Abstract
The intensification of aquaculture industries around the globe has led to increased susceptibility and exposure to diseases. To ensure the well-being of animals and the profitability of the industry, many aquaculture farms resort to antibiotic treatments. However, with the increasing presence of antimicrobial resistance (AMR), it has become important to regulate and limit the use of antibiotics, especially in animal production and regarding the antibiotics that are deemed as critically important for human health by the World Health Organization (WHO). This review describes how AMR mitigation strategies have developed over time in international settings and how they relate to aquaculture. Furthermore, we analyzed how different countries and regions abide by these statutes, as well as the antibiotic standards from a selection of certification schemes. Our results show that the role of aquaculture has been inexplicitly addressed in international guidance documents and that there is a need to further increase the activities of aquaculture operations in combating AMR, with an emphasis on alternatives to antibiotic use. We also found that most countries and regions allow the highest priority-, or critically important antibiotics in aquaculture, which could have detrimental effects on animal, environmental, and public health. As a result, most countries fail to comply with the recommendations and standards set by international organizations and certification schemes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The rapid expansion and intensification of aquaculture, have led to a significant increase in antibiotic use, posing a risk to the global public-, animal-, and environmental health due to antimicrobial resistance (AMR) (Tacon 2020; Lulijwa et al. 2020; Rigos et al. 2021; IFFO 2021; FAO 2020). AMR is defined as the ability of microorganisms like bacteria, viruses, parasites, and fungi to resist the effects of medications that once successfully treated them, making infections harder to control and increasing the risk of disease spread, severe illness, or even death (WHO 2021). The reason for the increased intake of antibiotics is largely due to greater exposure to pathogens as a result of intensiveness and animal density (Rico et al. 2013; Schar et al. 2020). Subsequently, the use and especially, misuseFootnote 1 of antibiotics in aquaculture operations have increased the emergence and rise of AMR (Henriksson et al. 2018; Leung et al. 2013; Reverter et al. 2020; Stentiford et al. 2012; Naylor et al. 2021). Aquaculture is not the only sector misusing antibiotics; the food production industry, particularly animal husbandry, accounts for 70% of global antibiotic use (Nelson et al. 2019; Léger et al. 2021; Byrne et al. 2019; Melo et al. 2021). In 2019, 1.27 million people died globally from AMR, highlighting the importance of appropriate antibiotic use (Murray et al. 2022).
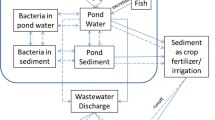
The reliance on antibiotics in aquaculture operations is forecasted to increase as climate change and warmer water temperatures benefit emerging infectious diseases (EIDs) (Reverter et al. 2020; Karvonen et al. 2010; Naylor et al. 2021). The open nature of aquaculture systems exposes adjacent waters, wild fish, flora, sediments, and plankton to antibiotic residues through dissemination (Lulijwa et al. 2020; Debois et al. 2024), with elevated frequencies of antimicrobial-resistant bacteria (ARG) in and near aquaculture environments as a consequence (see Table 1 for examples) (Choi et al. 2020; Gorito et al. 2022; Bondad-Reantaso et al. 2023). As a result, aquaculture is especially receptive to other industries’ effluents as well as contributing to increased antimicrobial residue levels in environments. To minimize the dependence on antibiotics and subsequently, the risk of AMR from spreading in aquatic environments an increased understanding of animal welfare practices is needed (Rodrigues da Costa and Diana 2022, Wright et al. 2023; Colonius and Earley 2013). This includes feeding techniques, feed composition (Herrera et al. 2022), stocking density (Bergqvist and Gunnarsson 2013), and handling of the animals (Ashley 2007).
Intergovernmental organizations (IGOs), non-governmental organizations (NGOs) as well as countries are aiming to combat this issue through regulations, recommendations, and national action plans (NAPs) as well as interdisciplinary collaborations, e.g., the Ad hoc Codex Intergovernmental Task Force on Antimicrobial Resistance which was initiated by the FAO and WHO in 2006 and aimed at creating guidelines to minimize the transmission of antimicrobial resistant bacteria through food and feed (FAO 2007). This involves a One Health approach that recognizes the interconnectedness of public health, animal health, wildlife, plants, and the environment (McEwen and Collignon 2018). The One Health approach aims to “design and implement programs, policies, legislation, and research in which multiple sectors communicate and work together to achieve better public health outcomes” (WHO 2017a). The importance of the interdisciplinary One Health approach is even more apparent as studies show that antibiotic residue in the environment alters soil microbiota and develops resistant genes, worsening control efforts (Pepi and Focardi 2021; Larsson et al. 2023).
Despite national and international commitments to address AMR, the misuse of antibiotics continues (Kirchhelle 2018). One challenge that has been highlighted is the lack of linkages and communication between different sectors (Kasimanickam et al. 2021), thus stressing the importance of multi-sectoral knowledge sharing as highlighted in the One Health approach (WHO 2017a). Absent regulations and enforcement mechanisms in low and lower middle-income countries have also been pointed out as a contributing factor to antibiotic misuse (Schar et al. 2018; Iskandar et al. 2020; Chokshi et al. 2019). Large, seafood-importing markets like the EU, USA, and Japan have much stricter regulations on antibiotic residues in imported food which causes detention and destruction of seafood products in border controls (Geetha et al. 2020; Karunasagar 2020). This also results in significant economic losses for exporting countries (Bondad-Reantaso et al. 2023). According to FAO data, antibiotic residues account for 28% of EU rejections and 20% of US rejections of aquaculture imports, with Viet Nam, China, Thailand, Bangladesh, and Indonesia as the countries whose aquaculture products are most frequently rejected on these grounds (FAO 2024). In many instances, rejections to markets are based on residue levels above the Maximum Residue Limits (MRLs) in the importing countries, and sometimes it is because of residue levels of banned antibiotics. In 2023, the EU rejected 13 shipments of shrimp due to traces of banned antibiotics, the US rejected 51, and Japan 21 shipments (ShrimpAlliance 2024). Most of these shipments were produced in Southeast and East Asia.
Having, and abiding by global standards on antibiotic usage and monitoring would not only decrease the presence of Antibiotic Resistant Genes (ARGs), but it would also increase predictability and transparency for importing countries and revenues for exporting countries. Apart from countries and IGOs, many NGOs, such as food certification bodies, have implemented stewardship programs based on recommendations and information provided by the World Health Organization (WHO) to increase awareness and incentivize better praxis (Umber and Moore 2021). In this paper, we analyze the presence of aquaculture in international commitments to combat the spreading of AMR. Furthermore, we compare IGO and best practice recommendations from two leading aquaculture certification programs (Aquaculture Stewardship Council & Best Aquaculture Practice) with national regulations and policies on antibiotic use for aquaculture operations in 17 of the largest aquaculture-producing countries (EU counted as one as they abide under the same jurisdiction on this). We considered regulations and recommendations on the usage of antibiotics in aquaculture systems, what antibiotic substances are recommended by IGOs and NGOs, and what substances are authorized in each country and the EU to increase transparency and knowledge on the subject. To achieve this, the following two research questions were addressed:
-
1.
How has the presence of AMR been translated into international steering documents and how does it relate to aquaculture?
-
2.
How do the antibiotic regulations in 17 of the largest aquaculture-producing countries/regions compare to these recommendations as defined by the WHO and two leading aquaculture certification programs?
Material and methodology
This study combines information from national steering documents, and research as well as from IGOs and NGOs to create a picture of current standards, recommendations, and regulations on antibiotic use for aquaculture. The 17 countries/regions were selected based on production volume, geographical location, and market influence (see Table 2), as well as by the availability of policies and regulations. For each of these countries, we tried to identify national regulations and policies related to antibiotic use and aquaculture. These policy documents and regulations were identified through search engine inquiries as well as through communications with company representatives, representatives from national agencies, and FAO representatives. Previous research on this topic was found through keyword searches such as aquaculture, antimicrobial resistance (ARM), antimicrobial resistant genes (ARG), antimicrobial resistant bacteria (ARB), policy, regulation, and alternatives to antibiotic use as well as through snowballing. Over 50 databases were used to scan recent scientific literature. The databases utilized for this paper included Elsevier ScienceDirect, JSTOR, PubMed, GoogleScholar, SAGE Journals, Taylor & Francis Online, and Wiley Online Library. The literature search was conducted during the fall of 2021 to summer 2022.
Policy mapping and comparative case study
We used policy mapping to systematically track and analyze antibiotic regulations and policies (Biggs et al. 2006; Helms and Biggs 2007, Bowen and Elizabeth 2020). Policy mapping is a type of content analysis that is used to make objective and replicable inferences from texts or other forms of communication in this particular context (Krippendorff 2019; Bengtsson 2016). More precisely, for this review, we looked at how the presence of AMR has developed in international commitments over time and if/how aquaculture has been included.
To address the second research question, "How do antibiotic regulations in 17 of the largest aquaculture-producing countries compare to the code of conduct defined by the WHO and two leading certification programs?", we employed a comparative case study methodology. This involved analyzing and synthesizing similarities and differences across multiple cases (Goodrick 2014), combining this approach with document analysis. Document analysis is a systematic review or evaluation of documents. It involves examining and interpreting data to elicit meaning, gain understanding, and develop empirical knowledge (Bowen 2009). To streamline and standardize information retrieval and analysis, we screened each document for the same information using five key questions on restriction and use characteristics, specifically addressing sustainability aspects of antibiotic usage in aquaculture. These questions were derived from previous research on effective policy implementations to combat AMR (Lulijwa et al. 2020; Haiping et al. 2021; Martin et al. 2015) and recommendations from intergovernmental organizations (IGOs).
These questions were based on previous research recommendations on successful policy implementations to combat AMR as well as IGO recommendations:
-
1.
Can antibiotics only be used if prescribed by a veterinarian or fish health expert?
-
2.
Is prophylactic use banned?
-
3.
Is using antibiotics as growth enhancers banned?
-
4.
a) What antibiotics are allowed? / b) Are highest-priority antibiotics allowed?Footnote 2
-
5.
Is there a limitation to the number of antibiotic treatments during each production cycle?
Since pharmaceuticals can have different names with the same chemical composition or active ingredients depending on the producer or market in different regions/countries each compound on each listed treatment was cross-referenced to see if the therapeutant was an antibiotic and what type of antibiotic (Merchant et al. 2020). The WHO has a list of important antibiotics for human health which serves as a cross-referencing list for each medicine to see whether or not it is listed as the highest critically important antibiotic or not (WHO 2018). Furthermore, both the ASC and BAP refer to this list in their standards to minimize antibiotic use in aquaculture.
Results
AMR on international agendas
Figure 1 presents a timeline showing the emergence of AMR in global agreements. The risk of using important antibiotics for human health in animal husbandry was first raised by the Swann Commission in 1969 (Swann et al. 1969). Sweden was the first country to ban antibiotics as growth enhancers in 1986 (Wierup 2001), followed by an EU ban in 2006 (Castanon 2007). The WHO recommended this ban in 1997 (WHO 1997), and in 2000, it adopted global principles to contain AMR in food-producing animals, emphasizing the restriction of critical antibiotics and good animal health management (WHO 2000).
The first expert committee meetings involving WHO, FAO, and the World Organisation for Animal Health (WOAH)Footnote 3 in 2003 and 2004 established a tripartite collaboration to combat AMR (FAO/WHO/OIE 2003, FAO/WHO/OIE 2018). This led to the creation of the first list of Critically Important Antibiotics (CIA) for human health (WHO 2017b) and for animal health (FAO/WHO/OIE 2004). In 2006, a report recommended that aquaculture follow existing global principles on antibiotic use (FAO/OIE/WHO 2006) (see Table 3).
Since then, multi-sectoral collaborations have emphasized the One Health approach (Evans and Leighton 2014), leading to the WHO's 2015 Global Action Plan to address AMR, including policies on antimicrobial use and reducing non-treatment uses (WHO 2015). In 2017, the UN General Assembly created the Ad hoc Interagency Coordination Group (IACG) on AMR, highlighting the need for a One Health approach and listing major concerns across humans, animals, food, plants, the environment, and water (IACG 2019).
The IACG report suggested strengthened accountability, global governance, and comprehensive national action plans to prevent AMR spread (IACG 2019). Since its inception, the tripartite has become a quadripartite with the inclusion of the United Nations Environmental Programme (UNEP). In their latest strategy, they emphasize individual countries' evidence-based One Health responses (FAO/WHO/OIE/UNEP 2022). Key concerns include mass medication of animals and unnecessary use of critical medicines, leading to mitigation steps like reducing antimicrobial use in food production and restricting medically important antimicrobials.
By adopting the One Health approach, aquaculture has been increasingly included in strategies to prevent AMR, highlighting the need for its inclusion alongside terrestrial animal husbandry.
National regulations compared to IGO & certification standards
The 17 countries analyzed in this paper all had significant aquaculture production and are exporting countries (see Table 2).
Most national antibiotic practice policies reviewed were issued by the Food and Drug Administration or the national veterinary institute of each country (see Appendix I for a list of documents). Two significant aquaculture-producing countries, Bangladesh and Egypt, lacked national antibiotic policies to the best of our knowledge. For Russia there was no specific information regarding antibiotics in aquaculture, but rather a list of all therapeutic substances available for animal husbandry in general. In India’s case, they have a list of banned antibiotics for shrimp farming and no official list of approved antibiotics (CAA 2022). Subsequently, we concluded that non-listed antibiotics are okay to use. The results were based on information from the regulations and policies listed in Appendix I. Table 3 summarizes the findings from each actor and makes the results easily comparable. Table 4 answers question 4a from the material and methodology section (also below).
-
Q1. Can antibiotics only be used if prescribed by a veterinarian or fish health expert?
-
Q2. Is prophylactic use of antibiotics banned?
-
Q3. Is using antibiotics as growth enhancers banned?
-
Q4. A) What antibiotic compounds are available for aquaculture? (See Table 3) / B) Are highest-priority antibiotics prohibited?
-
Q5. Are there limitations to the number of antibiotic treatments during each production cycle?
-
Q1. All countries and regions analyzed in this project require a prescription by a veterinarian or fish health expert before using antibiotics. As is the case for the UN bodies and certification programs studied in this paper.
-
Q2. Using antibiotics prophylactically for food-producing animals is one of the main issues raised by the WHO and FAO (WHO 2017b, 2015). Reducing this type of use is one of their main strategic goals in their efforts to combat AMR and is regarded as unnecessary as good animal health management should not be replaced by prophylactic antibiotic treatments (WHO 2000). This, however, seems to be difficult to implement and prophylactic treatments using antibiotics have not been banned in 8 countries in this study (Table 3). This issue is also raised by BAP and ASC and certified seafood cannot be treated prophylactically by antibiotics.
-
Q3. Using antibiotics, especially medically important antimicrobials as growth enhancers is another issue raised by the WHO and FAO (WHO 2017b, 2015). This type of usage is seen as irresponsible and is also prioritized as a main goal in their strategies to combat AMR. Most countries observed in this paper have policies and regulations against this. Each organization analyzed for this paper has a clear policy against this type of use.
-
Q4 a) The latest WHO list of critically important antimicrobials was published in 2018 (WHO 2018). It defines antimicrobials that are of the highest importance, critically important, and highly important for human medicine. These antimicrobials should not be used in food-producing industries unless there is no other option (WHO 2017b, 2015). As this analytical question was divided in two, we first present what antibiotics on that WHO CIA list are authorized for aquaculture in each country/region (Table 4).
-
Q4 b) Each country and region allow for medically important antibiotics to be used. However, the industry on the Faroe Islands uses no antibiotics even if there are no regulations against it (MFNR, 2020). Canada and the US do not allow any antimicrobials listed as critically important or highest priority (FSW 2020; GC 2010). Australia has no list of official antibiotics; it is case-dependent and is decided by the veterinarian (APVMA 2014). The Faroe Islands use no antibiotics in their aquaculture and Norway uses little to none (NORM/NORM-VET 2021, MFNR 2020).
-
Q5. None of the countries had any restrictions on the number of antimicrobial treatments per production cycle. However, the two certification programs have put a limit to three in most standards.
Discussion
Mitigating the spreading of AMR and reducing overall antibiotic use has been central for international organizations like the FAO, WHO, and WOAH over the last twenty-plus years. The recommendations on prudent use of antibiotics have remained unchanged throughout this period, with a focus on terminating antibiotic use for growth promotion and prophylactic use as well as ceasing the use of antibiotics that are of critical importance for human health. Even though these recommendations have been around for the last twenty years, many countries still fail to adopt laws to regulate this type of use. Some aspects are difficult to remove completely, such as prophylactic use for some healthy animals as sick individuals cannot easily be removed from aquaculture sites (Rigos et al. 2021). The difference between regulated and unregulated prophylactic use is that a diagnosis must be issued before antibiotics are applied in countries with regulations against prophylactic use.
A significant concern is that 13 out of the 17 countries allowed for at least one of the antibiotics listed as critically important or of the highest importance for human health (Table 4). Natural variations and local conditions play an important role in how antibiotics are used. However, through proper health management plans, it should be possible to reduce the use of antibiotics and avoid using critically important antibiotics. In some countries, e.g., Australia and the UK veterinarians can prescribe treatments that are not included in the official list of authorized antibiotics (VMD 2021b; APVMA 2014). In Australia, there is no official list of authorized antibiotics but is instead always case-dependent (APVMA 2014). In the UK there is a list, but through the cascade act veterinarians are allowed to prescribe unauthorized products if they can motivate why that would be the best course of action and if there are no other treatments (VMD 2021a). An example of unauthorized antibiotics that have been used in UK aquaculture is oxolonic acid (a quinolone, listed as the highest priority) and amoxicillin (a penicillin) (VMD 2021b).
The certification schemes, IGOs, and highest-performing countries have many similarities but differ in that the certifications have limits on the number of treatments per production cycle. The ASC and BAP have both limited the number of treatments to three for most species including salmon, seabass, flatfish, tilapia, and tropical marine finfish (ASC 2019e, 2019d, 2019b, 2019a, 2019c; BAP 2021). The logic behind having a maximum number of treatments is to incentivize producers to improve fish health management and it is argued that a well-managed farm should not need more than three treatments per production cycle (ASC 2019f).
The recommendations and standards set up by the certification programs and by the UN agencies aim to be general and applicable all over the globe. This indicates that these goals and standards should be attainable for all and are not restricted to only one geographical region or cultured species. Thus, countries should increase the stringency in their efforts to combat the spread of AMR and to better match the WHO, FAO, and WOAH recommendations, which in fact is their responsibility as all countries in this study are members of the UN. In some cases, like the Faroe Islands and Norway, the industry is outperforming the regulations by using little to no antimicrobials (NORM/NORM-VET 2021, MFNR 2020). However, as stated in the introduction, this paper analyzes the regulations and policies and not the actual performance of the industry. Both regulations and policies should be ambitious as well as reasonable, which is what the UN and certification programs’ best practice recommendations are striving towards. A ban on antibiotic use in aquaculture is not morally warranted due to how it would impact animal welfare (Karavolias et al. 2018). However, there is a need to increase awareness about how to minimize use and move away from using critically important antibiotics by improving animal welfare practices (Wright et al. 2023). This could be done by strengthening the presence of animal welfare in policies and strategies (Pinillos et al. 2016). Enforcing antibiotic regulations is also challenging in regions with many small-scale farms and in developing countries that lack surveillance resources. A total ban also increases the risks of “backyard” use production, with incorrect and inefficient use of antibiotics (Kirchhelle 2018). It is important to increase the level of enforcement of regulations and policies in countries where this is an issue (Schar et al. 2018). Thus, through improving enforcement methods, increasing the relevance of animal welfare in policies, and through higher monitoring capacities countries could make important strides in mitigating the risk of AMR development. Furthermore, an increased emphasis on knowledge sharing and collaborations with different stakeholders regarding alternatives to antibiotic treatments would be beneficial, such as vaccination, bacteriophages, quorum quenching, probiotics and prebiotics, chicken egg yolk antibody and medicinal plant derivative (Bondad-Reantaso et al. 2023).
Conclusion
This paper analyzed 17 countries (including the EU) regulations on antibiotic use for aquaculture production, the development of UN recommendations for reducing risks of AMR, and two certification programs’ standards on antibiotic use. The findings showed that several countries had appropriate regulations in place and international commitments seem to have affected both their policies and regulations. However, there is still room for improvement and there is a need for countries to increase the stringency in their antibiotic regulations, most notably relating to prophylactic use, what antibiotics are authorized, and the number of treatments. The FAO/WHO/WOAH strategies state that improving animal health management (i.e., animal welfare) would result in reduced use of antibiotics and the ASC argues that a well-managed farm should not need more than three antibiotic treatments per production cycle. Arguably, three treatments are still a rather high number which through proper animal health management practices could be limited further. As a result, one important steppingstone in minimizing antibiotic use is to have a thorough prescriptive management system in place with continuous analysis of animal and environmental health in the farm. This includes but is not limited to, using pre- and probiotics (Bondad-Reantaso et al. 2023), re-assessing and optimizing feed composition (Herrera et al. 2022), optimizing stocking density (Bergqvist and Gunnarsson 2013), and limiting human interaction (handling) (Ashley 2007).
An increased knowledge about how regulations on antibiotic use differ between countries is important as it indicates where the capacity and understanding may be missing.
Data availability
No datasets were generated or analysed during the current study.
Notes
Examples of misuse: Prophylactic use, growth promotion Durso and Cook 2014. Impacts of antibiotic use in agriculture: what are the benefits and risks? Curr Opin Microbiol, 19, 37–44, Kirchhelle 2018. Pharming animals: a global history of antibiotics in food production (1935–2017). Palgrave Communications, 4, 96, Van Boeckel et al. 2015. Global trends in antimicrobial use in food animals. Proceedings of the National Academy of Sciences, 112, 5649. And incomplete courses of treatment Martin et al. 2015. Antibiotics Overuse in Animal Agriculture: A Call to Action for Health Care Providers. Am J Public Health, 105, 2409–10., wrong dosage or antibiotic, and exposure to antibiotic residues Arsène et al. 2022. The public health issue of antibiotic residues in food and feed: Causes, consequences, and potential solutions. Vet World, 15, 662–671.
As defined by the WHO https://apps.who.int/iris/bitstream/handle/10665/325036/WHO-NMH-FOS-FZD-19.1-eng.pdf.
Previously the OIE.
References
Abdelazeem MA, Mahmoud M, Elayaraja S, Fatma MY, Mona HA, Ali WE-K, Helal FH, Wael NH (2020) Emerging MDR-Pseudomonas aeruginosa in fish commonly harbor oprL and toxA virulence genes and bla TEM, bla CTX-M, and tetA antibiotic-resistance genes. Sci Rep 10:1–12
APVMA (2014) Quantity of Antimicrobial Products Sold for Veterinary use In Australia. Canberra, Australia: Australian Pesticides and Veterinary Medicines Authority
Arsène MMJ, Davares AKL, Viktorovna PI, Andreevna SL, Sarra S, Khelifi I, Sergueïevna DM (2022) The public health issue of antibiotic residues in food and feed: Causes, consequences, and potential solutions. Vet World 15:662–671
ASC (2019a) ASC Flatfish Standard Version 1.0 – June 2019. Utrecht, Netherlands: Aquaculture Stewardship Council
ASC (2019b) ASC Freshwater Trout Standard Version 1.2. Utrecht, Netherlands: Aquaculture Stewardship Council
ASC (2019c) ASC Seabass, Seabream and Meagre Standard Version 1.1. Utrecht, Netherlands: Aquaculture Stewardship Council
ASC (2019d) ASC Tilapia Standard Version 1.2. Utrecht, Netherlands: Aquaculture Stewardship Council
ASC (2019e) ASC Tropical Marine Finfish Standard Version 1.0. Utrecht, Netherlands: Aquaculture Stewarship Council
ASC (2019f0 Salmon Standard 1.3. Utrecht, NL: Aquaculture Stewardship Council
Ashley PJ (2007) Fish welfare: Current issues in aquaculture. Appl Anim Behav Sci 104:199–235
BAP (2021) BAP Farm Standard. Issue 3.0 – 01-March-2021. Portsmouth, NH: Best Aquaculture Practices
Bengtsson M (2016) How to plan and perform a qualitative study using content analysis. NursingPlus Open 2:8–14
Bergqvist J, Gunnarsson S (2013) Finfish Aquaculture: Animal Welfare, the Environment, and Ethical Implications. J Agric Environ Ethics 26:75–99
Biggs, Selden Helms, Leila B (2006) The practice of American public policymaking. Armonk, N.Y. ; London, England : M.E. Sharpe
Bondad-Reantaso MG, Mackinnon B, Karunasagar I, Fridman S, Alday-Sanz V, Brun E, Le Groumellec M, Li A, Surachetpong W, Karunasagar I, Hao B, Dall’occo A, Urbani R, Caputo A (2023) Review of alternatives to antibiotic use in aquaculture. Rev Aquac 15(4):1421–51
Bowen G (2009) Document Analysis as a Qualitative Research Method. Qual Res J 9:27–40
Bowen E, Elizabeth L (2020) An Introduction to Policy Mapping Methodology to Inform Policy Research and Practice. Society for Social Work and Research 24th Annual Conference - Reducing Racial and Economic Inequality, January 17th 2020 Washington DC. Society for Social Work and Reseach. https://sswr.confex.com/sswr/2020/webprogram/Session10675.html
Byrne MK, Miellet S, McGlinn A, Fish J, Meedya S, Reynolds N, Van Oijen AM (2019) The drivers of antibiotic use and misuse: the development and investigation of a theory driven community measure. BMC Public Health 19:1425
CAA (2022) List of Antibiotics and other pharmacologically active substances banned for using in shrimp aquaculture [Online]. Channai: Coastal Aquaculture Authority. Available: https://caa.gov.in/uploaded/doc/Pharmacologically.pdf [Accessed November 3rd 2023]
Castanon JIR (2007) History of the Use of Antibiotic as Growth Promoters in European Poultry Feeds. Poult Sci 86:2466–2471
Choi S, Sim W, Jang D, Yoon Y, Ryu J, Oh J, Woo J-S, Kim YM, Lee Y (2020) Antibiotics in coastal aquaculture waters: Occurrence and elimination efficiency in oxidative water treatment processes. J Hazard Mater 396:122585
Chokshi A, Sifri Z, Cennimo D, Horng H (2019) Global contributors to antibiotic resistance. J Global Infect Dis 11:36–42
Colonius TJ, Earley RW (2013) One welfare: a call to develop a broader framework of thought and action. J Am Vet Med Assoc 242:309–310
RodriguesdaCosta M, Diana A (2022) A Systematic Review on the Link between Animal Welfare and Antimicrobial Use in Captive Animals. Animals (Basel) 12(8):1025
Debois ABL, Henriksson P, Luthman O, Troell M, Green D (2024) Aquaculture requires special consideration in National Action Plans for Antimicrobial Resistance. In: UNIVERSITY, S. (ed.)
Durso LM, Cook KL (2014) Impacts of antibiotic use in agriculture: what are the benefits and risks? Curr Opin Microbiol 19:37–44
Evans BR, Leighton FA (2014) A history of One Health. Rev Sci Tech 33:413–420
FAO (2007) Ad hoc Codex Intergovernmental Task Force on Antimicrobial Resistance (CX-804) [Online]. Seoul, Republic of Korea: Food and Agriculture Organization of the United Nations. Available: https://www.fao.org/unfao/govbodies/gsb-subject-matter/statutory-bodies-details/en/c/357/?no_cache=1 [Accessed June 26 2024]
FAO (2020) The State of World Fisheries and Aquaculture 2020. The State of World Fisheries and Aquaculture (SOFIA). Rome, Italy: Food and Agriculture Organization of the United Nations
FAO (2024) Antimicrobial Resistance: What is it? [Online]. Rome, Italy: Food and Agriculture Organization of the United Nations. Available: https://www.fao.org/antimicrobial-resistance/background/what-is-it/en/ [Accessed April 9th 2024]
FAO/OIE/WHO (2006) Antimicrobial Use in Aquaculture and Antimicrobial Resistance. Seoul, South Korea: Food and Agriculutre Organization/ World Organisation for Animal Health/ World Health Organisation
FAO/WHO/OIE (2003) Expert Workshop on Non-Human Antimicrobial Usage and Antimicrobial Resistance: Scientific assessment. In: HEALTH, F. A. A. O. O. T. U. N. W. H. O. W. O. F. A. (ed.). Geneva, Switzerland Food and Agriculture Organization of the United Nations; World Health Organization; World Organization for Animal Health
FAO/WHO/OIE (2004) Second Joint FAO/OIE/WHO Expert Workshop on Non-Human Antimicrobial Usage and Antimicrobial Resistance: Management options In: HEALTH, F. A. A. O. O. T. U. N. W. H. O. W. O. F. A. (ed.) 2 ed. Oslo, Norway: Food and Agriculutre Organization of the United Nations; World Health Organization; World Organization for Animal Health
FAO/WHO/OIE (2018) Critically Important Antimicrobials for Human Medicine. In: HEALTH, F. A. A. O. O. T. U. N. W. H. O. W. O. F. A. (ed.) 6 ed. Geneva, Switzerland Food and Agriculture Organization of the United Nations; World Health Organization; World Organization for Animal Health.
FAO/WHO/OIE/UNEP (2022) Strategic Framework for collaboration on antimicrobial resistance. Geneva, Switzerland Food and Agriculture Organization of the United Nations; World Health Orgnaization; World Organization for Animal Health; United Nations Environmental Program
FSW (2020) Approved Drugs for Use in Aquaculture [Online]. Washington DC: U.S. Fish and Wildlife Service. Available: https://www.fws.gov/sites/default/files/documents/3rd-Edition-Quick-Reference-Guide-to-Approved-for-Use-in-Aquaculture-Drugs.pdf [Accessed May 2nd 2023].
GC (2010) List of Veterinary Drugs that are Authorized for Sale by Health Canada for Use in Food-Producing Aquatic Animals - Health Canada. In: CANADA, G. O. (ed.). Ottawa, Canada
Geetha R, Ravisankar T, Patil PK, Avunje S, Vinoth S, Sairam CV, Vijayan KK (2020) Trends, causes, and indices of import rejections in international shrimp trade with special reference to India: a 15-year longitudinal analysis. Aquacult Int 28:1341–1369
Goodrick D (2014) Comparative Case Studies. Methodological Briefs: Impact Evaluation 9. Florence, Italy: UNICEF Office of Research. https://www.betterevaluation.org/sites/default/files/Comparative_Case_Studies_ENG.pdf
Gorito AM, Ribeiro ARL, Rodrigues P, PereirA MFR, Guimarães L, Almeida CMR, Silva AMT (2022) Antibiotics removal from aquaculture effluents by ozonation: chemical and toxicity descriptors. Water Res 218:118497
Haiping L, Jiangyue W, Fanping M, Aifeng L (2021) Immunochromatographic assay for the detection of antibiotics in animal-derived foods: A review. Food Control 130:108356
Helms L, Biggs S (2007) Policy Mapping: A New Framework for Teaching Policymaking and Policy Design through Case Studies. J Public Aff Educ 13:565–584
Henriksson P, Rico A, Troell M, Klinger D, Buschmann A, Saksida S, ChadagZhang MW (2018) Unpacking factors influencing antimicrobial use in global aquaculture and their implication for management: a review from a systems perspective. Sustain Sci 13:1105–1120
Herrera M, Costas B, Gisbert E (2022) Editorial: Animal welfare, Volume I: Animal welfare in aquaculture - Physiological basis and recent findings. Front Physiol 13:1097913–1097913
IACG (2019) NO TIME TO WAIT: SECURING THE FUTURE FROM DRUG-RESISTANT INFECTIONS. Switzerland Interagency Coordination Group on Antimicrobial Resistnace, Geneva
IFFO (2021) Aquaculture: Fed and unfed production systems [Online]. London, UK: The Marine Ingridients Organisation Available: https://www.iffo.com/aquaculture-fed-and-unfed-production-systems#:~:text=The%20FAO%20figures%20suggest%20that,of%20fed%20aquaculture%20production%20currently. [Accessed May 16th 2022]
Iskandar K, Molinier L, Hallit S, Sartelli M, Catena F, Coccolini F, Hardcastle TC, Roques C, Salameh P (2020) Drivers of antibiotic resistance transmission in low-and middle-income countries from a “one health” perspective—a review. Antibiotics (basel) 9:1–23
Jang HM, Kim YB, Choi S, Lee Y, Shin SG, Unno T, Kim YM (2018) Prevalence of antibiotic resistance genes from effluent of coastal aquaculture. South Korea Environ Pollut 1987(233):1049–1057
Karavolias J, Salois MJ, Baker KT, Watkins K (2018) Raised without antibiotics: Impact on animal welfare and implications for food policy. Transl Anim Sci 2:337–348
Karunasagar I (2020) Review of National Residue Control Programme for Aquaculture Drugs in Selected Countries. Asian Fish Sci 33:62–74
Karvonen A, Rintamäki P, Jokela J, Valtonen ET (2010) Increasing water temperature and disease risks in aquatic systems: Climate change increases the risk of some, but not all, diseases. Int J Parasitol 40:1483–1488
Kasimanickam V, Kasimanickam M, Kasimanickam R (2021) Antibiotics Use in Food Animal Production: Escalation of Antimicrobial Resistance: Where Are We Now in Combating AMR? Medical Sciences (basel) 9:14
Kirchhelle C (2018) Pharming animals: a global history of antibiotics in food production (1935–2017). Palgrave Commun 4:96
Krippendorff K (2019) Content analysis. Sage publications, Inc. https://doi.org/10.4135/9781071878781
Larsson DGJ, Gaze WH, Laxminarayan R, Topp E (2023) AMR, One Health and the environment. Nat Microbiol 8:754–755
Léger A, Lambraki I, Graells T, Cousins M, Henriksson PJG, Harbarth S, Carson C, Majowicz S, Troell M, Parmley EJ, Jørgensen PS, Wernli D (2021) AMR-Intervene: a social-ecological framework to capture the diversity of actions to tackle antimicrobial resistance from a One Health perspective. J Antimicrob Chemother 76:1
Leung TLF, Bates AE, Dulvy N (2013) More rapid and severe disease outbreaks for aquaculture at the tropics: implications for food security. J Appl Ecol 50:215–222
Lin X, Tan A, Deng Y, Liu W, Zhao F, Huang Z (2023) High occurrence of antibiotic resistance genes in intensive aquaculture of hybrid snakehead fish. Front Marine Sci 9:1088176
Lulijwa R, Rupia EJ, Alfaro AC (2020) Antibiotic use in aquaculture, policies and regulation, health and environmental risks: a review of the top 15 major producers. Rev Aquac 12:640–663
Martin MJ, Thottathil SE, Newman TB (2015) Antibiotics Overuse in Animal Agriculture: A Call to Action for Health Care Providers. Am J Public Health 105:2409–2410
Mcewen SA, Collignon PJ (2018) Antimicrobial Resistance: a One Health Perspective. Microbiol Spectr 6:521–47
Melo MCR, Maasch JRMA, De La Fuente-Nunez C (2021) Accelerating antibiotic discovery through artificial intelligence. Commun Biol 4:1050
Merchant L, Lutter R, Chang S (2020) Identical or similar brand names used in different countries for medications with different active ingredients: a descriptive analysis. BMJ Qual Saf 29:988–991
MFNR (2020) Aquaculture – Legislation and management [Online]. Tórshavn, faroe Islands: ministry of fisheries and natural resources. Available. https://www.faroeseseafood.com/fishery-aquaculture/aquaculture-legislation-and-management/. Accessed 30 Jan 2020
Murray CJL, Ikuta KS, Swetschinski L, Robles Aguilar G, Gray A, Han C, Bisignano C, Rao P, Wool E, Johnson SC, Chipeta MG, Fell F, Hackett S, Haines-Woodhouse G, Kashef Hamadani BH, Kumaran EAP, Mcmanigal B, Agarwal R, Akech S, Amuasi J, Andrews J, Aravkin A, Ashley E, Bailey F, Baker S, Basnyat B, Bekker A, Boonkasidecha S, Chansamouth V, Chaurasia S, Chiurchiù S, Chowdhury F, Cook AJ, Cressey TR, Criollo-Mora E, Cunningham M, Darboe S, Day NPJ, De Luca M, Dokova K, Dunachie SJ, Eckmanns T, Fisher-Pearson N, Forrest K, Garrett D, Gastmeier P, Giref AZ, Gupta V, Haller S, Holm M, Hopkins S, Iregbu KC, Jacobs J, Jarovsky D, Javanmardi F, Khorana M, Kobeissi E, Lim C, Limmathurotsakul D, Lunn M, Nakamura T, Newton P, Novotney A, Nwakanma D, Obiero CW, Olivas-Martinez A, Ooko E, Ortiz-Brizuela E, Perrone C, Plakkal N, Raad M, Ramdin T, Riddell A, Roberts T, Roca A, Rudd KE, Russell N, Schnall J, Scott JAG, Shivamallappa M, Sifuentes-Osornio J, Steenkeste N, Stewardson AJ, Stoeva T, Tasak N, Thaiprakong A, Turner C, Van Doorn HR, Velaphi S, Vongpradith A, Vu H, Walsh T, Waner S, Wozniak T, Zheng P, Sartorius B, Stergachis A, Moore C, Dolecek C, Naghavi M (2022) Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. The Lancet (British Edition) 399:629–655
Naylor RL, Hardy RW, Buschmann AH, BushCaoKlingerLittleLubchencoShumwayTroell SRIDHDCJSEM (2021) A 20-year retrospective review of global aquaculture. Nat 591:551–563
Nelson DW, Moore JE, Rao JR (2019) Antimicrobial resistance (AMR): significance to food quality and safety. Food Qual Saf 3:15–22
Nonaka L, Maruyama F, Suzuki S, Masuda M (2015) Novel macrolide-resistance genes, mef(C) and mph(G), carried by plasmids from Vibrio and Photobacterium isolated from sediment and seawater of a coastal aquaculture site. Lett Appl Microbiol 61:1–6
NORM/NORM-VET (2021) Usage of Antimicrobial Agents and Occurrence of Antimicrobial Resistance in Norway. Tromso/Oslo: NORM/NORM-VET
Oviedo-Bolaños K, Rodríguez-RodrígueZ JA, Sancho-Blanco C, Barquero-Chanto JE, Peña-Navarro N, Escobedo-Bonilla CM, Umaña-Castro R (2021) Molecular identification of Streptococcus sp. and antibiotic resistance genes present in Tilapia farms (Oreochromis niloticus) from the Northern Pacific region. Costa Rica Aquaculture Int 29:2337–2355
Pepi M, Focardi S (2021) Antibiotic-Resistant Bacteria in Aquaculture and Climate Change: A Challenge for Health in the Mediterranean Area. Int J Environ Res Public Health 18(11):5723
Pinillos RG, Appleby MC, Manteca X, Scott-Park F, Smith C, Velarde A (2016) One Welfare – a platform for improving human and animal welfare. Veterinary Record 179:412–413
Reverter M, Sarter S, Caruso D, Avarre J-C, Combe M, Pepey E, Pouyaud L, Vega-Heredía S, De Verdal H, Gozlan RE (2020) Aquaculture at the crossroads of global warming and antimicrobial resistance. Nat Commun 11:1870
Rico A, Phu TM, Satapornvanit K, Min J, Shahabuddin AM, Henriksson PJG, Murray FJ, Little DC, Dalsgaard A, Van Den Brink PJ (2013) Use of veterinary medicines, feed additives and probiotics in four major internationally traded aquaculture species farmed in Asia. Aquac 412–413:231–243
Rigos G, Kogiannou D, Padrós F, Cristòfol C, Florio D, Fioravanti M, Zarza C (2021) Best therapeutic practices for the use of antibacterial agents in finfish aquaculture: a particular view on European seabass (Dicentrarchus labrax) and gilthead seabream (Sparus aurata) in Mediterranean aquaculture. Rev Aquac 13:1285–1323
Schar D, SommanustweechaI A, Laxminarayan R, Tangcharoensathien V (2018) Surveillance of antimicrobial consumption in animal production sectors of low- and middle-income countries: Optimizing use and addressing antimicrobial resistance. PLoS Med 15:e1002521–e1002521
SchaR D, Klein EY, Laxminarayan R, Gilbert M, Van Boeckel TP (2020) Global trends in antimicrobial use in aquaculture. Sci Rep 10:21878
Shen X, Jin G, Zhao Y, Shao X (2020) Prevalence and distribution analysis of antibiotic resistance genes in a large-scale aquaculture environment. Sci Total Environ 711:134626–134626
Shrimpalliance (2024) Food Regulatory Authorities in the EU, Japan, and the United States Once Again Confirm that India and Vietnam Continue to Use Banned Antibiotics in their Shrimp Aquaculture [Online]. Southern Shrimp Alliance Available: https://shrimpalliance.com/food-regulatory-authorities-in-the-eu-japan-and-the-united-states-once-again-confirm-that-india-and-vietnam-continue-to-use-banned-antibiotics-in-their-shrimp-aquaculture/ [Accessed April 9th 2024]
Stentiford GD, Neil DM, Peeler EJ, Shields JD, Small HJ, FlegeL TW, Vlak JM, Jones B, Morado F, Moss S, Lotz J, Bartholomay L, Behringer DC, Hauton C, Lightner DV (2012) Disease will limit future food supply from the global crustacean fishery and aquaculture sectors. J Invertebr Pathol 110:141–157
Su H, Liu S, Hu X, Xu X, Xu W, Xu Y, Li Z, Wen G, Liu Y, Cao Y (2017) Occurrence and temporal variation of antibiotic resistance genes (ARGs) in shrimp aquaculture: ARGs dissemination from farming source to reared organisms. Sci Total Environ 607–608:357–366
Swann MBKL, Field HI, Howie JW, Lucas IAM, Millar ELM et al (1969) Report of the joint committee on the use of antibiotics in animal husbandry and veterinary medicine. London, UK: Her Majesty’s Stationery Office. https://wellcomecollection.org/works/cqvewh54/items
Tacon AGJ (2020) Trends in Global Aquaculture and Aquafeed Production: 2000–2017. Rev Fish Sci Aquaculture 28:43–56
Thiang EL, Lee CW, Takada H, Seki K, Takei A, Suzuki S, Wang A, Bong CW (2021) Antibiotic residues from aquaculture farms and their ecological risks in Southeast Asia: a case study from Malaysia. Ecosyst Health Sustain 7(1):1926337
Umber JK, Moore KA (2021) Assessment of Antibiotic Stewardship Components of Certification Programs in US Animal Agriculture Using the Antibiotic Stewardship Assessment Tool. Front Sustain Food Syst 5:724097
Van Boeckel TP, Brower C, Gilbert M, Grenfell BT, Levin SA, Robinson TP, Teillant A, Laxminarayan R (2015) Global trends in antimicrobial use in food animals. Proc Natl Acad Sci 112:5649
VMD (2021a) The cascade: prescribing unauthorised medicines. In: DIRECTORATE, V. M. (ed.). London, UK Government of the United Kingdom
VMD (2021b) UK Veterinary Antibiotic Resistance and Sales Surveillance Report. Edinburgh Veterinary Medicines Directorate
WHO (2018) WHO list of Critically Important Antimicrobials for Human Medicine (WHO CIA list). World Health Organization, Geneva, Switzerland
WHO (1997) The medical impact of antimicrobial use in food animals: report of a WHO meeting. Berlin, Germany, 13–17 October 1997. Geneva, Switzerland: World Health Organization
WHO (2000) WHO global principles for the containment of antimicrobial resistance in animals intended for food : report of a WHO consultation with the participation of the Food and Agriculture Organization of the United Nations and the Office International des Epizooties, Geneva, Switzerland 5-9 June 2000. Geneva: World Health Organization
WHO (2015) Global action plan on antimicrobial resistance. In: organization, W. H. (ed.). Geneva, Switzerland: World Health Organization
WHO (2017a) One Health [Online]. Geneve, Switzerland Available: https://www.who.int/news-room/q-a-detail/one-health [Accessed May 17 2021]
WHO (2017b) WHO guidelines on use of medically important antimicrobials in food-producing animals. In: ORGANIZATION, W. H. (ed.). Geneve, Switzerland: World Health Organization
WHO (2021) Antimicrobial resistance [Online]. Geneva, Switzerland: World Health Organization. Available: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance [Accessed May 25th 2022]
Wierup M (2001) The Swedish experience of the 1986 year ban of antimicrobial growth promoters, with special reference to animal health, disease prevention, productivity, and usage of antimicrobials. Microb Drug Resist 7:183–190
Wright A, Li X, Yang X, Soto E, Gross J (2023) Disease prevention and mitigation in US finfish aquaculture: A review of current approaches and new strategies. Rev Aquac 15(4):1638–53
Xu M, Huang X-H, Shen X-X, Chen H-Q, Li C, Jin G-Q, Cao J-S, Xue Z-X (2022) Metagenomic insights into the spatiotemporal responses of antibiotic resistance genes and microbial communities in aquaculture sediments. Chemosphere (oxford) 307:135596–135596
Acknowledgements
A special thank you should be extended to people who have contributed to finding the material analyzed in this paper; Dr. Pongsiri Vorapongse (Thai Union), Dr. Tipparat Pongthanapanich (FAO) and Dr Kohei Makita (Rakuno Gakuen University).
Funding
Open access funding provided by Södertörn University.
Author information
Authors and Affiliations
Contributions
O.L. and M.T. developed the idea. O.L. collected and analyzed the data. O.L. wrote the main manuscript text and prepared the figures and tables. O.L. reached out to different stakeholders to ensure that the data used was correct and that there were no misunderstandings or new policies or regulations since the manuscript was written. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
Employment: Since this manuscript was written, O.L. has started working at the Aquaculture Stewardship Council. However, this was after the manuscript was written and the affiliation does not affect the outcome of the study.
Additional information
Handling Editor: Gavin Burnell
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Luthman, O., Robb, D.H.F., Henriksson, P.J.G. et al. Global overview of national regulations for antibiotic use in aquaculture production. Aquacult Int (2024). https://doi.org/10.1007/s10499-024-01614-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10499-024-01614-0