2016)
Limaye, S.J., Mogul, R., Smith, D.J., Ansari, A.H., Słowik, G.P., Vaishampayan, P.: Venus’ spectral signatures and the potential for life in the clouds. Astrobiology 18, 1181–1198 (2018)
Maring, H., Savoie, D.L., Izaguirre, M.A., McCormick, C., Arimoto, R., Prospero, J.M., Pilinis, C.: Aerosol physical and optical properties and their relationship to aerosol composition in the free troposphere at Izaña, Tenerife, Canary Islands during July 1995. J. Geophys. Res. 105, 14,677–14,700 (2000)
Markiewicz, W.J., Petrova, E., Shalygina, O., Almeida, M., Titov, D.V., Limaye, S.S., Ignatiev, N., Roatsch, T., Matz, K.D.: Glory on Venus cloud tops and the unknown UV absorber. Icarus 234, 200–203 (2014)
Marov, M.H., Grinspoon, D.H., Owen, T., Levchenko, N., Mastaler, R.: The Planet Venus. Yale University Press, New Haven (1998)
Marquis, R.E., Shin, S.Y.: Mineralization and responses of bacterial spores to heat and oxidative agents FEMS. Microbiol. Rev. 14, 375–379 (2006)
Mastrapaa, R.M.E., Glanzbergb, H., Headc, J.N., Melosha, H.J., Nicholsonb, W.L.: Survival of bacteria exposed to extreme acceleration: implications for panspermia. Earth Planet. Sci. Lett. 189(30), 1–8 (2001)
Mautner, M.N.: Biological potential of extraterrestrial materials. 1. Nutrients in carbonaceous meteorites and effects on biological growth. Planet. Space Sci. 45, 653–664 (1997)
Mautner, M.N.: Planetary bioresources and astroecology. 1. Planetary microcosm bioassays of Martian and carbonaceous chondrite materials: nutrients, electrolyte solutions, and algal and plant responses. Icarus 158, 72–86 (2002)
McLean, R.J.C., McLean, M.A.C.: Microbial survival mechanisms and the interplanetary transfer of life through space. J. Cosmol. 7, 1802–1820 (2010)
McLean, R.J.C., Welsh, A.K., Casasanto, V.A.: Microbial survival in space shuttle crash. Icarus 181, 323–325 (2006)
Melosh, H.: The rocky road to panspermia. Nature 332, 687–688 (1988). https://doi.org/10.1038/332687a0
Melosh, H.J.: Impact Cratering—A Geological Process. Oxford University Press, Oxford (1989)
Melosh, H.J.: Exchange of meteorites (and life?) between stellar systems. Astrobiology 3, 207–215 (2003)
Meteoritical Bulletin Database: (2019)
Mileikowsky, C., Cucinotta, F.A., Wilson, J.W., Gladman, B., Horneck, G., Lindegren, L., Melosh, J., Rickman, H., Valtonen, M., Zheng, J.Q.: Natural transfer of viable microbes in space. Part 1: from Mars to Earth and Earth to Mars. Icarus 145, 391–427 (2000a)
Mileikowsky, C., Cucinotta, F.A., Wilson, J.W., Gladman, B., Horneck, G., Lindegren, L., Melosh, J., Rickman, H., Valtonen, M., Zheng, J.Q.: Risks threatening viable transfer of microbes between bodies in our solar system. Planet. Space Sci. 48, 1107–1115 (2000b)
Misconi, N.Y., Weinberg, J.L.: Is Venus concentrating interplanetary dust toward its orbital plane? Science 200, 1484–1485 (1978). https://doi.org/10.1126/science.200.4349.1484
Mitchell, F.J., Ellis, W.L.: Surveyor III: bacterium isolated from lunar retrieved TV camera. In: Levinson, A.A. (ed.) Proceedings of the Second Lunar Science Conference. MIT Press, Cambridge (1971)
Möhler, O., DeMott, P.J., Vali, G., Levin, Z.: Microbiology and atmospheric processes: the role of biological particles in cloud physics. Biogeosci. Discuss. 4, 2559–2591 (2007)
Moseley, B.E., Mattingly, A.: Repair of irradiated transforming deoxyribonucleic acid in wild type and a radiation- sensitive mutant of micrococcus radiodurans. J. Bacteriol. 105(3), 976–983 (1971). PMC 248526 Freely accessible. PMID 4929286
Moore, T.E., Horwitz, J.L.: Thirty Years of Ionospheric Outflow: Causes and Consequences. American Geophysical, Union, San Francisco (1998)
Moser, D.P., Gihring, T.M., Brockman, F.J., et al.: Desulfotomaculum and methanobacterium spp. Dominate a 4- to 5-kilometer-deep fault. Appl. Environ. Microbiol. 71, 8773–8783 (2005)
Mostafa, M., Afify, N., Gaber, A., Abu Zaid, E.: Investigation of thermal properties of some basalt samples in Egypt. J. Therm. Anal. Calorim. 75, 179–188 (2004). https://doi.org/10.1023/B:JTAN.000001734
Mueller, R.C., Belnap, J., Kuske, C.R.: Soil bacterial and fungal community responses to nitrogen addition are constrained by microhabitat in an arid shrubland. Front. Microbiol. 10, e0117026 (2015). https://doi.org/10.1371/journal.pone.0117026
Murray, N., Ménard, B., Thompson, T.A.: Radiation pressure from massive star clusters as a launching mechanism for super-galactic winds. Astrophys. J. 735, 66–71 (2011)
Narbonne, G.M.: The Ediacara Biota: Neoproterozoic origin of animals and their ecosystems. Annu. Rev. Earth Planet. Sci. 33, 421–442 (2005)
Narbonne, G.M., Gehling, J.G.: Life after snowball: the oldest complex Ediacaran fossils. Geology 31, 27–30 (2003)
Nicholson, W.L., Munakata, N., Horneck, G., Melosh, H.J., Setlow, P.: Resistance of bacillus endospores to extreme terrestrial and extraterrestrial environments. Microbiol. Mol. Biol. Rev. 64, 548–572 (2000)
Nicholson, W.L., et al.: Growth of carnobacterium spp. from permafrost under low pressure, temperature, and anoxic atmosphere has implications for Earth microbes on Mars. Proc. Natl. Acad. Sci. USA (2012). https://doi.org/10.1073/pnas.1209793110
Noffke, N.: Ancient sedimentary structures in the < 3.7b Ga gillespie lake member, Mars, that compare in macroscopic morphology, spatial associations, and temporal succession with terrestrial microbialites. Astrobiology 15(2), 1–24 (2015)
Novikova, N.: Mirobiological Research on Board the ISS, Planetary Protection. the Microbiological Factor of Space Flight. Institute for Biomedical Problems, Moscow (2009)
Novikova, N., et al.: Long-term spaceflight and microbiological safety issues. Space J. (2016). https://roomeu.com/article/long-term-spaceflight-and-microbiological-safety-issues
Onofri, S., de la Torre, J.-P., de Vera, E., et al.: Survival of rock-colonizing organisms after 1.5 years in outer space. Astrobiology 12, 508–516 (2012)
Onofri, S., Selbman, L., Pacelli, C., et al.: Survival, DNA and ultrastructural integrity of a cryptoendolithic Antarctic fungus on Mars and lunar rock analogues exposed outside the International Space Station. Astrobiology 19, 2 (2019). https://doi.org/10.1089/ast.2017.1728
Pacelli, C., Selbmann, L., Zucconi, L., De Vera, J.P.P., Rabbow, E., Horneck, G., de la Torre, R., Onofri, S.: BIOMEX experiment: ultrastructural alterations, molecular damage and survival of the fungus cryomyces antarcticus after the experiment verification tests. Orig. Life Evol. Biosph. 47(2), 187–202 (2016)
Pérez-Hoyos, S., Sánchez-Lavega, A., García-Muñoz, A., Irwin, P.G.J., Peralta, J., Holsclaw, G., McClintock, W.M., Sanz-Requena, J.F.: Venus upper clouds and the UV-absorber from MESSENGER/MASCS observations. J. Geophys. Res., Planets 123, 43 (2017)
Pirt, M.W., Pirt, S.J.: The influence of carbon dioxide and oxygen partial pressures on chlorella growth in photosynthetic stead-state cultures. J. Gen. Microbiol. 119, 321–326 (1980)
Plummer, W.T.: Venus clouds: test for hydrocarbons. Science 163, 1191–1192 (1969)
Pointing, S.B., Belnap, J.: Microbial colonization and controls in dryland systems. Nat. Rev., Microbiol. 10, 551–562 (2012)
Polymenakou, P.P.: Atmosphere: a source of pathogenic or beneficial microbes? Atmosphere 3(1), 87–102 (2012)
Preece, C., Phoenix, G.K.: Responses of sub-arctic dwarf shrubs to low oxygen and high carbon dioxide conditions. Environ. Exp. Bot. 85, 7–15 (2013)
Prospero, J.M., Blades, E., Mathison, G., Naidu, R.: Interhemispheric transport of viable fungi and bacteria from Africa to the Caribbean with soil dust. Aerobiologia 21, 1–19 (2005)
Puleo, J.R., Fields, N.D., Bergstrom, S.L., Oxborrow, G.S., Stabekis, P.D., Koukol, R.: Microbiological profiles of the Viking spacecraft. Appl. Environ. Microbiol. 33, 379–384 (1977)
Rabb, H.: Life on Mars, Astrobiology Society, SoCIA, vol. 14. University of Nevada, Reno (2018). 2018
Raggio, J., Pintado, A., Ascaso, C., De La Torre, R., De Los Ríos, A., Wierzchos, J., Horneck, G., Sancho, L.G.: Whole lichen thalli survive exposure to space conditions: results of lithopanspermia experiment with aspicilia fruticulosa. Astrobiology 2011 May(11), 281–292 (2011). https://doi.org/10.1089/ast.2010.0588. 4
Randel, W.J., Russell, J.M., Rochie, A., Waters, J.W.: Seasonal cycles and QBO variations in stratospheric CH4 and H2O observed in UARS HALOE data. J. Atmos. Sci. 55, 163–185 (1998)
Randel, W.J., Park, M., Emmons, L., Kinnison, D., Bernath, P., Walker, K.A., Boone, C., Pumphrey, H.: Asian monsoon transport of pollution to the stratosphere. Science 328, 611–613 (2010)
Reponen, T., Grinshpun, S.A., Conwell, K.L., Wiest, J., Anderson, M.: Aerodynamic versus physical size of spores: measurement and implication for respiratory deposition. Grana 40(3), 119–125 (2001)
Richter, K., Schicklberger, M., Gescher, J.: Dissimilatory reduction of extracellular electron acceptors in anaerobic respiration. Appl. Environ. Microbiol. 78(4), 913–921 (2012)
Rizzo, V., Cantasano, N.: Possible organosedimentary structures on Mars. Int. J. Astrobiol. 8(4), 267–280 (2009)
Rizzo, V., Cantasano, N.: Structural parallels between terrestrial microbialites and Martian sediments. Int. J. Astrobiol. (2016). https://doi.org/10.1017/S1473550416000355
Robb, F., Antranikian, G., Grogan, D., Driessen, A.: Thermophiles: Biology and Technology at High Temperatures. CRC Press, Boca Raton (2007)
Roffman, D.A.: Meteorological implications: evidence of life on Mars? J. Astrobiol. Space Sci. Rev. 1, 329–337 (2019). 2019
Rossi, L., Marcq, E., Montmessin, F., Fedorova, A., Stam, D., Bertaux, J.-L., Korablev, O.: Preliminary study of Venus cloud layers with polarimetric data from SPICAV/VEx planet. Space Sci. 113–114, 159–168 (2015). 2015
Ruffi, W., Farmer, J.D.: Silica deposits on mars with features resemblinghot spring biosignatures at El Tatio in Chile. Nat. Commun. 7, 13554 (2016)
Russell, C.T., Vaisberg, O.: The Interaction of the Solar Wind with Venus. University Of Arizona Press, Tucson (1983)
Sagan, C., Morowtz, H.: Life in the clouds of Venus. Nature 215, 1259–1260 (1967)
Sanchez, F.J., Mateo-Martí, E., Raggio, J., Meeßen, J., Martínez-Frías, J., Sancho, L.G., Ott, S., de la Torrea, R.: The resistance of the lichen circinaria gyrosa (nom. Provis.) towards simulated Mars conditions-a model test for the survival capacity of an eukaryotic extremophile. Planet. Space Sci. 2012(72), 102–110 (2012). 1
Sancho, L.G., de la Torre, R., Horneck, G., Ascaso, C., de los Rios, A., Pintado, A., Wierzchos, J., Schuster, M.: Lichens, survive in space: results from the 2005, LICHENS experiment. Astrobiology 7, 443–454 (2007)
Satoh, K.Y., Nishiyama, T., Yamazaki, T., Sugita, T., Tsukii, Y., Takatori, K., Benno, Y., Makimura, K.: Microbe-I (2011) fungal biota analyses of the Japanese experimental module KIBO of the International Space Station before launch and after being in orbit for about 460 days. Microbiol. Immunol. 55(12), 823–829 (2011). https://doi.org/10.1111/j.1348-0421.2011.00386.x
Sattler, B., Puxbaum, H., Psenner, R.: Bacterial growth in supercooled cloud droplets. Geophys. Res. Lett. 28, 239–242 (2001)
Schaber, G.G., Kirk, L., Strom, R.G.: Geology and distribution of impact craters on Venus: what are they telling us? J. Geophys. Res. 97(E8), 13257–13301 (1992)
Schroder, K-P., Smith, R.C.: Distant future of the Sun and Earth revisited. Mon. Not. R. Astron. Soc. 386, 155 (2008)
Schuerger, A.C., Ming, D.W., Golden, D.C.: Biotoxicity of Mars soils: 2. Survival of Bacillus subtilis and Enterococcus faecalis in aqueous extracts derived from six Mars analog soils. Icarus 290, 215–223 (2017)
Schulze-Makuch, D., Grinspoon, D.H., Abbas, O., Irwin, L.N., Bullock, M.A.: A sulfur-based survival strategy for putative phototrophic life in the Venusian atmosphere. Astrobiology 4, 11–18 (2004)
Schulze-Makuch, D., Irwin, L.N., Lips, J.H., LeMone, D., Dohm, J.M., Farien, A.G.: Scenarios for the evolution of life on Mars. J. Geophys. Res., Planets 110, E12 (2005)
Seckbach, J., Libby, W.F.: Vegetative life on Venus? Investigations with algae which grow under pure CO2 in hot acid media at elevated pressures. Space Life Sci. 2, 121–143 (1970)
Seckbach, J., Baker, F.A., Shugarman, P.M.: Algae thrive under pure CO2. Nature 227, 744–745 (1970)
Selbman, L., Isola, Z.D., Onofri, D.: Rock black fungi: excellence in the extremes. From the Antarctic to Space. Curr. Genet. 61, 335–345 (2015). https://doi.org/10.1007/s00294-014-0457-7. 2015
Setlow, P.: Spores of bacillus subtilis: their resistance to and killing by radiation, heat and chemicals. J. Appl. Microbiol. 101, 514–525 (2006)
Setlow, B., Setlow, P.: Small, acid-soluble proteins bound to DNA protect bacillus subtilis spores from killing by dry heat. Appl. Environ. Microbiol. 61, 2787–2790 (1995)
Shen, Y., Zhang, T., Hoffman, P.F.: On the coevolution of Ediacaran oceans and animals. Proc. Natl. Acad. Sci. USA 105, 7376–7381 (2008)
Small, L.W.: On Debris Flows and Mineral Veins—Where surface life resides on Mars (2015). eBook. https://www.scribd.com/doc/284247475/On-Debris-Flows-eBook
Smerdon, J.E., Pollack, H.N., Cermak, V., Enz, J.W., Kresl, K., Safanda, J., Wehmiller, J.F.: J. Geophys. Res. 109, 44–54 (2004)
Soffen, G.A.: NASA Technical Report N65-23980, (1965)
Stetter, K.O.: Hyperthermophiles in the history of life. Philos. Trans. R. Soc. Lond. B, Biol. Sci. 361(1474), 1837–1843 (2006)
Steven, B., Gallegos-Graves, L.V., Belnap, J., Kuske, C.R.: Dryland soil bacterial communities display spatial biogeographic patterns associated with soil depth and soil parent material. FEMS Microbiol. Ecol. 86, 101–113 (2013). https://doi.org/10.1111/1574-6941.12143
Sunde, E.P., Setlow, P., Hederstedt, L., Halle, B.: The physical state of water in bacterial spores. Proc. Natl. Acad. Sci. USA 106, 19334–19339 (2009)
Surkov, Y.A., Moskalyeva, L.P., Shcheglov, O.P., Kharyukova, V.P., Manvelyan, O.S., Kirichenko, V.S., Dudin, A.D.: Determination of the elemental composition of rocks on Venus by Venera 13 and Venera 14 (preliminary results). J. Geophys. Res. 13, suppl (1983). Part 2, 88, A481
Szewczyk, N.J., Mancinelli, R.L., McLamb, W., Reed, D., Blumberg, B.S., Conley, C.A.: Caenorhabditis elegans survives atmospheric breakup of STS-107, Space Shuttle Columbia. Astrobiology 5, 690–705 (2005)
Tegen, I., Lacis, A.A.: Modeling of particle size distribution and its influence on the radiative properties of mineral dust aerosol. Aerosol Atmos. Opt. 21, 19237–19244 (2012)
Thomas, W.H., Vernet, M., Gibson, C.H.: Effects of small-scale turbulence on photosynthesis, pigmentation, cell division, and cell size in the marine dinoflagellate gomaulax polyedra (dinophyceae)1. J. Phycol. 1995(31), 50–59 (1995)
Tugay, T., Zhdanova, N.N., Zheltonozhsky, V., Sadovnikov, L., Dighton, J.: The influence of ionizing radiation on spore germination and emergent hyphal growth response reactions of microfungi. Mycologia 98(4), 521–527 (2006)
Valdes, J., Pedroso, I., Quatrini, R., Dodson, R.J., Tettelin, H., Blake, R. II, Eisen, J.A., Holmes, D.S.: Acidithiobacillus ferrooxidans metabolism: from genome sequence to industrial applications. BMC Genomics 9, 597–601 (2008)
Van Den Bergh, S.: Life and death in the inner solar system. Publ. Astron. Soc. Pac. 101, 500–509 (1989)
Venkateswaran, K., La Duc, M.T., Vaishampay, M.T.: Genetic inventory task: final report. In: JPL Publication, California Institute of Technology, Pasadena, vol. 12-12 (2012)
Vesper, S.J., Wong, W., Kuo, C.M., Pierson, D.L.: Mold species in dust from the ISS identified and quantified by mold-specific quantitative PCR. Res. Microbiol. 159, 432–435 (2008)
Vreeland, R.N., Rosenzweig, W.D., Powers, D.W.: Isolation of a 250 million-year-old halotolerant bacterium from a primary salt crystal. Nature 407, 897–900 (2000)
Wainwright, M., Fawaz Alshammari, F., Alabri, K.: Are microbes currently arriving to Earth from space? J. Cosmol. 2010, Vol 7, 1692–1702 (2010)
Way, M.J., Del Genio, A.D.: A view to the possible habitability of ancient Venus over three billion years, IEPSC Abstracts, vol. 13, EPSC-DPS 2019-1846-1, 2019 EPSC-DPS Joint Meeting 2019
Way, M.J., Del Genio, A.D., Kiang, N.Y., Sohl, L.E., Grinspoon, D.H., Aleinov, I., Kelley, M., Clune, T.: Was Venus the first habitable world of our solar system? Geophys. Res. Lett. 43, 8376–8383 (2016)
Weirzchos, J.: Microorganisms in desert rocks: the edge of life on Earth. Int. Microbiol. 15, 171–181 (2012)
Wember, V.V., Zhdanova, N.N.: Peculiarities of linear growth of the melanin-containing fungi Cladosporium sphaerospermum Penz and Alternaria alternata (Fr.) Keissler. Mikrobiol. ž. 63, 3–12 (2001)
White, O., Eisen, J.A., Heidelberg, J.F., et al.: Genome sequence of the radioresistant bacterium deinococcus radiodurans R1. Science 286, 1571–1577 (1999)
Wibking, B.D., Thompson, T.A., Krumholz, M.R.: Radiation pressure in galactic discs: stability, turbulence, and winds in the single-scattering limit. Mon. Not. R. Astron. Soc. 477, 4665–4684 (2018)
Wickramasinghe, N.C., Wickramasinghe, D.T., Steele, E.J.: Comets, enceladus and panspermia. Astrophys. Space Sci. 363, 244 (2018). https://doi.org/10.1007/s10509-018-3465-0
Zakharova, K., Marzban, G., de Vera, J-P., Lorek, A., Sterflinger, K.: Protein patterns of black fungi under simulated Mars-like conditions. Sci. Rep. 4, 5114 (2014)
Zhdanova, N.N., Tugay, T., Dighton, J., Zheltonozhsky, V., McDermott, P.: Ionizing radiation attracts soil fungi. Mycol. Res. 2004(108), 1089–1096 (2004)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Compliance with ethical standards
The author has complied with all ethical standards and reports no conflicts of interest, financial or non-financial. This report and research was independently funded by the author.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Joseph, R.G. RETRACTED ARTICLE: Life on Venus and the interplanetary transfer of biota from Earth. Astrophys Space Sci 364, 191 (2019). https://doi.org/10.1007/s10509-019-3678-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10509-019-3678-x