Abstract
Heavy metals including cadmium (Cd) are one of the major persistent and non-biodegradable wastewater pollutants. However, Cd reaches the aquatic ecosystem via industrial and agricultural waste discharges and causes serious deterioration in the welfare status of aquatic animals. The use of feed supplements with immune-stimulants to mitigate the toxic influences of heavy metals including Cd is a much more intriguing point. Thus, the current experiment used the bio-synthetized chitosan nanoparticles derived from Bacillus subtilis (Bs-CNPs) as a feed supplement and evaluated its ameliorative impacts on the growth and welfare status of Cd-intoxicated Nile tilapia (Oreochromis niloticus). Bifactorial design (3 Bs-CNPs levels × 3 Cd levels) was used in the current study where Nile tilapia fingerlings (58–63 g) were fed on 0.0, 2, and 4 g Bs-CNPs/kg feed alongside with exposing to 0.0, 0.392, and 0.784 mg Cd/L for 60 days to represent nine treatments as follows: T1: control group (no Cd exposure; no Bs-CNPs supplement); T2 and T3: fish were intoxicated with 0.784 and 0.392 mg Cd/L, respectively; T4 and T5: fish fed on 2 and 4 g Bs-CNPs/kg feed, respectively; T6 and T7: fish were fed on 2 g Bs-CNPs/kg feed along with exposure to 0.784 and 0.392 mg Cd/L, respectively; and T8 and T9: fish were fed on 4 g Bs-CNPs/kg feed along with exposure to 0.784 and 0.392 mg Cd/L, respectively. It is noted that the Cd-intoxicated fish exhibited significant retardation in growth performance and digestive enzyme activities with a decline in their survival rate compared to the control group (T1). The results also revealed that exposing fish to Cd toxicity alone with no feed supplement (T2 and T3) experienced abnormal clinical signs and behavioral changes. Compared with the control group (no Cd with no Bs-CNPs), highest values of cortisol, glucose, aspartate and alanine aminotransferase, and acetylcholine esterase activity were found in fish fed on the control diet along with exposure to 0.784 mg Cd/L. Higher Cd restudies in liver, intestine, gills, kidney, and muscles tissues were detected in fish exposed to 0.784 mg Cd/L alone and the sequence order of Cd levels in different fish organs is intestine > gills > liver > kidney > muscles. Remarkable pathological alterations in hepatic and intestinal tissues were also observed. On the other hand, feeding Nile tilapia on Bs-CNPs-enriched diets alone with no Cd exposure enhanced their growth performance, digestive enzyme activities, and hematological parameters with no Cd residues in fish organs. Interestingly, feeding the Cd-intoxicated fish on diets with Bs-CNPs (4 g/kg feed) returned their growth, digestive enzymes, hematological, and biochemical parameters to approximate those of the control group. Furthermore, these treatments showed histopathological alteration recovery in the intestine and liver tissues is similar to those in the control group (no Cd with no Bs-CNPs). Fish fed on Bs-CNPs levels with no Cd exposure showed no Cd residues in different fish organs. The Cd levels in different organs of fish exposed to 0.392 mg Cd/L along with feeding on Bs-CNPs (4 g/kg feed) were lower than those in Cd-exposed fish treatments. Consequently, the current study evoked that feeding Nile tilapia fingerlings on Bs-CNPs (4 g/kg feed) could enhance their growth performance and protect the fish from the Cd toxicity that may occur in the aquatic ecosystem.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cadmium (Cd) is a non-biodegradable and non-digestible heavy metal that could accumulate in fish body leading to the deterioration of their performance and welfare status (Das et al. 2023; Elgendy et al. 2023; Abdel-Tawwab et al. 2024). Cd pollution may reach the fish farms and cages from rain-of drift, anthropogenic activities, industrial pollutants, domestic sewage, atmospheric deposition, and mining drifts, among others (Monier et al. 2023). In addition, Cd can result in pathological alterations causing skeletal abnormalities and damages of organ architecture in several aquatic animals (Putranto et al. 2014; Bukola et al. 2015; Abdel-Tawwab et al. 2024). However, the aquatic animals could accumulate and magnify Cd within their bodies, leading to oxidative damage of proteins and DNA, as well as the onset of diverse pathological alterations (Jia et al. 2011; Obaiah et al. 2020).
Recently, many studies used various feed supplements to enhance the performance, antioxidant, and immune functions in several fish species (Dawood et al. 2018; Mohan et al. 2019; Hoseinifar et al. 2020; Galindo-Villegas et al. 2022) and promote their resistance to water pollutants (Hamed et al. 2022; Abdel-Tawwab et al. 2024; Ahmed et al. 2024; Sabra et al. 2024). Chitosan is one of these feed additives, which is a polysaccharide compound that originated from the deacetylation of chitin found in crustacean exoskeletons (Chen and Chen 2019). It is used in different biomedical, drug delivery systems, and food industry applications because of its chemical and physical properties such as safety, biocompatibility, effectiveness, and gelling characteristics (El-Husseiny et al. 2022). Normal-sized chitosan or chitosan nanoparticles (CNPs) were used as feed supplements in aqua-feeds showing growth and immune-stimulatory effects with antioxidant, anti-inflammatory, antibacterial, antiviral, and anticancer activities in several fish species ( Abd El-Naby et al. 2019; Abdel-Tawwab et al. 2019; Abdel-Ghany and Salem 2020; El-Naggar et al. 2022a, b). Additionally, dietary CNPs showed ameliorative activities against waterborne pollutants. According to Hussein et al. (2021), feeding African catfish (Clarias gariepinus) on CNPs diets reduced the adverse effects of bisphenol-A toxicity. Naiel et al. (2020) and Ismael et al. (2021) also stated that the dietary supplementation of CNPs alleviated the imidacloprid toxicity in Nile tilapia, Oreochromis niloticus. Sabra et al. (2024) found beneficial effects of dietary CNPs against the doxycycline toxicity in Nile tilapia.
Nile tilapia (O. niloticus) represents one of the major freshwater fish species that is raised in different aquaculture systems in Egypt and exported to various nations across the globe (El-Sayed 2019; El-Sayed and Fitzsimmons 2023). Due to the adverse effects of Cd toxicity on the performance and health status of fish, enhancing the resistance of fish to Cd toxicity is essential. Therefore, this work investigated the ameliorative influences of feeding Nile tilapia on Bs-CNPs-supplemented diets on their growth and welfare indices and evaluated its defensive aspect against Cd impacts.
Materials and methods
Biosynthesis of chitosan NPs using B. subtilis
Chitosan, with a purity exceeding 93% and a viscosity range of 65–310 (Bio Basic Inc., Markham, Canada), and B. subtilis (Aquavet Co., Egypt) were used for B. subtilis bio-synthesized chitosan nanoparticles (Bs-CNPs) preparation, which were prepared using B. subtilis via autoclaving followed by centrifugation at 15,000 × g for 30 min as described in Fesharaki et al. (2010) and Saravanan et al. (2018). In brief, the trial procedure involved inoculating a 100 μL portion of bacterial suspension containing 1011 CFU into 100 mL of Luria–Bertani (LB) broth. This combination was subsequently subjected to incubation at 30 °C and 180 × g for 48 h. Following that incubation, the culture was centrifuged at 8000 × g for 20 min to separate the bacterial pellets from the supernatant. The liquid above the sediment was removed, and the bacterial clumps were re-suspended in 100 mL of enrichment medium (EM) liquid culture containing 10 μL of chitosan. This combination was subsequently subjected to incubation at 30 °C for 3 days in a shaking incubator operating at 180 × g. The confirmation of nano-sized chitosan biosynthesis was indicated by a color change from bright yellow to red. Afterward, Bs-CNPs in the supernatant were collected and preserved until use.
The chemical status of chitosan in Bs-CNPs
Around 2 g of chitosan was homogenized in 1000 mL of distilled water (DW) to create a basic stock solution. Additionally, solutions of 2N HCl and potassium iodine (KI; 2%) have been added, with the latter serving as a starch index. Various concentrations of the basic solutions were then prepared to establish a calibration curve or standard curve. In the testing process, 100 μL aliquot of the Bs-CNPs stock solution was added to test tubes, followed by the inclusion of 1 mL each of 2N HCl and KI solution. The contents were homogenized till a brown-yellow color changed. The chitosan content was determined by measuring the absorbance using a microtiter plate reader at 644 nm, and the absorbance values were then applied to the standard curve equation: y = 0.6633x + 0.0015 for Bs-CNPs. The same experimental conditions were applied in parallel for both chitosan and Bs-CNPs samples, and the starch indicator was combined with all tubes to facilitate color development.
Characterization of Bs-CNPs
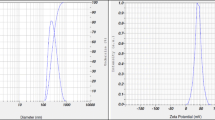
The shape and average size of Bs-CNPs were evaluated using transmission electron microscopy (TEM) images obtained with a JEM-2100 Plus microscope manufactured by JEOL Ltd., Japan, located at the Central Laboratory, City of Scientific Research and Technological Applications in Alexandria, Egypt. The absorption spectra of Bs-CNPs were recorded using a UV–visible spectrophotometer covering the range of 200–1000 nm. This analysis was conducted using equipment from Mettler-Toledo LLC., based in Columbus, OH, USA. Furthermore, the exact size and surface charge of Bs-CNPs were determined using a Zeta sizer analyzer, and the Zeta potential was measured using a Malvern 3000 Zetasizer Nano ZS instrument. These measurements were conducted at the Central Laboratories, City of Scientific Research and Technological Applications in Alexandria, Egypt.
Determination of the 96 h LC50 value of Cd
Cadmium chloride (CdCl2; purity > 95% and Mw: 183.32) was acquired from Al-Gomohra Co. in Alexandria, Egypt. A stock solution of Cd was fresh-made by dissolving CdCl2 in deionized water at a concentration of 1000 mg Cd/L. Nile tilapia (O. niloticus) fingerlings (55–65 g) were obtained from a private fish farm, Kafrelsheikh, Egypt, transported to the wet lab, Faculty of Veterinary Medicine, Alexandria University, Egypt, and acclimatized for wet lab conditions for 2 weeks. Then, 270 fish were divided into nine groups in triplicates (10 fish/100-L aquarium) and exposed to 0.0, 1, 2, 3, 4, 5, 6, 7, and 8 mg Cd/L for 96 h as described in Abdel-Tawwab et al. (2024). Aquaria were augmented with artificial aeration through the utilization of air pumps. The fish were provided with the control basal diet (25% crude protein; CP) three times daily until reaching apparent satiety. Every 2 days, remnants of fish feces and miscellaneous debris were eliminated through siphoning, and 50% of the water volume was replenished with fresh well-aerated tap-water harboring an equivalent initial concentration of Cd. Water quality parameters were monitored daily as documented by Boyd (1984), and recorded values as outlined as follows: water temperature, 27.0–27.5 °C; dissolved oxygen, 6.3–6.5 mg/L; pH, 8.2–8.4; total salinity of 2–2.2 ppt, total hardness level, 82.5–88.3 mg CaCO3/L, and photo-period 12 light: 12 dark. Fish were pronounced deceased upon cessation of gill opercula function and bodily motion (Abdel-Tawwab et al. 2024). Dead fish were then extracted and tallied on a daily basis in order to quantify their mortality rate. The 96 h-LC50 of Cd was calculated according to Behreus and Karber (1953), and it was found to be 7.84 mg/L. Accordingly, Cd values in this experiment were modified at sublethal doses of 0.784 and 0.392 mg Cd/L to represent the 1/10 and 1/20 of the 96 h-LC50 of Cd.
Diet formation and fish-rearing management
The Bs-CNPs were added to the control diet at 0.0, 2.0, and 4.0 g/kg feed (Table 1) and well-mixed with other diet’s ingredients for 30 min, during which 200 mL of water per each kg of diet was added to make a dough. Diet mixtures were prepared in a meat processor, and diets strings are dried at room temperature, at that point pulverized (2–3 mm diameter), and kept at − 4.0 °C until their utilization.
Healthy Nile tilapia fingerlings were bought from the same fish farm, Kafrelsheikh, Egypt, and transported to the wet lab, Faculty of Veterinary Medicine, Alexandria University, Egypt. After the acclimation to lab conditions for 2 weeks, fish (58–63 g) were arbitrarily distributed into 27 120-L aquaria (10 fish/aquarium) to represent nine treatments (T1–T9) in triplicates as follows: T1; control group (no Cd exposure; no Bs-CNPs supplement); T2 and T3: fish were intoxicated with 0.784 and 0.392 mg Cd/L, respectively; T4 and T5: fish fed on 2 and 4 g Bs-CNPs/kg feed, respectively; T6 and T7: fish were fed on 2 g Bs-CNPs/kg feed along with exposure to 0.784 and 0.392 mg Cd/L, respectively; and T8 and T9: fish were fed on 4 g Bs-CNPs/kg feed along with exposure to 0.784 and 0.392 mg Cd/L, respectively. Fish were provided with the trial diets at 9:00, 13:00, and 17:00 h until apparent satiety for 60 days. Fish feces were collected after 40 min of feeding, dried, and weighed. Every 2 days, remnants of fish feces and miscellaneous debris were eliminated through siphoning, and 50% of the aquarium’s water volume was replenished with fresh well-aerated tap-water containing the same initial concentration of Cd.
Water quality parameters were monitored on daily bases: all treatments had water temperature ranges of 27.0–27.5 °C; dissolved oxygen, 6.3–6.5 mg/L; pH, 8.2–8.4; total salinity of 2.0–2.2 ppt; total hardness level, 82.5–88.3 mg CaCO3/L; and photo-period 12 light: 12 dark. The values of water quality parameters are within the acceptable ranges for fish farming (Boyd and Tucker 2012).
Following the feeding period, the fish housed in every individual aquarium were subjected to a 1-day period of starvation prior to the sampling process. Subsequently, they were anesthetized through immersion in a solution containing 50 µL/L of clove oil for a duration of 5 min (Hamackova et al. 2006). All fish were enumerated and collectively weighed in order to calculate the metrics related to fish growth and utilization of feed as follows:
Fish behavior
Fish were individually marked with small plastic tags placed on their dorsal fins to facilitate individual identification. Behavioral observations were conducted using a focal sampling procedure at intervals of 1 h and 15 s, utilizing videotaping software called Observer, as outlined in Chatigny (2017). The observed behavior patterns were recorded for 3 h weekly. Mean duration and frequency of each behavior were reported based on the methods reported by Neetha et al. (2023). Swimming behavior encompassed both rapid and slow movements in the water without engaging in other activities, as described in Alonso and Valle-Torres (2018). Resting behavior was concluded when fish exhibited minimal activity in the aquarium and had their eyes closed, following the criteria established by Sekiguchi and Kohshima (2003).
Sampling
Fish samples were collected at the end of the experimental period (60 days). Prior to sampling, the fish were fasted for 24 h. Following this, a total of nine fish per treatment (3 fish/aquarium) were randomly selected, and blood samples were extracted from the caudal vein utilizing 2 mL sterilized hypodermic syringes. The collected blood samples were divided into two Eppendorf tubes; one containing heparin as an anticoagulant agent and immediately used for hematological investigation. The other tubes were left with no anticoagulant to clot at room temperature for 30 min and centrifuged at 1500 × g for 15 min to get sera samples, which were stored at − 20 °C for haemato-biochemical assessment.
After blood sampling, fish were dissected, and samples of gills, kidney, dorsal muscles, liver, and intestinal tissues were taken and subjected to freeze-drying for determining the Cd residues. Other parts of the liver and mid-intestine tissues were preserved in neutral formalin 10% for histopathological investigations.
Determination of digestive enzyme activity
The mid-intestine samples from different temperature groups were homogenized (1 g/9 mL) in phosphate-buffered saline (pH ~ 7.4) using a Potter–Elvehjem glass/Teflon homogenizer. Then, samples were centrifuged (5000 × g for 10 min) and the supernatants were saved at −80 °C until further use. Activities of lipase, α-amylase, and proteases were analyzed using kits supplied by Biodiagnostic (Giza, Egypt) according to the manufacturer guides.
Hematological assays
Blood samples were collected from the fish to assess various hematological parameters as described in Brown (1980). Counts of white blood cells (WBCs) and red blood cells (RBCs) were conducted using Neubauer hemocytometer. Hematocrit (Hct %) levels were determined using heparinized capillary tubes. Hemoglobin (Hb) was determined by the cyanmethemoglobin method according to Van Kampen and Zijlstra (1961).
Stress indices
The biochemical biomarkers were ascertained through the utilization of diagnostic reagent kits (Biodiagnostic Co., Cairo, Egypt) in accordance with the guidelines provided by the manufacturer. The assessment of blood cortisol and glucose levels was conducted utilizing the methodologies outlined by Trinder (1969) and Foster and Dunn (1974), respectively. The enzymatic activities of alanine (ALT) and aspartate (AST) aminotransferase were determined colorimetrically as per the protocol established by Reitman and Frankel (1957). Acetylcholinesterase (AChE) activity was measured using the method described in Golombieski et al. (2008).
Cd levels in water samples and fish organs
To quantify the concentrations of Cd in aquaria water, water samples (1 L) were obtained from each aquarium and passed through a 0.8-µm Millipore acetyl-cellulose filter paper (Millipore, Bedford, MA, USA). Subsequently, these samples were combined with 10 mL of concentrated H2SO4 and subjected to digestion on a hot plate set at 70 °C until the volume reached 50 mL. Following this process, the samples were moved to a volumetric flask and adjusted to a final volume of 100 mL using deionized water.
After decapitation, gills, kidney, dorsal muscles, liver, and intestine tissues were collected from the fish and subjected to freeze-drying. The tissues were washed three times in deionized water and rinsed in double-deionized water. Subsequently, the tissue samples were dried overnight at 80 °C until completely dry up to constant weights. Approximately 0.5 g of dried weight from each tissue sample was subsequently subjected to digestion with 5 mL of concentrated H2SO4 and placed on a hot plate set at 130 °C until complete desiccation. The resulting mixture was then diluted with 2N HCl to a consistent volume of 10 mL. The quantification of Cd levels was conducted through the utilization of an atomic absorption spectrophotometer (Thermo 6600, Thermo Electron Corporation, Cambridge, UK) after calibration with Cd standard solutions.
Histopathological alterations
After the rearing period, the liver and mid-intestines tissues from the control and treated fish were taken and washed then fixed in 10% neutral buffered formalin for at least 48 h. Fixed samples were subsequently dehydrated utilizing various concentrations of absolute ethanol, followed by embedding in paraffin wax, and subjected to standard histopathological assessment. Sections measuring 5 μm in thickness were generated, stained with hematoxylin and eosin, and scrutinized under a light microscope as outlined by Bancroft and Gamble (2008). The histopathological variations were graded based on the criteria established by Dommels et al. (2007), with each section being assigned a rating of ( −) denoting no changes, ( +) indicating mild alterations, (+ +) representing moderate changes, and (+ + +) signifying severe modifications.
Statistical analysis
The examination of variance homogeneity in the dependent variables was conducted through Levene’s test. Kolmogorov–Smirnov was employed to ascertain normality of data. The utilization of two-way ANOVA was implemented to clarify the impacts of administering Bs-CNPs and Cd exposure, as well as their interaction. Subsequently, the evaluation of significant differences among treatments at P < 0.05 was carried out using Tukey’s HSD test. All statistical analyses were executed utilizing SPSS statistical software version 26 (SPSS, Richmond, VA, USA) following the methodology outlined by Dytham (2011).
Results
Characterization of Bs-CNPs
The TEM observation of Bs-CNPs revealed that the nanoparticles exhibited a relatively homogeneous morphology, characterized by a consistent particle size distribution and spherical shape. Specifically, TEM analysis indicated that the average of Bs-CNPs size was 79 nm (Figs. 1 and 2).
Growth performance
It is noted that the final weight (g), weight gain%, SGR (%/day), and feed intake (g feed/fish) of Nile tilapia fingerlings, in the current study, were significantly (P ≤ 0.05) affected by dietary Bs-CNPs, Cd exposure, and their interaction (Table 2). Initial fish weight and FCR values are not significantly (P > 0.05) changed among different treatments (T1–T9) but fish survival was affected only by dietary Bs-CNPs and Cd exposure (Table 2). However, fish exposed to 0.784 and 0.392 mg Cd/L, respectively, (T2 and T3) notably (P ≤ 0.05) diminished their growth parameters and fish survival compared to the control group (T2–T3 vs T1; Table 2). On the other hand, feeding the fish on 2.0 and 4.0 g Bs-CNPs/kg feed alone (T4–T5) displayed marked (P ≤ 0.05) improvements in the growth indices and fish survival as contrasted to the control group (T1). Interestingly, feeding Cd-intoxicated Nile tilapia on diets with Bs-CNPs supplementation (T6, T7, T8, and T9) revealed significant improvements in their growth parameter as compared with T2 and T3 groups. The Cd toxic effects were minimized by feeding the fish on Bs-CNPs diets and become near the normal control group especially in case of feeding the fish on a 4.0 g Bs-CNPs/kg diet (T9; Tables 2 and 3).
Digestive enzymes
In a parallel way, the activities of proteases, lipase, and α-amylase of mid-intestinal parts of Nile tilapia fingerlings were significantly (P ≤ 0.05) affected by dietary Bs-CNPs, Cd exposure, and their interaction (Table 3). Activities of measured digestive enzymes (proteases, lipase, and α-amylase) were restricted in Cd-exposed fish (T2–T3) compared to the control group (T1; Table 3). Meanwhile, feeding Cd-intoxicated fish on diets with Bs-CNPs supplementation (T6–T9) revealed marked enhancements in the abovementioned digestive enzymes as compared with T2 and T3 groups (Table 3).
The fish behavior
Exposed Nile tilapia fingerlings to Cd toxicity throughout the 60-day experiment (T2–T3) exhibited adverse effects on certain behavior parameters (Table 4). It is noted that feeding time, feeding frequency, and swimming time were decreased in comparing with the control group (T1). Feeding the fish on Bs-CNPs (T4–T5) exhibited no abnormalities in their behavior as the control group (T1). The significant improvement in feeding time, feeding frequency, and swimming time were observed in the T9 group which fed on 4.0 g Bs-CNPs meanwhile exposed to 0.392 mg Cd/L. Overall, the severity of abnormalities went up in the highest Cd concentrations (T2 > T3) as erratic locomotion, loss of appetite, pale gills, skin color, gasping behavior, and sluggish movement. Using of Bs-CNPs in Cd-exposed Nile tilapia appeared to ameliorate the severity of behavioral signs, particularly at the T9 followed by the T8 (Table 4). Fish in T6 and T7 showed moderate effects on loss of appetite, erratic movement, skin color changes, and weak effect in sluggish movement, gasping air, and pale gills (Table 4).
Hematological profile
It is noted that WBC and RBC counts, Hb level, and Hct % were significantly (P ≤ 0.05) affected by dietary Bs-CNPs, Cd exposure, and their interaction as illustrated in Table 5. However, feeding Nile tilapia on Bs-CNP alone (T4–T5) exhibited notable (P < 0.05) increments in WBCs and RBCs counts, Hb level, and Hct % as compared with the control group (T1). On the other hand, significant (P < 0.05) decreases in these hematological indices were seen in Cd-intoxicated fish alone (T2 and T3) in relation to the control group (T1). Interestingly, feeding Nile tilapia fingerlings on Bs-CNPs diets along with Cd exposure showed mild increases in the abovementioned hematological indices, particularly at T9 as compared with T2 and T3 groups (Table 5).
Stress indices
The results of the current experiment evoke that blood cortisol, glucose, AST, ALT, and AChE levels in Nile tilapia fingerlings were significantly (P ≤ 0.05) affected by dietary Bs-CNPs, Cd exposure, and their interaction (Table 6). The current study evoked that exposing Nile tilapia to Cd toxicity alone (T2–T3) led to significant (P < 0.05) rises in blood cortisol, glucose, AST, ALT, and AChE levels (Table 6). Conversely, feed the fish with Bs-CNPs diets alone (T4 and T5) showed no changes in the above-mentioned stress indices compared with the control group (T1). The combination of Bs-CNPs feeding with Cd exposure resulted in slight increases in stress indices, particularly at T9 (Table 6) compared to the Cd exposure alone (T2–T3). This suggests potential protective effects of dietary Bs-CNPs against the toxic effects induced by Cd exposure on Nile tilapia.
Cd residues in fish organs
Regarding the Cd residues in different organs of Nile tilapia fingerlings, it is noted that Cd residues in liver, intestine, gills, kidney, and muscle tissues were significantly (P ≤ 0.05) affected by dietary Bs-CNPs, Cd exposure, and their interaction (Table 7). No Cd residues were detected in different fish organs of Nile tilapia fingerlings fed on the control diet (T1) as well as Bs-CNPs diets alone (T4 and T5) fed groups (Table 7). The levels of Cd residues in those fish tissues were notably (P < 0.05) greater in fish exposed to Cd toxicity alone (T2 and T3) compared to all other experimented groups. It is worth mentioning that feeding Cd-intoxicated Nile tilapia on Bs-CNPs diets (T6, T7, T8, and T9) significantly (P < 0.05) lowered Cd residues in different fish organs, especially fish in the T9 group that showed lowest Cd levels in different fish organs (Table 7). It is also noted that the order of Cd residues in the organs of T2 and T3 fish groups is as follows: intestine > gills > liver > kidney > muscles (Table 7).
Histopathological findings
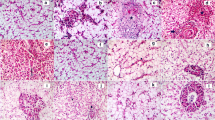
The hepatic tissues of Nile tilapia subjected to concentrations of 0.784 and 0.392 mg Cd/L (T2 and T3) manifested a loss of their distinctive architecture and prominently displayed heightened degrees of hepatocyte vacuolation compared to the control group (T1; Fig. 3). Erythrocytic infiltration of the liver was more considerable in T2 and T3 groups compared with the T1 fish group (Fig. 3). Additionally, the cytoplasm of the hepatocytes in both groups was characterized by densely stained coarse pink granules and vacuoles. On the other hand, feeding fish on Bs-CNPs did not exhibit abnormal structure of hepatic tissues compared with the T1 group (Fig. 3). Table 8 presents the results depicting the histopathological alterations observed in the hepatic tissues across various experimental groups. Healthy liver tissues with typical hexagonal hepatocytes, well-defined nuclei, and a plentiful presence of lipid droplets were observed in the control groups T1, T4, and T5 (Table 8). Various anomalies were observed in the groups of fish treated with Cd (T2 and T3), exhibiting hepatic hemorrhage, nuclear ruptures, necrosis, and infiltration of erythrocytes in blood sinusoids, along with the presence of sizable vacuoles resulting from cellular ruptures (Fig. 3, Table 8). It is noted that feeding Cd-intoxicated fish with Bs-CNPs diets (T6, T7, T8, and T9) minimized those features lowering their score (Fig. 3 and Table 8).
Histopathological alterations (H&E-stained sections) in hepatic tissues of Nile tilapia (O. niloticus) fed with B. subtilis bio-synthesized chitosan nanoparticles (Bs-CNPs) along with exposure to sub-lethal levels of cadmium (Cd) toxicity for 60 days. The marks (H) mean hemorrhage of the hepatocytes; (I) means infiltration of inflammatory cells; (DN) means disintegrated nucleus; (D) means dilated hepatic central vein; (V) means vacuolation of hepatocytes. T1: Fish fed on the control diet with no Cd exposure; T2–T3: Fish exposed to 0.784 and 0.392 mg Cd/L, respectively; T4–T5: Fish fed on 2.0 and 4.0 g Bio-CNPs/kg feed, respectively; T6–T7: Fish fed on 2.0 g Bio-CNPs/kg feed with exposure to 0.784 and 0.392 mg Cd/L, respectively; T8–T9: Fish fed on 4.0 g Bio-CNPs/kg feed with exposure to 0.784 and 0.392 mg Cd/L, respectively
The mid-intestinal tissue of the normal control fish (T1) displayed normal histological structures of the intestinal villi, goblet cells, and normal mucosal layers without any histopathological alterations (Fig. 4). In addition, no histopathological changes in the intestinal mucosal layers were observed in Bs-CNPs-fed fish (T4 and T5). In contrast, moderate improvements in the intestinal tissue histopathology are seen in Cd-exposed fish fed with Bs-CNPs (T6, T7, T8, and T9). Moderate to complete restoration and improvements of the intestinal tissues histopathology are shown in the T8 and T9 fish groups that showed mild inflammatory cell infiltration, low goblet cell hyperplasia with increase in the length of intestinal villi as compared to Cd-exposed fish alone (T2 and T3; Fig. 4). Furthermore, the histopathological lesion scores of the mid-intestinal tissues for each group (Table 9) revealed significant (P < 0.05) increases in lesion scoring in T2 and T3 as compared to the control group (T1) and Bs-CNPs-fed fish groups (T4 and T5), which has no lesions in the mid-intestine tissues. However, Cd-exposed fish in T2 and T3 exhibited moderate to severe enteritis, respectively, in the form of inflammatory cell infiltrations of mucosal and submucosal layers, hemorrhage, shortening of intestinal villi, and edema with degeneration and necrosis of some columnar epithelial cells (Table 9).
Histopathological alterations (H&E-stained sections) in mid-intestine of Nile tilapia (O. niloticus) fed with B. subtilis bio-synthesized chitosan nanoparticles (Bs-CNPs) along with exposure to sub-lethal levels of cadmium (Cd) toxicity for 60 days. The marks (H) mean hemorrhage of the hepatocytes; (I) means infiltration of inflammatory cells; (DN) means disintegrated nucleus; (D) means dilated hepatic central vein; (V) means vacuolation of hepatocytes. T1: Fish fed on the control diet with no Cd exposure; T2–T3: Fish exposed to 0.784 and 0.392 mg Cd/L, respectively; T4–T5: Fish fed on 2.0 and 4.0 g Bio-CNPs/kg feed, respectively; T6–T7: Fish fed on 2.0 g Bio-CNPs/kg feed with exposure to 0.784 and 0.392 mg Cd/L, respectively; T8–T9: Fish fed on 4.0 g Bio-CNPs/kg feed with exposure to 0.784 and 0.392 mg Cd/L, respectively
Discussion
The exposure of different aquatic organisms to Cd pollution results in various adverse physiological and pathological effects depending upon its dose, fish model system, and the length of exposure time (Das et al. 2023; Elgendy et al. 2023; Abdel-Tawwab et al. 2024). Searching for natural bioactive substances for mitigation of the Cd cytotoxicity is an effective and urgent strategy to counter and overcome the Cd toxicity. In parallel, CNPs have a high ability for adsorption of water pollutants and therefore may have a crucial role in water purification (Abou Kana et al. 2013; Olivera et al. 2016). Additionally, dietary normal-sized chitosan and CNPs are widely used in aqua-feeds to highlight the growth, antioxidant capacity, and immune functions in several aquatic animals (Abdel-Ghany and Salem 2020; El-Naggar et al. 2022a, b).
Growth performance
Dietary Bs-CNPs, in the present study, enhanced the growth performance of Nile tilapia in a dose-dependent manner, and the optimum performance was observed in fish fed on 4 g Bs-CNPs/kg feed (T4). The boosted growth in Bs-CNPs-fed fish may be linked to the enhancements of the intestinal histo-morphometry, which improved nutrient absorption and feed utilization. Additionally, dietary CNPs played a positive role in activating the secretion of digestive enzymes (as seen in Table 3) and inhibiting the pathogenic bacteria while promoting beneficial ones in hybrid tilapia (Qin et al. 2014) and Nile tilapia (Abd El-Naby et al. 2019; Abdel-Tawwab et al. 2019). Consequently, digested nutrients can be readily absorbed by intestinal villi, making them available for cellular uptake (Zhang 2019). In this regard, Abdel-Tawwab et al. (2019) found that feeding Nile tilapia on CNPs diets at levels of 1.0 g/kg diet produced the optimum growth performance and feed utilization.
On the other side, significant reductions in final weight, weight gain %, SGR, feed intake, and survival rate were noticed in Cd-intoxicated fish groups in T2 and T3 compared with the control group (T1). The negative impact of Cd exposure on the growth performance of Nile tilapia may be associated to the reduced feed intake, compromised physiological conditions, and the deterioration of intestinal histology in response to the stress induced by Cd toxicity (Abdel-Tawwab et al. 2024). This could be linked to the low feed intake, which was directly impacted with poor fish appetite and low swimming activities. Reduced feeding behavior, potentially influenced by Cd exposure, leads to decreased energy intake by the organism, ultimately affecting its growth and reproduction (Sabullah et al. 2015). The toxicity of Cd was found to elevate metabolic requirements and interfere with regular physiological processes, ultimately resulting in hindered growth (Hogstrand et al. 1996). On the other hand, the retarded growth in Cd-exposed fish may be linked to the down-regulation of the expression of growth genes (IGF-1 and GH) as reported by Abdel-Tawwab et al. (2024). Prior researches have been documented that the growth performance indices were hindered when exposed to Cd concentrations in rainbow trout, Oncorhynchus mykiss (Ricard et al. 1998), guppy, Poecilia reticulate (Miliou et al. 1998), juvenile bull trout, Salvelinus confluentus (Hansen et al. 2002), Nile tilapia (Abdel-Tawwab and Wafeek 2010, 2017; Elgendy et al. 2023), and gilthead seabream, Sparus aurata (Abdel-Tawwab et al. 2024).
The inclusion of Bs-CNPs in fish feeds is proposed as a strategy to mitigate the detrimental effects of Cd toxicity and enhance the growth performance of many fish species. However, feeding Cd-intoxicated fish on Bs-CNPs (T6–T9), especially at T9 (4 g/kg feed) minimized the adverse impacts of Cd toxicity showing significant enhancements in fish growth and hematology along with marked reductions in Cd residues in different fish organs. This suggests potential mitigating effects of dietary Bs-CNPs on chelating and adsorbing the Cd leading to noted reduction in its accumulation in fish tissues. Chitosan is a cationic polysaccharide that has large numbers of hydroxyl and amino groups, which provide high availability of active sites to enhance the adsorption capacity (Rani and Shanker 2020). Therefore, it can simply adsorb pollutants through hydrogen bonding and electrostatic interactions leading to the formation of more complex structures (Chelu et al. 2023; Abdelmeguid et al. 2024). According to Hussein et al. (2021), feeding African catfish CNPs reduced the adverse effects of bisphenol-A toxicity. Naiel et al. (2020) and Ismael et al. (2021) also stated that dietary supplementation of CNPs alleviated imidacloprid toxicity effects on Nile tilapia growth.
Fish behavior
Understanding the relationship between toxicants, physiological disturbances, and behavioral changes in fish is essential for assessing the health of aquatic animals (Iheanacho et al. 2021, 2023). In the current experiment, fish exposed to varying Cd concentrations displayed adverse effects on feeding and swimming behavior compared to the control group. Recording the abnormal behavior in the Cd-intoxicated fish groups in T2 and T3 is linked to neurotoxic effects and the irritation in fish body system. Cd, as a toxic heavy metal, can indeed have detrimental effects on nerve cells and degenerate the axons and myelin sheaths and is also responsible for changes in the synthesis and release of neurotransmitters, which may be associated with the appearance of behavioral changes (Jie et al. 2013; Miranda et al. 2019). The increase in AChE activities in T2 and T3 groups may disrupt the normal functioning of the nervous system in fish. This disturbance can lead to many behavioral alterations, including changes in swimming patterns, feeding behavior, social interactions, and overall activities (Iheanacho and Odo 2020a, b).
Measuring the AChE activity provides valuable insights into the neurotoxic effects of pollutants on fish and helps assess the health status of aquatic organisms (Romani et al. 2003; Vidal-Liñán et al. 2015; Iheanacho and Odo 2020a, b). AChE is an enzyme known for its high sensitivity, especially when subjected to extended periods of exposure to toxic substances such as heavy metals (Romani et al. 2003; Iheanacho and Odo 2020a, b; Eguiraun and Martinez 2023). The functionality of the catalytic triad in AChE has been compromised due to the presence of Cd ions, leading to the inhibition of the enzyme’s activity. As a result, there has been a subsequent rise in the accumulation of acetylcholine, causing alterations in behavior (DeForest et al. 2007; Iheanacho and Odo 2020a, b). Even in low doses, Cd toxicity could elicit early signs of behavioral and morphological changes in fish. Additionally, heavy metal exposure leads to the increase of their concentrations in blood resulting in the disturbance of such ions as Na+, K+, and Ca2+ in the plasma, which almost affects the fish behavior (Sharma et al. 2019). The resting time of fish with low swimming activities was observed in Cd-exposed groups (T2 and T3) and decreased in T8 and T9. Meanwhile, there were no significant changes in fish resting time in T1, T4, and T5.
It is noted that Cd-exposed groups (T2 and T3) displayed severe behavioral changes (loss of appetite, gasping, and sluggish movement), T6 and T7 showed moderate effects, while T8 and T9 had no observable effects. These results may be due to the fact that chitosan has the ability to regenerate peripheral nerve injuries; this regeneration is associated with improvements in the thickness of myelinated nerve fibers and muscle action potentials in nerves (Gong et al. 2009; Wang and Li 2011). The modulation of the behavior in fish exposed to Cd and fed on Bs-CNPs-supplemented diets may be associated with its ability to regulate AChE levels. In a similar study, Primo et al. (2020) asserted that zinc oxide nanoparticles (ZnONPs) exhibited an adsorptive capacity towards Cu ions, resulting in a decrease in their concentrations and ultimately mitigating the detrimental effects of the copper toxicity on the levels of brain AChE. Ahmed et al. (2024) additionally indicated that ZnONPs had the potentiality to mitigate behavioral impairment through the facilitation of neuronal synaptic plasticity in copper-intoxicated African catfish.
Hematological profile
Hematological parameters are used as indices of pollutant-induced stress, such as heavy metals (Elgendy et al. 2023; Abdel-Tawwab et al. 2024; Ahmed et al. 2024). These results were obtained from using different Cd concentrations exposure in T2 and T3 on blood parameters and showed significant decreases in WBCs, RBCs, Hb, and Hct levels compared to the control as well as Bs-CNPs-fed fish groups. These changes in erythrocyte profile suggest the onset of anemia, likely resulting from disruptions in hematopoiesis and erythrocyte damage within hemopoietic organs. Interestingly, our study shows that dietary Bs-CNPs modulated the erythrocyte profile positively. Similar beneficial effects on erythrogram have been reported in Gibel carp and Nile tilapia fed with CNPs-based diets (Chen and Chen 2019). Feeding the fish on Bs-CNPs respectively along with Cd exposure (T6–T9) displayed significant improvements in blood parameters, particularly at T9 as compared to T2 and T3 those were exposed to Cd alone. These findings suggest that Bs-CNPs, especially at higher concentrations, have a positive impact on blood parameters in Cd-exposed fish. This aligns with the idea that CNPs can contribute in improving the erythrogram under conditions of heavy metal exposure (Wang and Li 2011). Sabra et al. (2024) observed that the co-exposure of doxycycline (40 mg/L) along with feeding of Nile tilapia on 0.5% of normal chitosan (CS) and CNPs significantly restored most hematological parameters to be closer to those observed in the control group.
Stress biomarkers
Cortisol and glucose levels represent significant and valuable stress biomarkers in fish (Barton and Iwama 1991; Barton A, 2002; Aliko et al. 2013). The present study demonstrates elevated concentrations of cortisol and glucose in Cd-exposed fish (T2–T3), suggesting an escalating physiological reaction to Cd-induced toxicity. Nevertheless, cortisol-mediated glycogenolysis or gluconeogenesis could also be responsible for hyperglycemia (Wendelaar Bonga 1997; Mommsen et al. 1999; Barton 2002). The outcomes observed herein could potentially be attributed to glycogenolysis, which involves the breakdown of glycogen in muscle and liver tissues, leading to the release of glucose; this process is stimulated by stress-inducing hormones such as cortisol and catecholamines (Wendelaar Bonga 1997; Mommsen et al. 1999; Barton 2002; Iheanacho and Odo 2020a, b). In a similar line, elevated levels of blood glucose were detected in common carp (Cicik and Engin 2005), Nile tilapia (Abdel-Tawwab and Wafeek 2010, 2017), and gilthead seabream (Abdel-Tawwab et al. 2024) exposed to Cd in comparison to the non-exposed control groups.
Exposing Nile tilapia to Cd toxicity (T2 and T3), in the current study, resulted in high levels of serum AST and ALT. This implies the presence of a cytoplasmic enzyme released into the bloodstream as a result of potential liver damage (Bernet et al. 2001; Iheanacho and Odo 2020a, b). The accumulation of Cd in hepatic tissues induces an oxidative stress state leading to a disruption in liver cellular processes; thereby, enhancing membrane permeability and subsequently raising levels of hepatic enzymes in the bloodstream (Abdel-Tawwab et al. 2024). Other studies reported that Cd exposure increased AST and ALT values in Bluespot mullet, Mugil seheli (El-Naga et al. 2005), Mozambique tilapia, O. mossambicus (van Dyk et al. 2007), Nile tilapia (Abdel-Tawwab and Wafeek 2010, 2017), and gilthead seabream (Abdel-Tawwab et al. (2024). This could be attributed to hepatic tissue necrosis that increased cell membrane permeability and caused damage of hepatic tissues.
On the other side, dietary Bs-CNPs declined cortisol, glucose, AST, and ALT levels in the current experiment. This is related to the potential role of dietary Bs-CNPs in adsorbing Cd ions leading to its low accumulation in different organs of Cd-intoxicated Nile tilapia. In a similar study, Sabra et al. (2024) observed that feeding of Nile tilapia on 0.5% of CS or 0.5% of CNPs along with co-exposure to doxycycline (40 mg/L) significantly lowered uric acid, creatinine, AST, and ALT levels in the fish to be near those observed in the control group. Abdel-Tawwab et al. (2010) and (2024) discovered similar outcomes, indicating that the inclusion of dietary yeast (Saccharomyces cerevisiae) in the diets of Galilee tilapia and gilthead bream led to a decrease in the levels of the aforementioned variables in fish exposed to copper and Cd, respectively.
Cd residues in fish organs
Cadmium tends to accumulate differentially in fish tissues, where certain tissues may have higher concentrations of Cd than others (Giacomin et al. 2018; Monier et al. 2023). Moreover, the allocation of amassed metals in different fish organs may differ based on variables including the mode of exposure, level of concentration, duration of exposure to pollutants, and the particular fish species in question. Our findings indicate that exposing Nile tilapia to Cd-polluted water in T2 and T3 groups resulted in a notable rise in Cd residue levels in fish tissues compared to all other groups and the Cd accumulation order was as follows: intestine > gills > liver > kidney > muscles. It demonstrated a high accumulation of Cd in the gastrointestinal tract of fish, with subsequent distribution to gills, kidney, liver, and muscle tissues (Kraal et al. 1995). These findings align with previous researches. Hollis et al. (2001) reported that in rainbow trout juvenile exposed to 0.3 μg Cd/mL for 30 days, kidney tissues accumulated more Cd, followed by gills and liver tissues. De Smet et al. (2001) established a sequence of Cd accrual in common carp subjected to 0.06–0.79 μg Cd/mL for 29 days as kidney > liver > gills. Guardiola et al. (2013) stated that the Cd concentration in liver tissues was greater than the muscle tissues in gilthead seabream. Lee et al. (2019) demonstrated that Cd accumulation in distinct tissues of common carp (Cyprinus carpio) follows the order of intestine > gills > kidney > liver > muscle when fish are exposed to Cd-contaminated water. Abdel-Tawwab et al. (2024) found that Cd accumulation order was liver > gills > muscles of gilthead seabream exposed to 1.0 and 2.0 mg Cd/L for 60 days. Finally, it is probable that the absorbed Cd undergoes translocation to storage organs like the liver or kidney during extended periods of exposure (Annabi et al. 2013). The lowest Cd levels were detected in muscle tissues. In this regard, Monier et al. (2023) observed that the highest Cd residue levels were in liver tissues, while it was the lowest in muscle tissues of grey mullet (Mugil cephalus), red seabream (Pagrus pagrus), and sardine (Sardinella aruita). Once the storage capacities of liver and kidney tissues were saturated, Cd deposition commenced in the muscle tissues of fish. Generally, the accumulation of metals in fish organs can be affected by various factors, including the type of metal ion exposed, metal concentration, and duration of exposure, along with fluctuations in environmental conditions such as temperature, salinity, hardness, and pH of water, all of which can influence the levels of heavy metals accumulation (Monier et al. 2023).
Interestingly, the fish group fed on Bs-CNPs diets with Cd-polluted water (T6–T9), particularly T9 that fed on 4 g/kg feed, exhibited significant decreases in Cd residue levels in fish tissues, reaching levels comparable to the control fish (T1) group. This suggests potential mitigating effects of dietary Bs-CNPs on Cd accumulation in fish tissues. Chitosan is a cationic polysaccharide that has large numbers of hydroxyl and amino groups, which provide high availability of active sites to enhance the adsorption capacity (Rani and Shanker 2020). Therefore, it can simply adsorb pollutants through hydrogen bonding and electrostatic interactions leading to the formation of more complex structures (Chelu et al. 2023; Abdelmeguid et al. 2024). In this view, El-Naggar et al. (2022a, b ) stated that feeding Nile tilapia on chitosan and CNPs reduced the residues of heavy metals including Cd. In a similar study, Abdel-Tawwab and Wafeek (2010) used organic selenium to reduce the Cd residue in the whole-body of Nile tilapia. Abdel-Tawwab et al. (2024) reported that dietary yeast (S. cerevisiae) had the capability to reduce Cd accumulation in the organs of Cd-intoxicated gilthead seabream.
The histopathological alterations of liver and intestine tissues
Histopathology has frequently been utilized as a marker in the evaluation of fish health, given its effectiveness in assessing the impacts of heavy metals on fish tissues (Ahmed et al. 2024). In the present study, no significant alterations were observed in both organs in the case of the control (T1) as well as the Bs-CNPs-fed fish (T4 and T5); meanwhile, marked deterioration in liver and intestine tissues was observed in Cd-intoxicated fish (T2 and T3). The examination of liver tissues from Nile tilapia exposed to Cd revealed various histopathological changes, such as loss of typical cellular organization; increased vacuolation, atrophy, necrosis, and peripheral displacement of nuclei; decreased size of nuclei and nucleoli; indistinguishable cell membranes; increased connective tissue; and the presence of macrophage granulomas or congestion in blood vessels. Related outcomes were observed with other fish species exposed to Cd toxicity (van Dyk et al. 2007; Patnaik et al. 2011; Das et al. 2023). Patnaik et al. (2011) asserted that the exposure of common carp, C. carpio communis, to 1.6 mg/L of CdCl2 for a duration of 4 days resulted in the manifestation of severe necrosis in hepatic cells, pyknotic nuclei, and indistinct cell boundaries across various regions. In the case of freshwater climbing perch (Anabas testudineus) subjected to 1.0 mg Cd/L over a 4-day period, the occurrence of vacuolations with darker nucleoli was limited to a small number of hepatocytes; however, the exposure to 2 mg Cd/L led to the observation of dilated blood capillaries, infiltration of a small number of macrophages, and a higher prevalence of vacuolated hepatocytes (Ahmed et al. 2014). In another study, juvenile Senegalese soles (Solea senegalensis) were exposed to 0.5, 5, and 10 mg Cd/L for 28 days and exhibited hyperemia, conjugation of blood vessels causing sinusoidal swelling and a few small foci in liver tissues (Costa et al. 2013). Guardiola et al. (2013) reported that exposing gilthead seabream to 1.0 mg/L of CdCl2 for 2, 10, or 30 days shows vacuolated, eosinophilic cytoplasm, and a very patent and round nucleus, and these progressive deleterious changes increased with the exposure time. Abdel-Tawwab et al. (2024) found that exposing gilthead seabream to 1.0 and 2.0 mg Cd/L for 60 days caused serious alterations in gills, liver, and kidney tissues, and these alterations increased with the high Cd exposure level.
The intestinal tract of fish plays a crucial role in the processes of nutrient absorption, digestion, metabolism, and immune defense while also maintaining the balance of electrolytes. In the present study, intestine tissues of Cd-intoxicated Nile tilapia (T2 and T3) accumulated more Cd than other fish organs. Numerous pathological alterations are evident based on the concentrations, as demonstrated. In Cd-exposed fish, the intestine displayed focal degenerated villi, swollen villi tips, myelinoid bodies within enterocytes’ cytoplasm, and rodlet cells in the intestinal mucosa of European sea bass, Dicentrarchus labrax (Giari et al. 2007). Younis et al. (2015) observed an increase in degenerated nuclei and apoptotic cells within the intestinal submucosal layer of Cd-intoxicated Nile tilapia. Yu et al. (2021) stated that crucian carp exposed to 0.1 and 0.5 mg Cd/L for 28 days manifested intestinal inflammation; reduced height of enterocytes; heightened mucosal folds, degeneration, necrosis, and atrophic changes in the mucosal folds; and a decrease in the width of the lamina propria.
Interestingly, the dietary supplementation of Bs-CNPs succeeded in alleviating and reducing damages in hepatic and intestinal tissues of Nile tilapia caused by Cd toxicity, especially at T9. These results are due to the potential role of dietary Bs-CNPs in chelating the waterborne Cd where the Cd residue in fish organs especially liver and intestine tissues was significantly reduced to be close to those of the control group (T1). The ability of dietary CNPs to regenerate and restore the normal histological structure of the various body tissues in Cd-exposed fish emphasizes its crucial role in the enhancement and modulation of inflammatory biomarkers and oxidative stress (Elbahnaswy et al. 2021). Sabra et al. (2024) reported that feeding Nile tilapia on CS and CSNPs ameliorated the adverse effects of doxycycline on gills, liver, and kidney tissues. The dietary supplementations of CS and CSNPs to gluten meal-based diets have enhanced the histological architecture of the liver (El-Naggar et al. 2021). Banaee et al. (2015) used chitosan alone and in combination with vitamin C to restore the levels of antioxidant enzymes and minimize the Cd levels in common carp. In a similar study, Ahmed et al. (2024) used dietary ZnONPs to reduce the Cu residues and minimize the tissue alterations in African catfish exposed to Cu toxicity for 60 days.
Conclusion
The current study proved that waterborne Cd exposure negatively impacts growth, hematology, and histopathological appraisal in Nile tilapia fingerlings. Meanwhile, feeding fish on Bs-CNPs diets improved the fish performance and ameliorated the adverse effects and pathological alterations of hepatic and intestinal tissues in Cd-exposed fish. Therefore, this study emphasizes the potential applications of dietary Bs-CNPs (4 g/kg feed) in aquaculture for improving fish health and performance even in the presence of environmental pollutants.
Data availability
No datasets were generated or analysed during the current study.
References
Abd El-Naby FS, Naiel MA, Al-Sagheer AA, Negm SS (2019) Dietary chitosan nanoparticles enhance the growth, production performance, and immunity in Oreochromis niloticus. Aquaculture 501:82–89
Abdel-Ghany HM, Salem ME-S (2020) Effects of dietary chitosan supplementation on farmed fish; a review. Rev Aquacult 12:438–452
Abdelmeguid NE, Ghanem SF, Assem SS, Abou Shabana NM, Ismail RF, Sultan AS (2024) Ameliorative effects of chitosan in water remediation, endocrine disruption and reproductive impairment of Solea solea after exposure to benzo (a) pyrene. Int Aquat Res 16:71–90
Abdel-Tawwab M, Wafeek M (2010) Response of Nile tilapia, Oreochromis niloticus (L.) fed dietary organic selenium to environmental cadmium toxicity. J World Aquacult Soc 41(1):106–114
Abdel-Tawwab M, Wafeek M (2014) Influence of water temperature and waterborne cadmium toxicity on growth performance and metallothionein– cadmium distribution in different organs of Nile tilapia, Oreochromis niloticus (L.). J Therm Biol 45:157–162
Abdel-Tawwab M, Wafeek M (2017) Fluctuations in water temperature affected waterborne cadmium toxicity: hematology, anaerobic glucose pathway, and oxidative stress status of Nile tilapia, Oreochromis niloticus (L.). Aquaculture 477:106–111
Abdel-Tawwab M, Mousa MAA, Mohammed MA (2010) Effect of yeast supplement on the growth and resistance of Galilee tilapia, Sarotherodon galilaeus (L.) to environmental copper toxicity. J World Aquacult Soc 41(2):214–223
Abdel-Tawwab M, Abdel-Razek N, Abdel-Rahman AM (2019) Immunostimulatory effect of dietary chitosan nanoparticles on growth performance of Nile tilapia, Oreochromis niloticus (L.). Fish Shellfish Immunol 88:254–258
Abdel-Tawwab M, Khalil RH, Younis NA et al (2024) Saccharomyces cerevisiae supplemented diets mitigate the effects of waterborne cadmium toxicity on gilthead seabream (Sparus aurata L.): growth performance, haemato-biochemical, stress biomarkers, and histopathological investigations. Vet Res Commun 48(1):69–84
Abou Kana M, Radi M, Elsabee MZ (2013) Wastewater treatment with chitosan nano-particles. Int J Nanotechnol Appl 3:39–50
Ahmed MK, Parvin E, Islam MM, Akter MS, Khan S, Al-Mamun MH (2014) Lead and cadmium-induced histopathological changes in gill, kidney and liver tissue of freshwater climbing perch Anabas testudineus (Bloch, 1792). Chem Ecol 30:532–540
Ahmed SAA, Ibrahim RE, Younis EMA, Abdelwarith A-W et al (2024) Antagonistic effect of zinc oxide nanoparticles dietary supplementation against chronic copper waterborne exposure on growth, behavioral, biochemical, and gene expression alterations of African catfish, Clarias gariepinus (Burchell, 1822). Biol Trace Elem Res. https://doi.org/10.1007/s12011-024-04115-6
Aliko V, Sula E, Gavazaj F, Maxhuni Q, Kastrati D (2013) Evaluation of environmental pollution applying oxidative stress biomarkers bioindicators of water pollution in fish from Sitnica river in Kosovo. Pol J Environ Stud 22:1519–1523
Alonso Á, Valle-Torres G (2018) Feeding behavior of an aquatic snail as a simple endpoint to assess the exposure to cadmium. Bull Environ Contam Toxicol 100:82–88
Annabi A, Said K, Messaoudi I (2013) Cadmium: bioaccumulation, histopathology and detoxifying mechanisms in fish. American J Res Com 1(4):60–79
Banaee M, Mehrpak M, Hagi BBN, Noori A (2015) Amelioration of cadmium-induced changes in biochemical parameters of the muscle of common carp (Cyprinus carpio) by vitamin C and chitosan. Int J Aquat Biol 3:362–371
Bancroft JD, Gamble M (2008) Theory and practice of histological techniques. Churchill Livingstone, Elsevier Health Sciences
Barton BA (2002) Stress in fishes: a diversity of responses with particular reference to changes in circulating corticosteroids. Integr Comp Biol 42:517–525
Barton BA, Iwama GK (1991) Physiological changes in fish from stress in aquaculture with emphasis on the response and effects of corticosteroids. Ann Rev Fish Dis 1:3–26
Behreus AS, Karber L (1953) Determination of LC50. Arch Exp Pathol Pharm 28:177
Bernet D, Schmidt H, Wahli T, Burkhardt-Holm P (2001) Effluent from a sewage treatment works causes changes in serum chemistry of brown trout (Salmo trutta L). Ecotoxicol Environ Saf 48:140–147
Boyd CE (1984) Water quality in warm water fishponds. Auburn University Agriculture Experimental Station, Auburn, AL, USA
Boyd CE, Tucker CS (2012) Pond aquaculture water quality management. Kluwer Academic Publishers, Boston, MA, USA
Brown BA (1980) Hematology: principles and procedures. Lea and Febiger, Philadelphia, PA, USA
Bukola D, Zaid A, Olalekan E, Falilu A (2015) Consequences of anthropogenic activities on fish and the aquatic environment. Poultry, Fisheries & Wildlife Sciences 3(2):1–12
Chatigny, F., 2017. Assessing the efficacy and side effects of lidocaine used as an analgesic on rainbow trout (Oncorhynchus mykiss). PhD. thesis, Department of Biomedical Sciences, Faculty of Veterinary Medicine, University of Prince Edward Island.
Chelu M, Musuc AM, Popa M, Calderon Moreno JM (2023) Chitosan hydrogels for water purification applications. Gels 9(8):664
Chen J, Chen L (2019) Effects of chitosan-supplemented diets on the growth performance, nonspecific immunity and health of loach fish (Misgurnus anguillicadatus). Carbohyd Polym 225:115227
Cicik B, Engin K (2005) The effects of cadmium on levels of glucose in serum and glycogen reserves in the liver and muscle tissues of Cyprinus carpio (L., 1758). Turk J Vet Anim Sci 29:113–117
Costa PM, Caeiro S, Costa MH (2013) Multi-organ histological observations on juvenile Senegalese soles exposed to low concentrations of waterborne cadmium. Fish Physiol Biochem 39:143–158
Das S, Kar I, Patra AK (2023) Cadmium induced bioaccumulation, histopathology, gene regulation in fish and its amelioration – a review. J Trace Elem Med Biol 79:127202
Dawood MAO, Koshio S, Esteban MA (2018) Beneficial roles of feed additives as immunostimulants in aquaculture: a review. Rev Aquacult 10:950–974
De Smet H, De Wachter B, Lobinski R, Blust R (2001) Dynamics of (Cd, Zn)-metallothionein in gills, liver, and kidney of common carp Cyrpinus carpio during cadmium exposure. Aquat Toxicol 52:269–281
DeForest DK, Brix KV, Adams WJ (2007) Assessing metal bioaccumulation in aquatic environments: the inverse relationship between bioaccumulation factors, trophic transfer factors and exposure concentration. Aquat Toxicol 84(2):236–246
Dommels Y, Butts C, Zhu S, Davy M, Martell S, Hedderley D, Barnett M, McNabb W, Roy N (2007) Characterization of intestinal inflammation and identification of related gene expression changes in mdr1a−/− mice. Genes Nutr 2:209–223
Dytham, C., 2011. Choosing and using statistics: a biologist’s guide. Blackwell Science Ltd,
Eguiraun H, Martinez I (2023) Non-linear analyses of fish behaviours in response to aquatic environmental pollutants—a review. Fishes 8:311
Elbahnaswy S, Elshopakey GE, Ibrahim I, Habotta OA (2021) Potential role of dietary chitosan nanoparticles against immunosuppression, inflammation, oxidative stress, and histopathological alterations induced by pendimethalin toxicity in Nile tilapia. Fish Shellfish Immunol 118:270–282
Elgendy MY, Ali SE, Abdelsalam M, Abd ElAziz TH et al (2023) Onion (Allium cepa) improves Nile tilapia (Oreochromis niloticus) resistance to saprolegniasis (Saprolegnia parasitica) and reduces immunosuppressive effects of cadmium. Aquacult Intern 31:1457–1481
El-Husseiny HM, Mady EA, El-Dakroury WA, Zewail MB, Noshy M, Abdelfatah AM, Doghish AS (2022) Smart/stimuli-responsive hydrogels: state-of-the-art platforms for bone tissue engineering. Appl Mater Today 29:101560
El-Naga A, El-Moselhy KM, Hamed MA (2005) Toxicity of cadmium and copper and their effect on some biochemical parameters of marine fish Mugil sheheli. Egypt J Aquat Res 31(2):60–71
El-Naggar MM, Salaah S, El-Shabaka H, Abd El-Rahman F, Khalil M, Suloma A (2021) Efficacy of dietary chitosan and chitosan nanoparticles supplementation on health status of Nile tilapia, Oreochromis niloticus (L). Aquac Rep 19:100628
El-Naggar MM, Medhat F, Taha A (2022) Applications of chitosan and chitosan nanoparticles in fish aquaculture. Egypt J Aquat Biol Fish 26(1):23–43
El-Naggar MM, Salaah S, Suloma A, Khalil MT, Emam WW (2022) Impact of chitosan and chitosan nanoparticles on reducing heavy metals in the Nile tilapia, Oreochromis niloticus. Egypt J Aquat Biol Fish 26(2):859–874
El-Sayed A-FM, Fitzsimmons K (2023) 2023 From Africa to the world—the journey of Nile tilapia. Rev Aquac. 15(Suppl. 1):6–21
El-Sayed A-FM (2019) Tilapia culture, 2nd edn. Academic Press, Elsevier Science Publishing Co. Inc, San Diego, USA
Fesharaki PJ, Nazari P, Shakibaie M, Rezaie S, Banoee M, Abdollahi M, Shahverdi AR (2010) Biosynthesis of selenium nanoparticles using Klebsiella pneumoniae and their recovery by a simple sterilization process. Braz J Microbiol 41(2):461–466
Foster L, Dunn R (1974) Single antibody technique for radioimmunoassay of cortisol in extracted serum or plasma. Clin Chem 20:365
Galindo-Villegas J, Bossier P, Reyes-López FE (2022) Editorial: Oral immune-enhancing research in fish. Front Immunol 13:850026
Giacomin M, Vilarinho GC, Castro KF, Ferreira M, Duarte RM, Wood CM, Val AL (2018) Physiological impacts and bioaccumulation of dietary Cu and Cd in a model teleost: the Amazonian tambaqui (Colossoma macropomum). Aquat Toxicol 199:30–45
Giari L, Manera M, Simoni E, Dezfuli BS (2007) Cellular alterations in different organs of European sea bass Dicentrarchus labrax (L.) exposed to cadmium. Chemosphere 67:1171–1181
Golombieski JI, Marchesan E, Camargo ER, Salbego J et al (2008) Acetylcholinesterase enzyme activity in carp brain and muscle after acute exposure to diafuran. Scientia Agricola 65:340–345
Gong Y, Gong L, Gu X, Ding F (2009) Chitooligosaccharides promote peripheral nerve regeneration in a rabbit common peroneal nerve crush injury model. Microsurgery 29:650–656
Guardiola FA, Cuesta A, Meseguer J, Martínez S et al (2013) Accumulation, histopathology and immunotoxicological effects of waterborne cadmium on gilthead seabream (Sparus aurata. Fish Shellfish Immunol 35:792–800
Hamackova J, Kouril J, Kozak P, Stupka Z (2006) Clove oil as an anaesthetic for different freshwater fish species. Bulgarian J Agr Sci 12(2):185–194
Hamed HS, Ismal SM, Abdel-Tawwab M (2022) Modulatory effects of dietary cinnamon (Cinnamomum zeylanicum) against waterborne lead toxicity in Nile tilapia fingerlings: growth performance, haemato-biochemical, innate immunity, and hepatic antioxidant indices. Aquacult Rep 25:101190
Hansen JA, Welsh PG, Lipton J, Cacela D, Dailey AD (2002) Relative sensitivity of bull trout (Salvelinus confuentus) and rainbow trout (Oncorhynchus mykiss) to acute exposures of cadmium and zinc. Environ Toxicol Chem 21:67–75
Hogstrand C, Verbost PM, Bonga SE, Wood CM (1996) Mechanisms of zinc uptake in gills of freshwater rainbow trout: interplay with calcium transport. Am J Physiol Integr Comp Physiol 270:1141–1147
Hollis L, Hogstrand C, Wood CM (2001) Tissue-specific cadmium accumulation, metallothionein induction, and tissue zinc and copper levels during chronic sublethal cadmium exposure in juvenile rainbow trout. Arch Environ Contam Toxicol 41(4):468–474
Hoseinifar SH, Sun Y-Z, Zhou Z, Van Doan H, Davies SJ, Harikrishnan R (2020) Boosting immune function and disease bio-control through environment-friendly and sustainable approaches in finfish aquaculture: herbal therapy scenarios. Rev Fish Sci Aquac 28:303–321
Hussein NM, Ma Saeed R, Shaheen AS, Hamed HS (2021) Ameliorative role of chitosan nanoparticles against bisphenol-A induced behavioral, biochemical changes and nephrotoxicity in the African catfish, Clarias gariepinus. Egypt J Aquat Biol Fish 25:493–510
Iheanacho SC, Odo GE (2020a) b). Dietary exposure to polyvinyl chloride microparticles induced oxidative stress and hepatic damage in Clarias gariepinus (Burchell, 1822). Environ Sci Pollut Res 27:21159–21173
Iheanacho SC, Odo GE (2020b) Neurotoxicity, oxidative stress biomarkers and haematological responses in African catfish (Clarias gariepinus) exposed to polyvinyl chloride microparticles. Compar Biochem Physiol (part c) 232:108741
Iheanacho SC, Adeolu AI, Nwose R, Ekpenyong J, Offu P, Amadi-Eke A, Iheanacho AC, Ogunji J (2021) Genotoxicity, oxidative stress and lysozyme induction in Clarias gariepinus chronically exposed to water-soluble fraction of burnt tire ash. Ecotoxicology 30(10):1983–1996
Iheanacho SC, Ekpenyong J, Nwose R, Adeolu AI, Offu P, Amadi-Eke A, … Ogunji J (2023). Effects of burnt tire-ash on Na+/K+, Ca2+-ATPase, serum immunoglobulin and brain acetylcholinesterase activities in Clarias gariepinus (Burchell, 1822). Drug and Chemical Toxicology, 46(3), 503–509
Ismael NE, Abd El-Hameed SA, Salama AM, Naiel MA, Abdel-Latif HM (2021) The effects of dietary clinoptilolite and chitosan nanoparticles on growth, body composition, haemato-biochemical parameters, immune responses, and antioxidative status of Nile tilapia exposed to imidacloprid. Environ Sci Pollut Res 28:29535–29550
Jia X, Zhang H, Liu X (2011) Low levels of cadmium exposure induce DNA damage and oxidative stress in the liver of Oujiang coloured common carp Cyprinus carpio var. color. Fish Physiol Biochem 37:97–103
Jie X, Jianmei L, Zheng F, Lei G, Biao Z, Jie Y (2013) Neurotoxic effects of nonylphenol: a review. Wien Klin Wochenschr 125(3–4):61–70
Kraal MH, Kraak MH, de Groot CJ, Davids C (1995) Uptake and tissue distribution of dietary and aqueous cadmium by carp (Cyprinus carpio). Ecotoxicol Environ Saf 31(2):179–183
Lee J-W, Choi H, Hwang U-K, Kang J-C, Kang YJ, Kim KI, Kim J-H (2019) Toxic effects of lead exposure on bioaccumulation, oxidative stress, neurotoxicity, and immune responses in fish: a review. Environ Toxicol Pharmacol 68:101–108
Miliou H, Zaboukas N, Moraitou-Apostolopoulou M (1998) Biochemical composition, growth, and survival of the guppy, Poecilia reticulata, during chronic sublethal exposure to cadmium. Arch Environ Contam Toxicol 35:58–63
Miranda T, Vieira LR, Guilhermino L (2019) Neurotoxicity, behavior, and lethal effects of cadmium, microplastics, and their mixtures on Pomatoschistus microps juveniles from two wild populations exposed under laboratory conditions-implications to environmental and human risk assessment. Int J Environ Res Public Health 16(16):2857
Mohan K, Ravichandran S, Muralisankar T, Uthayakumar V, Chandirasekar R, Seedevi P, Abirami RG, Rajan DK (2019) Application of marine-derived polysaccharides as immunostimulants in aquaculture: a review of current knowledge and further perspectives. Fish Shellfish Immunol 86:1177–1193
Mommsen TP, Vijayan MM, Moon TW (1999) Cortisol in teleosts: dynamics mechanisms of action, and metabolic regulation. Rev Fish Biol Fish 9:211–268
Monier MN, Soliman AM, Al-Halani AA (2023) The seasonal assessment of heavy metals pollution in water, sediments, and fish of grey mullet, red seabream, and sardine from the Mediterranean coast, Damietta. North Egypt Reg Stud Mar Sci 57:102744
Naiel MA, Ismael NE, Abd El-Hameed SA, Amer MS (2020) The antioxidative and immunity roles of chitosan nanoparticle and vitamin C-supplemented diets against imidacloprid toxicity on Oreochromis niloticus. Aquaculture 523:735219
Neetha R, Nikhil J, Chitra KC (2023) A comparative study on the sensitivity of selected freshwater fishes against triclosan exposure. Drug Chem Toxicol 46(6):1116–1129
NRC (2011) National Research Council, Nutrient requirements of fish and shrimp. National Council of the National Academies, Washington DC, USA
Obaiah J, Vivek C, Padmaja B, Sridhar D, Peera K (2020) Cadmium toxicity impact on aquatic organisms oxidative stress: implications for human health safety and environmental aspects. A review. Intl J Sci Res 9:2277–8616
Olivera S, Muralidhara HB, Venkatesh K, Guna VK, Gopalakrishna K, Kumar Y (2016) Potential applications of cellulose and chitosan nanoparticles/composites in wastewater treatment: a review. Carbohyd Polym 153:600–618
Patnaik BB, Howrelia H, Mathews T, Selvanayagam M (2011) Histopathology of gill, liver, muscle and brain of Cyprinus carpio communis L. exposed to sublethal concentration of lead and cadmium. Afr J Biotechnol 10:12218–12223
Primo JO, Bittencourt C, Acosta S, Sierra-Castillo A et al (2020) Synthesis of zinc oxide nanoparticles by ecofriendly routes: adsorbent for copper removal from wastewater. Front Chem 8:571790
Putranto TWC, Andriani R, Munawwaroh A, Irawan B, Soegianto A (2014) Effect of cadmium on survival, osmoregulation and gill structure of the Sunda prawn, Macrobrachium sintangense (de Man), at different salinities. Mar Freshw Behav Physiol 47(5):349–360
Qin C, Zhang Y, Liu W, Xu L, Yang Y, Zhou Z (2014) Effects of chito-oligosaccharides supplementation on growth performance, intestinal cytokine expression, autochthonous gut bacteria and disease resistance in hybrid tilapia Oreochromis niloticus ♀ × Oreochromis aureus ♂. Fish Shellfish Immunol 40(1):267–274
Rani M, Shanker U (2020) Metal oxide-chitosan based nanocomposites for efficient degradation of carcinogenic PAHs. J Environ Chem Eng 8:103810
Reitman S, Frankel S (1957) A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol 28:56–63
Ricard AC, Daniel C, Anderson P, Hontela A (1998) Effects of subchronic exposure to cadmium chloride on endocrine and metabolic functions in rainbow trout Oncorhynchus mykiss. Arch Environ Contam Toxicol 34:377–381
Romani R, Antognelli C, Baldracchini F, De Santis A, Isani G, Giovannini E, Rosi G (2003) Increased acetylcholinesterase activities in specimens of Sparus auratus exposed to sublethal copper concentrations. Chem Biol Interact 145(3):321–329
Sabra MS, Abd El-Aal M, Idriss SKA, Soliman HAM, Salaah SM, Sayed AH (2024) Possible beneficial effects of nano chitosan against doxycycline toxicity in Nile tilapia (Oreochromis niloticus). Aquaculture 587:740855
Sabullah M, Ahmad S, Shukor M, Gansau A, Syed M, Sulaiman M, Shamaan N (2015) Heavy metal biomarker: fish behavior, cellular alteration, enzymatic reaction and proteomics approaches. Int Food Res J 22(2):435–454
Saravanan M, Barik SK, Mubarak Ali D, Prakash P, Pugazhendhi A (2018) Synthesis of silver nanoparticles from Bacillus brevis (NCIM 2533) and their antibacterial activity against pathogenic bacteria. Microb Pathog 116:221–226
Sekiguchi Y, Kohshima S (2003) Resting behaviors of captive bottlenose dolphins (Tursiops truncatus). Physiol Behav 79(4–5):643–653
Sharma M, Thakur J, Verma S (2019) Behavioural responses in effect to chemical stress in fish: a review. Intl J Fish Aquatic Stud 7(1):1–5
Trinder P (1969) Determination of blood glucose using 4-amino phenazone as oxygen acceptor. J Clin Pathol 22:246
van Kampen EJ, Zijlstra WG (1961) Recommendations for haemoglobinometry in Human blood. Br J Haematol 13:71
van Dyk JC, Pieterse GM, van Vuren JH (2007) Histological changes in the liver of Oreochromis mossambicus (Cichlidae) after exposure to cadmium and zinc. Ecotoxicol Environ Saf 66(3):432–440
Vidal-Liñán L, Bellas J, Fumega J, Beiras R (2015) Bioaccumulation of BDE-47 and effects on molecular biomarkers acetylcholinesterase, glutathione-S-transferase and glutathione peroxidase in Mytilus galloprovincialis mussels. Ecotoxicology 24(2):292–300
Wang Y, Li J (2011) Effects of chitosan nanoparticles on survival, growth and meat quality of tilapia. Oreochromis Nilotica Nanotoxicology 5(3):425–431
Wendelaar Bonga SE (1997) The stress response in fish. Physiol Rev 7:591–662
Younis E, Abdel-Warith A-W, Al-Asgah N, Ebaid H (2015) Histopathological alterations in the liver and intestine of Nile tilapia Oreochromis niloticus exposed to long-term sublethal concentrations of cadmium chloride. Chin J Oceanol Limnol 33:846–852
Yu Y, Tong B, Liu Y, Liu H, Yu H (2021) Bioaccumulation, histopathological and apoptotic effects of waterborne cadmium in the intestine of crucian carp Carassius auratus gibelio. Aquac Rep 20:100669
Zhang B (2019) Dietary chitosan oligosaccharides modulate the growth, intestine digestive enzymes, body composition and nonspecific immunity of loach Paramisgurnus dabryanus. Fish Shellfish Immunol 88:359–363
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Mohsen Abdel-Tawwab: conceptualization, validation, supervision, formal analysis, and writing—review and editing. Elsayed A. Eldessouki: methodology and formal analysis. Hoda A. Abd-Ellatieff: investigation, validation, and writing—review and editing. Riad H. Khalil: conceptualization, methodology, and formal analysis. Nasser M. El-Sabbagh: conceptualization, methodology, and formal analysis. Hamida Saleh: validation and writing—original draft. Nehad A. Saleh: methodology and formal analysis. Taghrid M.N. Abdelhakim: methodology and formal analysis. Dalia H. Samak: investigation, validation, supervision, formal analysis, and writing—original draft.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
All methods in the study were performed according to relevant guidelines and regulations. All experiments were carried out according to ARRIVE 2.0 guidelines and were approved by the Institutional Animal Care and Use Committee (IACUC) in the Faculty of Veterinary Medicine, Damanhour University, Egypt (Code: DMU/VetMed-2024/007).
Additional information
Handling Editor: Brian Austin
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abdel-Tawwab, M., Eldessouki, E.A., Abd-Ellatieff, H.A. et al. Antagonistic effects of Bacillus subtilis-derived chitosan nanoparticles on growth performance, stress biomarkers, and histological alterations of cadmium-intoxicated Nile tilapia fingerlings. Aquacult Int (2024). https://doi.org/10.1007/s10499-024-01661-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10499-024-01661-7