Abstract
Self-reported sexual orientation of transgender individuals occasionally changes over transition. Using functional magnetic resonance imaging, we tested the hypothesis that neural and behavioral patterns of sexual arousal in transgender individuals would shift from the assigned to the experienced gender (e.g., trans women’s responses becoming more dissimilar to those of cis men and more similar to those of cis women). To this aim, trans women (N = 12) and trans men (N = 20) as well as cisgender women (N = 24) and cisgender men (N = 14) rated visual stimuli showing male–female, female–female or male–male intercourse for sexual arousal before and after four months of gender-affirming hormone therapy. A Bayesian framework allowed us to incorporate previous behavioral findings. The hypothesized changes could indeed be observed in the behavioral responses with the strongest results for trans men and female–female scenes. Activation of the ventral striatum supported our hypothesis only for female–female scenes in trans women. The respective application or depletion of androgens in trans men and trans women might partly explain this observation. The prominent role of female–female stimuli might be based on the differential responses they elicit in cis women and men or, in theory, the controversial concept of autogynephilia. We show that correlates of sexual arousal in transgender individuals might change in the direction of the experienced gender. Future investigations should elucidate the mechanistic role of sex hormones and the cause of the differential neural and behavioral findings.
The study was registered at ClinicalTrials.gov (NCT02715232), March 22, 2016.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Self-reported sexual orientation and/or attraction has been found to change for transgender persons during their transition process (Auer et al., 2014; Katz-Wise et al., 2016; Meier et al., 2013). In a study by Auer et al. (2014), 33% of trans women (TW) and 22% of trans men (TM) reported a change in sexual orientation. Of those reporting changes, 39% of the TW and 60% of the TM reported that the changes occurred prior to sex reassignment surgery. Self-reported changes were more likely in individuals previously attracted to the opposite biological sex (i.e., gynephilic TW and androphilic TM) compared with those previously attracted to the same biological sex (i.e., androphilic TW and gynephilic TM). A study by Meier et al. (2013) found that originally gynephilic TM were especially likely to report a change in sexual attraction, suggesting an opposite pattern. In that study, overall 40% of all TM who had begun transition reported a change in sexual orientation. Despite being contradictory, these results point toward an interaction between gender and pre-transition sexual preferences in mediating the temporal dynamics of self-reported sexual attraction/orientation over transition. These factors must thus both be taken into account when assessing changes to sexual arousal over transition.
The use of myriad self-report and physiological measures is limiting comparability between studies. In addition, inter-individual variance in genital arousal patterns is high, particularly in case of bisexuality (Slettevold et al., 2019). Also, patterns of sexual attraction in self-report or physiological measures might not always be interchangeable with sexual orientation, resulting in cases where, e.g., self-reported sexual orientation changes but patterns of sexual arousal do not (Lawrence et al., 2005). Similarly, behavioral and peripheral (genital) measures do not necessarily carry over to brain activity patterns associated with sexual arousal. For example, differences between cis men (CM) and cis women (CW) in subjective arousal (Murnen & Stockton, 1997) were not detected when assessing brain activity during sexual arousal with functional magnetic resonance imaging (fMRI) (Mitricheva et al., 2019). This apparent contradiction between different measures of sexual arousal might be related to a desynchrony in genital and subjective response to erotic stimulation, which might even be stronger in CW than in CM (Meston & Stanton, 2019; Sierra et al., 2019). Thus, comparative investigations of sexual arousal should consider different measures to capture the full spectrum of responses. Moreover, besides genital measures, for which an ascertainment bias needs to be taken into account (Chivers et al., 2004), neural responses might provide an alternative way to quantify sexual arousal (Ponseti et al., 2006).
Regarding the brain regions involved in sexual arousal, only the hypothalamus and the ventral striatum (VS) showed activation specifically corresponding to sexual and not general emotional arousal (Walter et al., 2008). The VS has been employed extensively for investigating brain correlates of sexual arousal across genders (Safron et al., 2007, 2017, 2020; Sylva et al., 2013), whereas hypothalamus activity is specifically related to male sexuality (Brunetti et al., 2008; Karama et al., 2002). Furthermore, the hypothalamus is functionally heterogeneous and sexually dimorphic (Makris et al., 2013), making it a suboptimal fMRI target (Ogawa et al., 2020; Osada et al., 2017). It was shown that gender and sexual orientation not only influence the magnitude of the behavioral and VS response but also its specificity for the stimulation material (Safron et al., 2017, 2020). However, longitudinal studies with transgender individuals investigating the influence of transition in general, or gender-affirming hormone therapy (GHT) in particular, on the changes in brain function related to sexual arousal are missing.
While studying transgender individuals receiving GHT offers the possibility to jointly investigate the roles of assigned gender, experienced gender, sexual orientation, sex hormones and social aspects of transition on sexual arousal (Kranz et al., 2020), insufficient emphasis has been placed on the confounding influence of sex hormones on brain function when their effect was not of primary interest (for reviews, see Heany et al. (2016); Slettevold et al. (2019)). Beyond the effect of sex hormones on neural activity, their effect on sexual behavior is well known (Schober & Pfaff, 2007). For instance, low-dose androgen therapy can improve sexual function in post-menopausal women (Vegunta et al., 2020) and hypogonadal men (Corona et al., 2016), whereas decreased testosterone or increased estrogen levels are associated with erectile dysfunction (Schulster et al., 2016). Moreover, testosterone use was also associated with changes in sexual attraction in TM but not independent of their preferences before beginning transition (Meier et al., 2013).
Here we assess changes to brain measures of sexual arousal related to transition using fMRI, focusing on the VS, based on previous reports of its specific role in this function (Safron et al., 2007, 2017, 2020; Sylva et al., 2013; Walter et al., 2008). We hypothesized that the patterns of behavioral responses and VS activation to erotic stimuli shift from assigned to experienced gender over the course of GHT, in analogy to patterns detected using other MRI based outcome parameters, such as white and gray matter structure (Flores et al., 2022; Kilpatrick et al., 2019; Kranz et al., 2017, 2018). To this aim, we assessed the subjective arousal ratings and VS activation in a sample of TW and TM before and after four months of GHT as well as CW and CM control groups, all undergoing an fMRI sexual arousal task. We then tested whether the rating and VS activation patterns of the TW and TM became more similar to the experienced and more dissimilar to the assigned gender.
Method
Participants
Transgender individuals seeking GHT were recruited from the Unit for Gender Identity Disorder, Department of Gynecology and Obstetrics at the General Hospital in Vienna. Cisgender control subjects were recruited via social media, designated message boards at the Medical University of Vienna and from a Neuroimaging Labs database. CM and CW were age-matched ± 3 years to TW and TM, respectively. Inclusion criteria comprised: a diagnosis of gender dysphoria (TM, TW only) according to the Diagnostic and Statistical Manual of Mental Disorders, version 5 (DSM-5: 302.85); general health based on medical history, physical examination, electrocardiogram, laboratory screening and structural clinical interview (SCID) for DSM-IV Axis-I. Participants were excluded in case of major neurological or internal illnesses, pregnancy, severe Axis-I comorbidities (TM, TW) or any Axis-I disorder (CM, CW), steroid hormone treatment within six months prior to inclusion (including hormonal contraceptives, with the exception of low dose progesterone in TM for cessation of menstruation prior to transition), treatment with psychotropic agents three months prior to inclusion, clinically relevant abnormal laboratory values, MRI contraindications, current substance abuse (excluding nicotine), current or past substance-related disorders and insufficient compliance.
Procedure
The study followed a controlled longitudinal observational design. All participants underwent two MRI sessions with an interval of (median ± interquartile range) 138 ± 35 days. After the first MRI, hormone treatment following an individualized regimen according to protocols of the Department of Obstetrics and Gynecology at the Medical University of Vienna was initiated. MRI sessions included structural, diffusion-weighted, task, resting-state, and spectroscopy scans. Only the task data are presented here. Blood for determining testosterone, progesterone, and estradiol plasma levels was drawn on the day of the MRI assessments. The Klein Sexual Orientation Grid was administered 14 ± 24 days before the first and 14 ± 23 days after the second MRI appointment.
Measures
In the MRI scanner, participants were visually presented a sequence of blocks with five explicit images of male–female, female–female or male–male intercourse. Sports scenes with two women (sports–female) or two men (sports–male) constituted the control for general arousal. Each image was shown for 4 s. Participants were instructed to indicate whether they are “strongly turned on,” “turned on,” “turned off,” or “strongly turned off” by the erotic stimuli using fingers of their right hand and an MRI-compatible four-button response device. For the sports scenes, participants were instructed to indicate how much they liked the image since rating them for arousal led to confusion in pilot runs. Four smiley faces with strong positive to strong negative expressions were depicted below the stimuli and circled in red after pressing the respective button. The 20-s blocks were flanked with baseline periods of the same length showing a fixation cross in the center of the stimulus frame. The task contained four blocks of each sexual arousal and two of each control condition in random order. The stimuli from Safron et al. (2007, 2017), Sylva et al. (2013) and normative scores were kindly provided by the authors. Since the material did not contain male–female scenes, we added these from a database of pornographic movie stills. This was done in order to prevent generalized aversive reactions to homosexual stimuli, which might be expected for the CW group (Sylva et al., 2013). An internal validation ensured comparable style and explicitness to the existing stimuli.
MRI scanning was conducted on a Siemens Prisma 3 T machine: TE/TR = 30/2050 ms, parallel acquisition with GRAPPA 2, 210.0 × 210.0 × 121.8 mm field of view with 100 × 100 pixel in-plane resolution and 35 axial slices of 2.8 mm (25% gap), resulting in 2.1 × 2.1 × 2.8 mm voxel size, flip angle 90°, 2275 Hz/pixel bandwidth, orientation parallel to the anterior–posterior commissure line.
Data Analysis
fMRI Preprocessing and Modeling
Data preprocessing was conducted using PESTICA (Beall & Lowe, 2007), SPM12 (http://www.fil.ion.ucl.ac.uk/spm/; RRID:SCR_007037) and the BrainWavelet Toolbox (Patel et al., 2014) (see supplement for single steps and parameters).
Single subject models contained one regressor per condition (male–female, female–female, male–male, sports–female, sports–male), the Friston-24 model of motion (Friston et al., 1996), and an automatically derived number of combined white matter and cerebrospinal fluid regressors for an adapted CompCor approach (Behzadi et al., 2007; Klöbl et al., 2020). Contrasts for raw erotic (male–female, female–female, male–male) and sports scenes (sports–female, sports–male) as well as category specific activations (|male–female—female–female|, |male–female—male–male|, |male–male—female–female|, i.e., the absolute difference between the raw erotic stimuli) were calculated. Even though previous works employed sports scenes to control for general arousal when investigating only a single sex or gender (e.g., Arnow et al., 2002, 2009; Safron et al., 2007, 2017), we refrained from contrasting the erotic to the sports stimuli for three reasons: first, the above-mentioned necessity of different instructions, second, the reactions to watching sports is gender-specific (Apostolou et al., 2014; Gantz & Wenner, 1991), and third, VS activation might vary with the sex of the athletes (Sylva et al., 2013). However, we provide an analysis of the contrasts between erotic and sports stimuli in the supplement.
Median activation was extracted from the VS region in the Automated Anatomical Labeling atlas 3 (AAL3) (Rolls et al., 2020) using the MarsBaR toolbox, version 0.44 (http://marsbar.sourceforge.net/; RRID:SCR_009605). Despite its activation specific to sexual arousal, the hypothalamus was not investigated as target region due to its heterogeneous structure.
Statistical Modeling and Inference
To test whether behavioral and neural (VS) response patterns shift from assigned to experienced gender, Bayesian multivariate mixed-effects models (brms_2.15.0 R package (Bürkner, 2017) run in RStudio 1.4.1717 (http://www.rstudio.com/; RRID:SCR_000432)) were used as statistical framework for our analyses for the following reasons: First, we aimed to incorporate previous behavioral results from comparable studies in order to partly compensate for the typically low sample sizes in studies on transgender populations. Second, the posterior probabilities (PPs) provided by Bayesian statistics facilitate more fine-grained interpretations, in accordance with the spectrum perspective on gender. In other words, though Bayesian models do not provide p-values for dichotomous decisions on significance, they provide probabilities on the outcomes of tests. The PP quantifies the support for our hypotheses after the model “has seen” the data, with higher percentages indicating stronger support, though no cutoff, such as the 5% typically used for the p-value. Third, complex hypotheses like measures of sexual arousal approaching the experienced gender and simultaneously distancing from the assigned gender are not easily evaluable in many other approaches, whereas the brms package allows for non-linear hypothesis testing. Bayesian multilevel models further protect against multiplicity issues (Gelman et al., 2012), which would have arisen for frequentist comparisons of four groups and two time points, allowing for more in-depth inferences. See Flores et al. (2022) for a detailed comparison of frequentist versus Bayesian mixed models and the supplement for a complementary frequentist analysis.
It was first tested whether the responses of all transgender participants over all sexual arousal conditions shifted from the assigned to the experienced gender. This means that the response pattern of TW would become more dissimilar to that of CM and more similar to that of CW. In turn, the response pattern of TM would become more dissimilar to that of CW and more similar to that of CM.
Second, since neither similar behavior between sexes and genders, nor across fMRI stimulus groups (i.e., male–female, female–female, and male–male scenes) can be assumed, sub-hypotheses separating TW and TM as well as the stimuli were further evaluated. We first investigated behavioral and VS category specificities of sexual arousal since this was shown to differ between sexes and orientations (Safron et al., 2017, 2020). Category specificity is calculated as the absolute difference between the responses to varying stimulus types (i.e., \( \left| {{\text{male}}{-}{{{\text{female}}}} - {\text{female}}{-}{{{\text{female}}}} } \right| \), \( \left| {{\text{male}}{-}{{{\text{female}}}} - {\text{male}}{-}{{{\text{male}}}} } \right| \), \( \left| {{\text{male}}{-}{{{\text{male}}}} - {\text{female}}{-}{{{\text{female}}}} } \right| \)) and thus denotes how different responses to the individual stimulus types are. Subsequently, since no particularly strong evidence was found for changes in category specificity of sexual arousal, the raw responses, i.e., the responses to all five conditions (i.e., male–female, female–female, male–male, sports–female, sports–male) were considered separately to unveil whether the absence of more specific effects resulted from generally comparable responses, added variance or a general lack of effects. Lastly, since the VS is generally sensitive to rewarding stimulation (which might be given by seeing individuals of a particular sex) and there might be latent driving factors underlying subjective ratings not covered by the experiment, we conducted an arousal-specific analysis, contrasting the erotic to the sports stimuli. The shifts in responses and VS activation in all three cases above were formally assessed using the following inequation:
The sexual preference of the participants at each time point was quantified as the mean of the Klein Sexual Orientation Grid items “current sexual attraction,” “current sexual behavior,” and “current sexual phantasies,” which were rated on a 7-point Likert scale from “women only” to “men only.” The score was inverse-coded for CW and TW to reflect the distance to heterosexuality with respect to the experienced gender in all groups, mean-centered per group and time point and used as covariate in all models to account for the effects on orientation-specific sexual arousal. This also controls for potentially mediating effects of the initial sexual preferences of the transgender participants (Auer et al., 2014; Meier et al., 2013). Furthermore, assuming that there is a shift in sexual arousal over the observed transition period while receiving GHT, the temporal dynamics of this effect will vary between individuals. We thus used the above-mentioned items of the Klein Sexual Orientation Grid to account for varying magnitude and rate of the hypothesized effect. Participant age was mean-centered within groups as well to avoid collinearity due to differences between the groups. To correct for the varying GHT regimens, the first principal components of the standardized log-transformed testosterone, progesterone and estradiol levels were included as separate covariates for TM and TW at the second measurement since group-specific effects were likely (Mueller et al., 2020). Missing hormone and Klein Sexual Orientation Grid values were estimated using the Missing Data Imputation toolbox, version 2, in MATLAB (Folch-Fortuny et al., 2016) (see supplement for details). The models further contained fixed group and time point factors, their interactions, and a random intercept per participant with correlations between the conditions. Per protocol, subjects were assessed after approximately 4 months of GHT (median ± interquartile range = 127 ± 14.25 days). We did not adjust for the time of receiving gender-affirming hormone therapy, as this was comparable between the subjects. Priors for behavioral data were calculated based on the sexual orientation score, the results of Gizewski et al. (2009), Ku et al. (2013), Safron et al. (2007), and the normative data from Safron et al. (2007) kindly provided by the authors (see supplement for mathematical details). To examine the influence of the priors on the behavioral results, we additionally conducted a complementary analysis using flat priors (as for the VS activation) presented in the supplement. Pairwise comparisons of the factors gave 95% highest posterior density (HPD) regions, i.e., areas of the posterior distributions containing 95% of the estimated samples. The nonlinear hypothesis tests return posterior probabilities of support by the data.
Results
The demographics and hormone levels of the study sample are provided in Table 1. Overall, data from 20 TW, 12 TM, 24 CW, and 14 CM was included in this analysis. Partially available data from the first session was included from ten participants. For two participants, only the second session could be used due to a date recording error. Less TW and matched CM could be recruited compared to TM and CW. Due to age matching, the former groups are also older. On average, subjective responses indicate that male–female stimuli were received as turning on over all groups except for TM before undergoing GHT. In contrast, male–male stimuli were received as turning off except for TM after undergoing GHT. The female–female stimuli show a marked difference between the cisgender groups with CW being turned off and CM being turned on. TW and TM reported an increase in subjective sexual arousal for female–female stimuli. At the second assessment, TW had numerically lower testosterone and higher progesterone and estradiol levels, whereas TM had higher testosterone, lower progesterone and lower estradiol levels. The hormone levels of the cisgender groups were more stable with slight variations in progesterone as well as estradiol in CW only. Demographics and covariates grouped by individuals participating only in the first or both MRI sessions are presented in the Supplementary Table S1.
Subjective Sexual Arousal Ratings
The behavioral model showed more pronounced category specificity for the comparisons male–female versus male–male and female–female versus male–male stimuli for CM at both time points (visualized for 95%-HPDs not covering zero in Fig. 1). The most category specificity for female–female versus male–female stimuli was found for CW, again at both time points. Category specificity for female–female versus male–female stimuli was stronger in TW than TM. The category specificity for male–female versus male–male stimuli decreased for TM, whereas the raw response increased for all erotic stimulus types.
Results of the behavioral analyses. For visualization purposes, the chord diagrams (Gu et al., 2014) only show the differences between groups and time points with a 95% highest posterior density not covering 0 (this is very roughly similar to a corrected significance of p < .05). The top row shows the category specific and the bottom row the raw differences. Colored arrow directions in the chord diagrams indicate “greater than” relations (i.e., the arrows represent the “>” relation with the smaller value at the tip and the larger at the shaft). For instance, the top left chord diagram shows that the category specificity of CW for heterosexual versus female–female stimuli is greater than for almost all other groups and time points, whereas, for TW at both time points, it is greater only than that of TM after the observed period of gender-affirming hormone therapy. Black solid arrows between the diagrams show which raw results fed into the calculation of the category specific results. Dotted lines and arrows indicate the results used for hypothesis testing. The hypothesis tests for response patterns shifting from the assigned to the experienced gender are presented on the top left (category specific responses) and right (raw responses) with their respective posterior probabilities. The central circle represents the support for the main hypothesis with the sectors fanning out representing the derived sub-hypotheses. For instance, the support for our hypothesis of a shift from the assigned to the experienced gender including all raw stimuli and groups was 96%, where it was even 99% for female–female stimuli and 98% for TM. For the responses of TM to female–female stimuli, it was 96%. The results for the sports scenes showing female (SF) or male (SM) athletes were not included in the hypothesis tests. AG: assigned gender, TX: transgender, EG: experienced gender, TW: trans women, TM: trans men, CW: cis women, CM: cis men, pre: pre-treatment assessment, post: post-treatment assessment, ⚤: scenes of male–female intercourse, ⚢: scenes of female–female intercourse, ⚣: scenes of male–male intercourse
Category specific behavioral data provided weak to moderate support for our hypothesis of transgender sexual arousal response patterns moving from the assigned to the experienced gender. This was strongest in TW for the category specificity of female–female versus male–male (PP = 81%) and weak in TW and TM for the category specificity of male–female versus male–male stimuli (PP = 23% and PP = 57%, respectively). In summary, after GHT, TW became more similar to CW and/or more dissimilar to CM in their varying responses (i.e., category specificity) to female–female versus male–male stimuli.
Raw behavioral data clearly showed the hypothesized changes. This effect was especially strong for female–female stimuli (PP = 99%) in both TW (PP = 85%) and TM (PP = 96%). TM showed the hypothesized shift in sexual arousal patterns across all erotic stimuli categories (PP = 98%) but least for the male–female stimuli (PP = 62%). An overview of the behavioral results including PPs showing to which degree the data supports our hypothesis is given in Fig. 1. Moderate to strong correlations of random intercepts were found for raw male–female and female–female (r = 0.57, 95% credible interval [0.32, 0.76]), male–female and female sports (r = 0.36, [0.01, 0.68]), female–female and female sports (r = 0.42, [0.06, 0.73]), and female sports and male sports scenes (r = 0.73, [0.33, 0.95]; see supplement for full data).
Ventral Striatum Activation
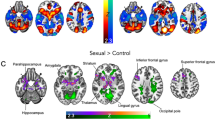
After four months of GHT, TM displayed clearly weaker category specificity of VS activation for male–female versus female–female stimuli than the other groups (see 95%-HPDs in Fig. 2). Distinct changes in the raw data according to our hypotheses were found mostly for TW (PP = 90%), especially with female–female stimuli (PP = 97%). An overview of the results for VS activation is given in Fig. 2. Correlations between the categories were weaker with considerably larger credible intervals. Complete details on the models and tests are provided in the supplementary tables.
Results of the ventral striatum activation analyses. For visualization purposes, the chord diagrams (Gu et al., 2014) only show the differences between groups and time points with a 95% highest posterior density not covering 0. The top row shows the category specific and the bottom row the raw differences. Colored arrow directions in the chord diagrams indicate “greater than” relations (i.e., the arrows represent the “>” relation with the smaller value at the tip and the larger at the shaft). Black solid arrows between the diagrams show which raw results fed into the calculation of the category specific results. Dotted lines and arrows indicate the results used for hypothesis testing. The hypothesis tests for ventral striatum activation patterns shifting from the assigned to the experienced gender are presented on the top left (category specific activation) and right (raw activation) with their respective posterior probabilities. The central circle represents the support for the main hypothesis with the sectors fanning out representing the derived sub-hypotheses. The results for the sports scenes showing female (SF) or male (SM) athletes were not included in the hypothesis tests. AG: assigned gender, TX: transgender, EG: experienced gender, TW: trans women, TM: trans men, CW: cis women, CM: cis men, pre: pre-treatment assessment, post: post-treatment assessment, ⚤: scenes of male–female intercourse, ⚢: scenes of female–female intercourse, ⚣: scenes of male–male intercourse
Discussion
We investigated whether the behavioral and neural responses to sexual arousal in transgender individuals shift from the assigned to the experienced gender over the course of four months of GHT. Overall strong support for this hypothesis was found in the raw behavioral responses, especially for the TM group and female–female scenes. For VS activation, a comparable effect was observed for female–female stimuli presented to the TW group. The support for category specific changes was markedly weaker as indicated by the lower PPs.
In general, the cisgender groups showed markedly stronger category specificity of their behavioral responses than the transgender groups. We included male–female stimuli to avoid receiving primarily aversive responses from CW (Sylva et al., 2013). Indeed, the most prominent behavioral category specificity among all subject groups and stimulus types was found for male–female versus female–female scenes in CW and male–female or female–female versus male–male scenes in CM at both time points. These findings are in accordance with Safron et al. (2020), insofar as that they show strong male–male versus female–female category specificity for CM. Our results now extend the strong category specificity of CM toward male–female versus male–male stimuli. In contrast, CW exhibit strong category specificity for male–female versus female–female stimuli, where this group signaled subjective arousal only for male–female images.
Effects in Trans Men
After four months of GHT, the TM group displayed a strong reduction in category specificity of male–female versus male–male stimuli as well as an increase spanning all raw responses to erotic stimuli. Furthermore, VS activation showed particularly less category specificity for male–female versus female–female stimuli after GHT compared to CM and TW at the first and CW at the second time point. The raw increases were absent in the neural data. Altogether, these results provided at most weak support for our hypothesis on category specific responses in TM shifting over GHT, but might point toward generally higher subjective sexual arousal after treatment. This interpretation is in line with previous reports of increased sexual desire and arousal in TM after sex reassignment surgery and GHT (Mueller et al., 2020) and might be explained by the general positive relationship between sexual arousal and testosterone levels (Klein & Gorzalka, 2009).
The lack of further category specific changes in TM might be attributable to a stronger expression of bisexualism among androphilic and ambiphilic/bisexual transitioned TM compared to CM with the same orientations (Bockting et al., 2009). Even though we adjusted our analyses for self-reported orientation using the Klein Sexual Orientation Grid, such traits might exist beyond what could be captured by the questionnaire. While measures of genital arousal displayed traits similar to CW and CM in a mixed pre- and post-surgical TM sample and the male-typical preference for orientation-congruent stimuli was present, more female-typical response to all stimuli was nevertheless observed (Raines et al., 2021). Our TM sample identified as mostly gynephilic, but existing bisexual rather than monosexual traits could partly explain the lack of clear changes in category specific responses to sexual arousal.
Of note, TM also had the least aversive behavioral responses to the sports scenes. Despite the correlations found between erotic and sports stimuli, a latent influence of general emotional arousal is unlikely, since the participants were instructed to rate the sports stimuli on how much they liked the image in general and not for sexual arousal (see section Measures). The observation that TM and CM responded the most positively to the sports scenes with the strongest difference to CW is potentially based on the generally higher appeal of watching sports in men. The evolutionary perspective of sports as a primarily male communication tool (Apostolou et al., 2014) as well as gender-related social norms and roles (Gantz & Wenner, 1991) might provide explanations here. Data from transgender individuals on the experience of watching sports is missing but our results suggest a difference between TW and TW as well as a potential influence of four months of GHT (see chord diagrams in Figs. 1 and 2 and also supplementary tables). Specific research on this topic is, however, necessary to confirm or refute these deliberations.
The raw behavioral responses to the erotic stimuli provide general support for our hypothesis, particularly with the TM pattern becoming more CM-/less CW-typical across erotic stimuli of all orientations. In detail, the increase in arousal for female–female scenes can be explained by the response approaching a CM and for the male–male scenes by distancing from a CW pattern. On the contrary, the VS activation does not show any changes in the hypothesized direction. This stark difference between behavioral and neural measures offers two potential explanations: First, the activation for sexual arousal does not change in the VS for TM after GHT. Second, there is incongruence between subjectively perceived and neurally controlled level of arousal. In an fMRI study with accompanying behavioral data, a small sample of gynephilic-leaning post-operative TM showed a significantly higher level of reported arousal to pictures showing two nude women compared to nude men (Kim et al., 2016a, 2016b). Activation of the hypothalamus, but not the VS was reported. Where the hypothalamus was found, like the VS, to react to sexual rather than general arousal (Walter et al., 2008), this reaction is potentially sex-specific and predominantly found in men (Brunetti et al., 2008; Karama et al., 2002). While the hypothalamus was not investigated here, this might be done in future studies on a voxelwise basis or after refining the target region (Osada et al., 2017), keeping structural sex differences in mind (Makris et al., 2013).
Effects in Trans Women
The TW group showed stronger behavioral category specificity for male–female versus female–female scenes compared to the TM group at and across both time points. This effect is also found for VS activation but only for pre-treatment TW and post-treatment TM. Pre-treatment, TW’s raw response to female–female stimuli was more positive than that of the CW or the TM group before GHT, establishing a certain similarity to the CM group. Contrary to TM, before undergoing GHT, TW showed increased raw VS activation for all conditions except the male sports scenes. This general level of VS activation and the resulting similarity across stimuli also explains the lack of neural category specificity in TW. Despite weaker changes on the behavioral level, the neural data indicates some shift from the assigned toward the experienced gender in TW, which was strongest for female–female scenes.
In a study on post-operative TW, premenopausal and menopausal CW, similar activation patterns to nude pictures were found in the former two but not in the last group (Kim & Jeong, 2014). These results should be interpreted with caution due to the age difference between groups (despite correction) and its highly exploratory nature. However, they point toward similarities in TW after transitioning and CW, as we hypothesized in our data set, as well as hormonal influences on sexual arousal. A large fMRI study comparing transgender individuals living in their experienced gender to cisgender controls reported significantly lower activation for erotic stimuli in the right superior temporal gyrus for TW as compared to CM (Mueller et al., 2020). The investigation was based on the “neurophenomenological model of sexual arousal” proposed by Stoléru et al. (2012). According to this model, the superior temporal gyrus belongs to the “inhibition and devaluation” component, the VS to the “motivational” component (like the hypothalamus). Interestingly, among all groups, the highest interhemispheric task connectivity for the superior temporal gyrus was also found in TW. Given the relationship between VS activation and whole-brain functional connectivity (Mori et al., 2019), seed-based analyses might shed further light on its particular importance for sexual arousal in TW.
The commonalities in behavioral and neural responses (i.e., both being strongest for female–female stimuli) could, in theory, be related to the controversial concept of autogynephilia in TW (Blanchard, 1989). While the self-reported orientation of our TW sample is gynephilic on average before and after GHT (Auer et al., 2014), the perceived sexual arousal by watching the intercourse of other women might have experienced a potentially hormone-induced actualization. Laube et al. (2020) reported a statistical trend that the desire for intercourse with a partner was particularly strong in gynephilic TW (as was our sample on average) before undergoing sex reassignment surgery and, to a lesser extent, before GHT. If such an increased sexual desire were mirrored in stronger VS activation, this would provide an alternative perspective on our findings to autogynephilia: The comparably weaker VS activation in TW after undergoing GHT matches the gradient in sexual desire indicated by Laube et al. (2020). In turn, the weaker VS activation as well as diminished sexual desire are in line with the argumentation of Blanchard (1991) that the effects of estrogen treatment and surgical castration reduce the sex drive in TW. Another possible explanation for the VS activation shift in TW strongest for female–female stimuli would be the group’s generally higher activation levels being reduced after GHT and this coinciding with the strongest difference in activation between CM and CW for female–female stimuli. In total, this could create the impression that the VS activation of the TW group shifts from a CM to a CW pattern only for female–female scenes.
Autogynephilia could, in theory, explain the higher VS activation in TW group to the female sports stimuli before undergoing GHT, which is in stark contrast to the more positive subjective ratings of the TM group, explainable by stronger interest in sports. The recently formulated “brain gender dissonance model” (Altinay & Anand, 2020) hypothesizes a close relationship between VS activation and cognitive dissonance in transgender individuals for stimuli inducing gender dysphoria. This might have happened in TW particularly before undergoing GHT when viewing the female sports scenes since these lacked a stronger stimulation such as sexual content. The authors of the model admit that the relation between VS and cognitive dissonance has not yet been investigated in transgender individuals. However, previous studies found correlations between attitude changes after decisions inducing cognitive dissonance (e.g., having to decide between two similarly liked options) and VS (Jarcho et al., 2010) and caudate nucleus activation (sometimes seen as part of the VS; Sharot et al. (2009)).
Whether these findings are comparable to cognitive dissonance induced by gender dysphoria and what this means for stimuli of coinciding hedonic content and cognitive dissonance needs to be answered by future research. Further points needing consideration are the more negative experience of sexuality in TW (Gil-Llario et al., 2021) and the suppression of female sexuality per se (Muggleton et al., 2019). Both could introduce biases in the neural and behavioral correlates of sexual arousal and might have contributed to the lack of behavioral effects.
Limitations
Less TW than TM could be recruited, which is in line with the reported incidence of gender-confirming surgery (Nolan et al., 2019), and a considerable amount of TW dropped out of the study. Even though this seems to be a general trend, there are societal and age-related influences on the ratio of gender dysphoria in biological males and females. The higher estimated prevalence of gender dysphoria in assigned male gender might be driven by the more complex developmental sex differentiation processes on a hormonal but also psychosocial level (Zucker, 2017). However, psychosocial factors might also lead to a distortion of the true prevalence ratio. Since we also found support for our hypothesis in TW, this likely did not lead to an underpowered sample. Still, simplifications were necessary when correcting for the potential influence of the varying treatment regimens. Since this study on transgender individuals rather than sexual preferences, mixed orientations are found especially in the TW and TM groups. Also, some cisgender participants might be described as heterosexual-leaning rather than strictly heterosexual. Correcting for the influence of varying sexual orientation leads to results for average responses, which are gynephilic-leaning in both transgender groups. Lastly, it is not possible to conclude whether the effects found solely stem from the influence of the hormones on brain and behavior or result, from an increased self-perception of the experienced gender, or from a wide range of potential social and personal influences during transition. In addition, due to methodological restrictions, we assumed categorical, not fluid gender, i.e. the extent to which individuals may experience themselves as belonging to a certain gender would affect experienced sexual orientation.
Conclusion
In line with our hypothesis, we propose that raw behavioral and neural measures of sexual arousal in transgender individuals might approach the pattern of the experienced gender during GHT. These effects were considerably stronger for raw responses (especially for female–female stimuli) than for the category specificity of stimuli (i.e., how specific the responses were for one orientation of the stimuli compared to another). Potential explanations for the more pronounced evidence within the raw responses might be, that the arousal to different stimulus categories changed in the same direction or, that the participants’ diverse sexual orientations together with the contrasting of their responses introduced additional variance. Where behavioral analyses showed generally strong support for our hypothesis (slightly stronger in TM), for VS activation, this was only the case in TW, with the most prominent changes found for female–female scenes. While we cannot offer a definite explanation for this observation, the general difference between male–female CM and CW responses could provide increased sensitivity to changes for this category. In TW, the controversial concept of autogynephilia could also offer a perspective. GHT may also play a role in facilitating some of our results. Stronger reported arousal across all erotic stimuli orientations within the TM group might be attributable to the effect of testosterone, which is known to increase libido. In a similar fashion, the diminished VS activation in the TW group might be caused by the application of anti-androgens. Here, we abstained from analyzing the hypothalamus due to its functional heterogeneity and sex-specific organization. However, future studies particularly focusing on this region might shed light on its role in transgender individuals, since the hypothalamus constitutes a promising target given its role in male sexuality. Furthermore, in-depth analyses of the role of the sexual orientation in the transitioning process would shed more light on changes in perceived sexuality.
Data Availability
Due to data protection, preprocessed fMRI and behavioral data are available only upon reasonable request to the corresponding author.
Code Availability
In-house code is available upon reasonable request to the corresponding author.
References
Altinay, M., & Anand, A. (2020). Neuroimaging gender dysphoria: A novel psychobiological model. Brain Imaging and Behavior, 14(4), 1281–1297. https://doi.org/10.1007/s11682-019-00121-8
Apostolou, M., Frantzides, N., & Pavlidou, A. (2014). Men competing, men watching: Exploring watching-pattern contingencies in sports. International Journal of Sport Communication, 7(4), 462–476. https://doi.org/10.1123/ijsc.2014-0033
Arnow, B. A., Desmond, J. E., Banner, L. L., Glover, G. H., Solomon, A., Polan, M. L., Lue, T. F., & Atlas, S. W. (2002). Brain activation and sexual arousal in healthy, heterosexual males. Brain, 125(5), 1014–1023. https://doi.org/10.1093/brain/awf108
Arnow, B. A., Millheiser, L., Garrett, A., Polan, M. L., Glover, G. H., Hill, K. R., Lightbody, A., Watson, C., Banner, L., Smart, T., & Buchanan, T. (2009). Women with hypoactive sexual desire disorder compared to normal females: A functional magnetic resonance imaging study. Neuroscience, 158(2), 484–502. https://doi.org/10.1016/j.neuroscience.2008.09.044
Auer, M. K., Fuss, J., Höhne, N., Stalla, G. K., & Sievers, C. (2014). Transgender transitioning and change of self-reported sexual orientation. PLoS ONE, 9(10), e110016. https://doi.org/10.1371/journal.pone.0110016
Beall, E. B., & Lowe, M. J. (2007). Isolating physiologic noise sources with independently determined spatial measures. NeuroImage, 37(4), 1286–1300. https://doi.org/10.1016/j.neuroimage.2007.07.004
Behzadi, Y., Restom, K., Liau, J., & Liu, T. T. (2007). A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. NeuroImage, 37(1), 90–101. https://doi.org/10.1016/j.neuroimage.2007.04.042
Blanchard, R. (1989). The concept of autogynephilia and the typology of male gender dysphoria. Journal of Nervous and Mental Disease, 177(10), 616–623. https://doi.org/10.1097/00005053-198910000-00004
Blanchard, R. (1991). Clinical observations and systematic studies of autogynephilia. Journal of Sex & Marital Therapy, 17(4), 235–251. https://doi.org/10.1080/00926239108404348
Bockting, W., Benner, A., & Coleman, E. (2009). Gay and bisexual identity development among female-to-male transsexuals in North America: Emergence of a transgender sexuality. Archives of Sexual Behavior, 38(5), 688–701. https://doi.org/10.1007/s10508-009-9489-3
Brunetti, M., Babiloni, C., Ferretti, A., Del Gratta, C., Merla, A., Olivetti Belardinelli, M., & Romani, G. L. (2008). Hypothalamus, sexual arousal and psychosexual identity in human males: A functional magnetic resonance imaging study. European Journal of Neuroscience, 27(11), 2922–2927. https://doi.org/10.1111/j.1460-9568.2008.06241.x
Bürkner, P.-C. (2017). Brms: An R package for Bayesian multilevel models using Stan. Journal of Statistical Software, 80(1), 1–28. https://doi.org/10.18637/jss.v080.i01
Chivers, M. L., Rieger, G., Latty, E., & Bailey, J. M. (2004). A sex difference in the specificity of sexual arousal. Psychological Science, 15(11), 736–744. https://doi.org/10.1111/j.0956-7976.2004.00750.x
Corona, G., Isidori, A. M., Aversa, A., Burnett, A. L., & Maggi, M. (2016). Endocrinologic control of men’s sexual desire and arousal/erection. Journal of Sexual Medicine, 13(3), 317–337. https://doi.org/10.1016/j.jsxm.2016.01.007
Flores, R. D., Sanders, C. A., Duan, S. X., Bishop-Chrzanowski, B. M., Oyler, D. L., Shim, H., Clocksin, H. E., Miller, A. P., & Merkle, E. C. (2022). Before/after Bayes: A comparison of frequentist and Bayesian mixed-effects models in applied psychological research. British Journal of Psychology, 113(4), 1164–1194. https://doi.org/10.1111/bjop.12585
Folch-Fortuny, A., Arteaga, F., & Ferrer, A. (2016). Missing data imputation toolbox for MATLAB. Chemometrics and Intelligent Laboratory Systems, 154, 93–100. https://doi.org/10.1016/j.chemolab.2016.03.019
Friston, K. J., Williams, S., Howard, R., Frackowiak, R. S., & Turner, R. (1996). Movement-related effects in fMRI time-series. Magnetic Resonance in Medicine, 35(3), 346–355. https://doi.org/10.1002/mrm.1910350312
Gantz, W., & Wenner, L. A. (1991). Men, women, and sports: Audience experiences and effects. Journal of Broadcasting & Electronic Media, 35(2), 233–243. https://doi.org/10.1080/08838159109364120
Gelman, A., Hill, J., & Yajima, M. (2012). Why we (usually) don’t have to worry about multiple comparisons. Journal of Research on Educational Effectiveness, 5(2), 189–211. https://doi.org/10.1080/19345747.2011.618213
Gil-Llario, M. D., Gil-Juliá, B., Giménez-García, C., Bergero-Miguel, T., & Ballester-Arnal, R. (2021). Sexual behavior and sexual health of transgender women and men before treatment: Similarities and differences. International Journal of Transgender Health, 22(3), 304–315. https://doi.org/10.1080/26895269.2020.1838386
Gizewski, E. R., Krause, E., Schlamann, M., Happich, F., Ladd, M. E., Forsting, M., & Senf, W. (2009). Specific cerebral activation due to visual erotic stimuli in male-to-female transsexuals compared with male and female controls: An fMRI study. Journal of Sexual Medicine, 6(2), 440–448. https://doi.org/10.1111/j.1743-6109.2008.00981.x
Gu, Z., Gu, L., Eils, R., Schlesner, M., & Brors, B. (2014). Circlize implements and enhances circular visualization in R. Bioinformatics, 30(19), 2811–2812. https://doi.org/10.1093/bioinformatics/btu393
Heany, S. J., van Honk, J., Stein, D. J., & Brooks, S. J. (2016). A quantitative and qualitative review of the effects of testosterone on the function and structure of the human social-emotional brain. Metabolic Brain Disease, 31(1), 157–167. https://doi.org/10.1007/s11011-015-9692-y
Jarcho, J. M., Berkman, E. T., & Lieberman, M. D. (2010). The neural basis of rationalization: Cognitive dissonance reduction during decision-making. Social Cognitive and Affective Neuroscience, 6(4), 460–467. https://doi.org/10.1093/scan/nsq054
Karama, S., Lecours, A. R., Leroux, J. M., Bourgouin, P., Beaudoin, G., Joubert, S., & Beauregard, M. (2002). Areas of brain activation in males and females during viewing of erotic film excerpts. Human Brain Mapping, 16(1), 1–13. https://doi.org/10.1002/hbm.10014
Katz-Wise, S. L., Reisner, S. L., Hughto, J. W., & St. Amand, C. (2016). Differences in sexual orientation diversity and sexual fluidity in attractions among gender minority adults in Massachusetts. Journal of Sex Research, 53(1), 74–84. https://doi.org/10.1080/00224499.2014.1003028
Kilpatrick, L. A., Holmberg, M., Manzouri, A., & Savic, I. (2019). Cross sex hormone treatment is linked with a reversal of cerebral patterns associated with gender dysphoria to the baseline of cisgender controls. European Journal of Neuroscience, 50(8), 3269–3281. https://doi.org/10.1111/ejn.14420
Kim, G.-W., & Jeong, G.-W. (2014). Neural mechanisms underlying sexual arousal in connection with sexual hormone levels: A comparative study of the postoperative male-to-female transsexuals and premenopausal and menopausal women. NeuroReport, 25(9), 693–700. https://doi.org/10.1097/wnr.0000000000000159
Kim, G.-W., Kim, S.-K., & Jeong, G.-W. (2016a). Neural activation-based sexual orientation and its correlation with free testosterone level in postoperative female-to-male transsexuals: Preliminary study with 3.0-T fMRI. Surgical and Radiologic Anatomy, 38(2), 245–252. https://doi.org/10.1007/s00276-015-1547-z
Kim, T. H., Kim, G. W., Kim, S. K., & Jeong, G. W. (2016b). Brain activation-based sexual orientation in female-to-male transsexuals. International Journal of Impotence Research, 28(1), 31–38. https://doi.org/10.1038/ijir.2015.29
Klein, C., & Gorzalka, B. B. (2009). Sexual functioning in transsexuals following hormone therapy and genital surgery: A review. Journal of Sexual Medicine, 6(11), 2922–2939. https://doi.org/10.1111/j.1743-6109.2009.01370.x
Klöbl, M., Michenthaler, P., Godbersen, G. M., Robinson, S., Hahn, A., & Lanzenberger, R. (2020). Reinforcement and punishment shape the learning dynamics in fMRI neurofeedback. Frontiers in Human Neuroscience, 14, 304. https://doi.org/10.3389/fnhum.2020.00304
Kranz, G. S., Hahn, A., Kaufmann, U., Tik, M., Ganger, S., Seiger, R., Hummer, A., Windischberger, C., Kasper, S., & Lanzenberger, R. (2018). Effects of testosterone treatment on hypothalamic neuroplasticity in female-to-male transgender individuals. Brain Structure and Function, 223(1), 321–328. https://doi.org/10.1007/s00429-017-1494-z
Kranz, G. S., Seiger, R., Kaufmann, U., Hummer, A., Hahn, A., Ganger, S., Tik, M., Windischberger, C., Kasper, S., & Lanzenberger, R. (2017). Effects of sex hormone treatment on white matter microstructure in individuals with gender dysphoria. NeuroImage, 150, 60–67. https://doi.org/10.1016/j.neuroimage.2017.02.027
Kranz, G. S., Zhang, B. B. B., Handschuh, P., Ritter, V., & Lanzenberger, R. (2020). Gender-affirming hormone treatment: A unique approach to study the effects of sex hormones on brain structure and function. Cortex, 129, 68–79. https://doi.org/10.1016/j.cortex.2020.04.005
Ku, H. L., Lin, C. S., Chao, H. T., Tu, P. C., Li, C. T., Cheng, C. M., Su, T. P., Lee, Y. C., & Hsieh, J. C. (2013). Brain signature characterizing the body-brain-mind axis of transsexuals. PLoS ONE, 8(7), e70808. https://doi.org/10.1371/journal.pone.0070808
Laube, J. S., Auer, M. K., Biedermann, S. V., Schröder, J., Hildebrandt, T., Nieder, T. O., Briken, P., & Fuss, J. (2020). Sexual behavior, desire, and psychosexual experience in gynephilic and androphilic trans women: A cross-sectional multicenter study. Journal of Sexual Medicine, 17(6), 1182–1194. https://doi.org/10.1016/j.jsxm.2020.01.030
Lawrence, A. A., Latty, E. M., Chivers, M. L., & Bailey, J. M. (2005). Measurement of sexual arousal in postoperative male-to-female transsexuals using vaginal photoplethysmography. Archives of Sexual Behavior, 34(2), 135–145. https://doi.org/10.1007/s10508-005-1792-z
Makris, N., Swaab, D. F., van der Kouwe, A., Abbs, B., Boriel, D., Handa, R. J., Tobet, S., & Goldstein, J. M. (2013). Volumetric parcellation methodology of the human hypothalamus in neuroimaging: Normative data and sex differences. NeuroImage, 69, 1–10. https://doi.org/10.1016/j.neuroimage.2012.12.008
Meier, S. C., Pardo, S. T., Labuski, C., & Babcock, J. (2013). Measures of clinical health among female-to-male transgender persons as a function of sexual orientation. Archives of Sexual Behavior, 42(3), 463–474. https://doi.org/10.1007/s10508-012-0052-2
Meston, C. M., & Stanton, A. M. (2019). Understanding sexual arousal and subjective-genital arousal desynchrony in women. Nature Reviews Urology, 16(2), 107–120. https://doi.org/10.1038/s41585-018-0142-6
Mitricheva, E., Kimura, R., Logothetis, N. K., & Noori, H. R. (2019). Neural substrates of sexual arousal are not sex dependent. Proceedings of the National Academy of Sciences, 116(31), 15671–15676. https://doi.org/10.1073/pnas.1904975116
Mori, A., Klöbl, M., Okada, G., Reed, M. B., Takamura, M., Michenthaler, P., Takagaki, K., Handschuh, P. A., Yokoyama, S., Murgas, M., Ichikawa, N., Gryglewski, G., Shibasaki, C., Spies, M., Yoshino, A., Hahn, A., Okamoto, Y., Lanzenberger, R., Yamawaki, S., & Kasper, S. (2019). Predicting ventral striatal activation during reward anticipation from functional connectivity at rest. Frontiers in Human Neuroscience, 13, 289. https://doi.org/10.3389/fnhum.2019.00289
Mueller, S. C., Wierckx, K., & T’Sjoen, G. (2020). Neural and hormonal correlates of sexual arousal in transgender persons. Journal of Sexual Medicine, 17(12), 2495–2507. https://doi.org/10.1016/j.jsxm.2020.08.021
Muggleton, N. K., Tarran, S. R., & Fincher, C. L. (2019). Who punishes promiscuous women? Both women and men are prejudiced towards sexually-accessible women, but only women inflict costly punishment. Evolution and Human Behavior, 40(3), 259–268. https://doi.org/10.1016/j.evolhumbehav.2018.12.003
Murnen, S. K., & Stockton, M. (1997). Gender and self-reported sexual arousal in response to sexual stimuli: A meta-analytic review. Sex Roles, 37(3), 135–153. https://doi.org/10.1023/A:1025639609402
Nolan, I. T., Kuhner, C. J., & Dy, G. W. (2019). Demographic and temporal trends in transgender identities and gender confirming surgery. Translational Andrology and Urology, 8(3), 184–190. https://doi.org/10.21037/tau.2019.04.09
Ogawa, A., Osada, T., Tanaka, M., Kamagata, K., Aoki, S., & Konishi, S. (2020). Connectivity-based localization of human hypothalamic nuclei in functional images of standard voxel size. NeuroImage, 221, 117205. https://doi.org/10.1016/j.neuroimage.2020.117205
Osada, T., Suzuki, R., Ogawa, A., Tanaka, M., Hori, M., Aoki, S., Tamura, Y., Watada, H., Kawamori, R., & Konishi, S. (2017). Functional subdivisions of the hypothalamus using areal parcellation and their signal changes related to glucose metabolism. NeuroImage, 162, 1–12. https://doi.org/10.1016/j.neuroimage.2017.08.056
Patel, A. X., Kundu, P., Rubinov, M., Jones, P. S., Vértes, P. E., Ersche, K. D., Suckling, J., & Bullmore, E. T. (2014). A wavelet method for modeling and despiking motion artifacts from resting-state fMRI time series. NeuroImage, 95(100), 287–304. https://doi.org/10.1016/j.neuroimage.2014.03.012
Ponseti, J., Bosinski, H. A., Wolff, S., Peller, M., Jansen, O., Mehdorn, H. M., Büchel, C., & Siebner, H. R. (2006). A functional endophenotype for sexual orientation in humans. NeuroImage, 33(3), 825–833. https://doi.org/10.1016/j.neuroimage.2006.08.002
Raines, J., Holmes, L., Watts-Overall, T. M., Slettevold, E., Gruia, D. C., Orbell, S., & Rieger, G. (2021). Patterns of genital sexual arousal in transgender men. Psychological Science, 32(4), 485–495. https://doi.org/10.1177/0956797620971654
Rolls, E. T., Huang, C. C., Lin, C. P., Feng, J., & Joliot, M. (2020). Automated anatomical labelling atlas 3. NeuroImage, 206, 116189. https://doi.org/10.1016/j.neuroimage.2019.116189
Safron, A., Barch, B., Bailey, J. M., Gitelman, D. R., Parrish, T. B., & Reber, P. J. (2007). Neural correlates of sexual arousal in homosexual and heterosexual men. Behavioral Neuroscience, 121(2), 237–248. https://doi.org/10.1037/0735-7044.121.2.237
Safron, A., Sylva, D., Klimaj, V., Rosenthal, A. M., & Bailey, J. M. (2020). Neural responses to sexual stimuli in heterosexual and homosexual men and women: Men’s responses are more specific. Archives of Sexual Behavior, 49(2), 433–445. https://doi.org/10.1007/s10508-019-01521-z
Safron, A., Sylva, D., Klimaj, V., Rosenthal, A. M., Li, M., Walter, M., & Bailey, J. M. (2017). Neural correlates of sexual orientation in heterosexual, bisexual, and homosexual men. Scientific Reports, 7, 41314. https://doi.org/10.1038/srep41314
Schober, J. M., & Pfaff, D. (2007). The neurophysiology of sexual arousal. Best Practice & Research Clinical Endocrinology & Metabolism, 21(3), 445–461. https://doi.org/10.1016/j.beem.2007.04.006
Schulster, M., Bernie, A. M., & Ramasamy, R. (2016). The role of estradiol in male reproductive function. Asian Journal of Andrology, 18(3), 435–440. https://doi.org/10.4103/1008-682x.173932
Sharot, T., De Martino, B., & Dolan, R. J. (2009). How choice reveals and shapes expected hedonic outcome. Journal of Neuroscience, 29(12), 3760–3765. https://doi.org/10.1523/jneurosci.4972-08.2009
Sierra, J. C., Álvarez-Muelas, A., Arcos-Romero, A. I., Calvillo, C., Torres-Obregón, R., & Granados, R. (2019). Relación entre la excitación sexual subjetiva y la respuesta genital: Diferencias entre hombres y mujeres. Revista Internacional de Andrologia, 17(1), 24–30. https://doi.org/10.1016/j.androl.2017.12.004
Slettevold, E., Holmes, L., Gruia, D., Nyssen, C. P., Watts-Overall, T. M., & Rieger, G. (2019). Bisexual men with bisexual and monosexual genital arousal patterns. Biological Psychology, 148, 107763. https://doi.org/10.1016/j.biopsycho.2019.107763
Stoléru, S., Fonteille, V., Cornélis, C., Joyal, C., & Moulier, V. (2012). Functional neuroimaging studies of sexual arousal and orgasm in healthy men and women: A review and meta-analysis. Neuroscience & Biobehavioral Reviews, 36(6), 1481–1509. https://doi.org/10.1016/j.neubiorev.2012.03.006
Sylva, D., Safron, A., Rosenthal, A. M., Reber, P. J., Parrish, T. B., & Bailey, J. M. (2013). Neural correlates of sexual arousal in heterosexual and homosexual women and men. Hormones and Behavior, 64(4), 673–684. https://doi.org/10.1016/j.yhbeh.2013.08.003
Vegunta, S., Kling, J. M., & Kapoor, E. (2020). Androgen therapy in women. Journal of Women’s Health, 29(1), 57–64. https://doi.org/10.1089/jwh.2018.7494
Walter, M., Bermpohl, F., Mouras, H., Schiltz, K., Tempelmann, C., Rotte, M., Heinze, H. J., Bogerts, B., & Northoff, G. (2008). Distinguishing specific sexual and general emotional effects in fMRI-subcortical and cortical arousal during erotic picture viewing. NeuroImage, 40(4), 1482–1494. https://doi.org/10.1016/j.neuroimage.2008.01.040
Zucker, K. J. (2017). Epidemiology of gender dysphoria and transgender identity. Sexual Health, 14(5), 404–411. https://doi.org/10.1071/SH17067
Acknowledgements
We thank the students of the Neuroimaging Labs for their continuous support throughout the study. We are grateful toward the Center of Excellence for MR Research at the Medical University of Vienna for the received support and supply with consumables.
Funding
Open access funding provided by Medical University of Vienna. This research was funded as whole or in part by the Austrian Science Fund (FWF) [KLI 504 and KLI 516 PI: Rupert Lanzenberger], a Brain and Behavior Research Foundation (formerly NARSAD) Young Investigator Grant (23741, PI: Marie Spies), the Medical Imaging Cluster of the Medical University of Vienna, and by the Grant “Interdisciplinary translational brain research cluster (ITHC) with highfield MR” from the Federal Ministry of Science, Research and Economy (BMWFW), Austria. Manfred Klöbl and Murray Bruce Reed are recipients of a DOC Fellowship of the Austrian Academy of Sciences.
Author information
Authors and Affiliations
Contributions
The study was planned by RL and GK. The fMRI task presented here was conventionalized by GK and MK. Magnetic resonance imaging data were acquired by MK, MBR and BS-D. Clinical data were acquired by MS, PH and MEK. UK, VR and MEK managed subject recruitment and handling. UK further provided medical support. MS and PH supervised the study. MK planned and conducted the statistical analysis and wrote the first draft of the manuscript. GK and MS provided methodological supervision.
Corresponding author
Ethics declarations
Conflict of interest
Rupert Lanzenberger received travel grants and/or conference speaker honoraria within the last three years from Bruker BioSpin MR and Heel and has served as a consultant for Ono Pharmaceutical. He received investigator-initiated research funding from Siemens Healthcare regarding clinical research using PET/MR. He is a shareholder of the start-up company BM Health GmbH since 2019. Marie Spies received travel grants from Janssen, AOP Orphan Pharmaceuticals; Lecture Honoraria: Heel, Janssen and Austroplant. She further spoke at a workshop organized by Eli Lilly. All other authors have no financial disclosures to state. Preliminary results of the analyses in this work were presented as poster at the 17th Austrian Neuroscience Association meeting in 2021 in Salzburg, Austria. A preprint of this work was published on medRxiv (doi: https://doi.org/https://doi.org/10.1101/2021.11.22.21266679). All authors reported no biomedical financial interests or potential conflicts of interest.
Ethical approval
This study was conducted according to the Declaration of Helsinki including all current revisions and the good scientific practice guidelines of the Medical University of Vienna. The protocol was approved by the institutional review board (EK Nr.: 1104/2015) and registered at clinicaltrials.gov (NCT02715232).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Klöbl, M., Reed, M.B., Handschuh, P. et al. Gender Dysphoria and Sexual Euphoria: A Bayesian Perspective on the Influence of Gender-Affirming Hormone Therapy on Sexual Arousal. Arch Sex Behav 53, 1859–1871 (2024). https://doi.org/10.1007/s10508-023-02778-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10508-023-02778-1