Abstract
The potential impacts of diet composition on water quality in recirculating aquaculture systems (RAS) have been studied only to a limited extent. This study examined the effects of different dietary C:N ratios on physiochemical water quality and associated microbiology in 12 pilot scale freshwater RAS with rainbow trout (Oncorhynchus mykiss). By adjusting the proportions of protein, carbohydrate, and fat, four diets with different C:N ratios—fully embracing the span observed in commercial diets—were formulated (CN8.5, CN9.3, CN10.0, and CN12.2). Each diet was administered to three separate RAS for 4 weeks at a daily feed loading of 1.67 kg/m3 make-up water. At the end of the trial (in week 4), concentrations of nitrite-N, nitrate–N, and dissolved COD, and microbial activity were significantly lower and water clarity was simultaneously higher in systems fed the CN12.2 diet as compared to the other diets. Microbial community compositions in system water and biofilter biofilms and the overall microbial abundance in system water were not affected. This was likely due to the molar C:N ratios in system water not correlating to the dietary C:N ratios and being very low (0.27–0.34) throughout all dietary C:N levels, suggesting that microorganisms were carbon limited in all RAS. Altogether, the results demonstrate that changing dietary C:N ratios can affect physicochemical water quality and microbial activity in RAS. In contrast, changing dietary C:N ratios did not affect water C:N ratios or water or biofilter microbial community composition at least within the span of dietary C:N ratios observed for commercial juvenile rainbow trout feeds.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recirculating aquaculture systems (RAS), feed—through fish digestion (and potentially feed spill)—is the main source of nutrient input. The ingredients and macronutrient composition of a diet (i.e., the content of proteins, lipids, and carbohydrates), the nutrient digestibility by fish, and the feed conversion ratio (FCR) obtained determine the form, quantity, and composition of the nutrient waste produced (Bureau and Hua 2010; Cho and Bureau 2001). In terms of the form of nutrient waste, indigestible nutrients are excreted as particulate waste (i.e., fecal waste), while digested but non-retained nutrients are excreted as dissolved and suspended waste (Dalsgaard and Pedersen 2011). Correspondingly, fecal waste is characterized by high concentrations of organic carbon (C) and comparatively low concentrations of nitrogen (N) when using high-performance and energy-dense diets. Dissolved and suspended waste, on the other hand, is characterized by high concentrations of N (principally ammonia-N and urea-N derived from protein metabolism) and more moderate concentrations of organic C as compared to fecal waste (Dalsgaard and Pedersen 2011, 2016).
In RAS, nutrients in dissolved and suspended waste as well as unremoved fecal waste will accumulate to equilibrium levels, which are determined by the relative amount of make-up water (i.e., the feed loading) and microbial degradation and assimilation activity (Pedersen et al. 2012). Given the close relationship between diet composition and waste excreted, changes in dietary C:N ratios could be expected to be reflected on C:N ratios in RAS water and/or on physicochemical water quality parameters, such as the concentration of inorganic nitrogen compounds and organic matter. Indeed, the impacts of dietary C:N ratios on water quality and water C:N ratios have been described in biofloc technology systems (Avnimelech 1999; Xu and Pan 2013). However, dietary effects on water quality in RAS are largely unknown, and a better understanding of the extent to which changes in the relative composition of dietary macronutrients and the associated production of nutrient waste affect the physicochemical water quality is important to improve RAS water and waste management.
Diet-induced changes in the availability of C and N in water could also lead to changes in the activity and composition of RAS microbial community. Ecological studies have found the optimum molar C:N ratios for aquatic microbes to vary between 3.6:1 and 12.7:1 (Goldman and Dennett 2000; Vrede et al. 2002). However, microbial communities in RAS are expected to be C-limited rather than N-limited due to an excessive accumulation of nitrate–N (NO3−-N) in the system (Leonard et al. 2002) and the majority of microbes present in water being heterotrophs, which use organic carbon as their energy source (Fossmark et al. 2020). Previously, decreasing the molar water C:N ratios from 0.56 to 0.33 by using membrane filtration to remove organic matter has been found to alter the microbial community composition and decrease the abundance of heterotrophic microbes (Bugten et al. 2022; Fossmark et al. 2020); but there is no knowledge on the effects of directly modifying the dietary C and N content. In addition to potentially affecting C-limited microbial communities in RAS water, diet-induced changes in water C:N ratios could affect biofilter communities. In two studies conducted with laboratory-scale biofilters, nitrification rates were shown to decrease by 50% when increasing molar C:N ratios from 0 to 0.6 (Ling and Chen 2005), and by 70% when increasing the ratio from 0 to 1.2 (Zhu and Chen 2001). This was explained with higher C availability promoting heterotrophic growth that would reduce the activity of autotrophic nitrifiers. However, in real-life RAS conditions, diet-induced changes in water C:N ratios and other physicochemical parameters might be quenched before biofilters, due to the high activity of (heterotrophic) microbes in the water phase.
This study aimed at building a better understanding between dietary C:N ratios and physicochemical water quality and associated microbiology in RAS. We speculated whether changes in dietary C:N ratios would affect elemental C:N ratios in the system and thereby the C:N ratios available to microbes, potentially affecting microbial community structure and/or abundance. This was studied by changing the relative proportions of proteins, lipids, and carbohydrates in four rainbow trout (Oncorhynchus mykiss) diets and feeding them to juvenile rainbow trout in triplicate pilot-scale RAS for 4 weeks. The microbial compositions were examined at both biofilter and water levels.
Materials and Methods
Feed compositions and system setup and operation
Using the same ingredients but adjusting the proportions of protein, fat, and carbohydrate, four diets were formulated to fully embrace the range of macronutrients and C:N ratios found in commercial diets for juvenile rainbow trout. Four experimental diets were named according to their C:N ratio (as per wet weight): CN8.5, CN9.3, CN10.0, and CN12.2 (equivalent to molar C:N ratios of 9.9, 10.8, 11.6, and 14.2, respectively) (Table 1). The diets, extruded as 3-mm pellets, were formulated and produced by Biomar A/S, Denmark.
The four diets were administered in triplicate to 12 identical pilot scale freshwater RAS at DTU Aqua, Hirtshals, Denmark. System configuration is illustrated in de Jesus Gregersen et al. (2021). Each system was composed of a 500-l fish rearing tank, a 140-l water sump, and a 75-l moving bed biofilm reactor (MBBR) filled with 40 l bioelements (750 m2/m3) (RK BioElements, Denmark). In 3 weeks of acclimation, a mix of the four diets was given to all systems stocked with juvenile rainbow trout. Afterwards, each system (fish biomass 7.31 ± 0.04 kg) was randomly assigned one of the four feed types and 100 g feed/day was fed for 4 weeks. Water exchange rate in each RAS was set at 60 l make-up water/day, equivalent to a feed loading of 1.67 kg feed/m3 make-up water. Dissolved oxygen level was automatically controlled at 85–100% by a monitoring system (Pacific System, OxyGuard International A/S, Denmark). Sodium bicarbonate was added into the sump as required to maintain pH at 7.0–7.4, and settled faeces were removed and discarded every day from the sedimentation columns attached to the bottom of rearing tanks. Water temperature depended on ambient temperatures, averaging 18.3 ± 0.7 °C in the 4 weeks.
Sampling
Prior to daily system maintenance, grab samples (5 l) were collected from the sump in each system and stored at 4 °C until analyzed. Water samples for dissolved nitrogenous compound analysis (nitrate–N (NO3−-N), nitrite-N (NO2−-N), total ammonia nitrogen (TAN), and urea-N), microparticle analysis, and free-living (FL) microbial abundance analysis were collected once a week. Water samples for total and dissolved chemical oxygen demand (COD) analysis, 5-day biochemical oxygen demand (BOD5) analysis, microbial activity analysis, water clarity measurements (i.e., turbidity and UV transmittance), and total N analysis were collected once in weeks 0, 1, 2, and 4. Samples for dissolved nitrogenous compound analysis were filtered at 0.20 μm (Filtropur S, Sarstedt, Germany), while samples for dissolved BOD5 and COD analysis were filtered at 0.45 μm (Adcantec, Toyo Roshi Kaisha, Japan).
For microbial community analysis, water samples were collected in the sump prior to daily system maintenance in week 0, 1, and 4 using syringe filters (0.22-μm Millipore Express® PLUS PES membrane, Merck KGaA, Germany). In addition, seven carrier media from each MBBR were collected in week 4. All microbiological samples were stored at – 20 °C for subsequent DNA extraction.
Physicochemical and microbial activity and community analysis
Table 2 summarizes the different methodologies applied for analyzing water samples, feeds, and fecal samples. Microbial activity in water samples were measured using two different methods: (1) a hydrogen peroxide (H2O2) degradation assay using the rate constant k (1/h) as an expression of microbial catalase activity (Pedersen et al. 2019) and (2) a fluorometric detection method (BactiQuant®) expressing microbial hydrolase activity in standardized BactiQuant® values (BQV) (Pedersen et al. 2017; Robertson et al. 2016). Microparticle size distribution (PSD) was measured by two instruments: (1) an AccuSizer 780 SIS (Particle Sizing Systems, USA) measuring the quantity of microparticles between 10 and 100 μm and (2) a Multisizer 4e Coulter Counter (Beckman Coulter Inc., USA) measuring the quantity of microparticles between 1 and 30 μm.
To measure the abundance of free-living (FL) microbes, 10 ml of sampled water from each system was pre-filtered through a cell strainer (40-µm FisherBrand, Thermo Fisher Scientific, USA) and diluted 10 times. Of this, 500 µl of filtrate was labelled with 5 µl of SYBR Green (100x, MilliporeSigma, Germany) and 5 µl of propidium iodide (PI, 600 µM, MilliporeSigma, Germany), and incubated at 37 °C for 10 min. Total cell abundance (cells/ml) was subsequently measured with a BD Accuri C6 Plus flow cytometer (Becton, Dickinson and Company, USA).
The DNeasy PowerLyzer™ PowerSoil DNA Isolation Kit (Qiagen, Germany) was used to extract DNA from samples, and the DNA quantity measured with a Qubit 2.0 Fluorometer (Thermo Fischer Scientific, USA). Microbial community compositions were studied using Ion Torrent PGM next-generation sequencing, targeting the V4 region of the 16S rRNA gene with primers 515F-Y (Parada et al. 2016) and 806R (Caporaso et al. 2011) as in Aalto et al. (2022). The total amount of sequences obtained was 1,140,089. For calculating alpha diversity indices (Shannon diversity, Chao1) and beta diversity (Bray–Curtis dissimilarity index), each sample was subsampled to 13,346 sequences. Sequences have been submitted to NCBI Sequence Read Archive under BioProject PRJNA877850.
Calculations
The content of carbohydrates in feed was calculated as nitrogen-free extract (NFE), i.e., as dry matter subtracted by protein, fat, and ash. Furthermore, macronutrients in the feed were converted to COD based on the oxycalorific coefficient (Dalsgaard and Pedersen 2011; Jobling 1995): 1.77 g O2/g protein, 2.88 g O2/g fat, and 1.16 g O2/g NFE. Feed crude energy was calculated based on the heat of combustion: 23.66 kJ/g protein, 39.57 kJ/g fat, and 17.17 kJ/g NFE. Total organic carbon (TOC) was calculated from COD using a COD:TOC ratio of 2.67 (Khanal and Huang 2003), and C:N ratios in feed and RAS water were subsequently calculated as TOC concentrations divided by N concentrations (total N in feed and total dissolved N in water, respectively). In water samples, particulate COD was calculated as the difference between total and dissolved COD. The same principle was used to calculate particulate BOD5. Particulate N suspended in the water was calculated as the difference between total N (unfiltered) subtracted by total dissolved N (filtered).
To generate a complete PSD between 1 and 100 μm, PSD data were categorized according to size class boundaries as suggested in Patterson et al. (1999). As the two PSD instruments used in this trial had different detection ranges, data in the size class between 12.7 and 32.0 μm were merged and averaged as described by de Jesus Gregersen et al. (2019). Particle β value, extracted from the power-law distribution model by Patterson et al. (1999), was used to indicate the status of particle size distribution.
Statistical analysis
Statistical analysis was performed in R (version 3.6.3; R Core Team, 2020) using the R packages: FSA (Ogle 2018), ggplot2 (Wickham 2016), rstatix (Kassambara 2020), vegan (Oksanen et al. 2019), and phyloseq (McMurdie and Holmes 2013). The effects of dietary C:N ratios on water quality in week 4 were tested by one-way ANOVA followed by Tukey test for pairwise multiple comparisons in case of significant differences. In case data did not meet the normality (Shapiro–Wilk test) and variance homogeneity (Levene test) assumptions, a Kruskal–Wallis test followed by a Dunn test was carried out. To test the effects of dietary C:N ratios on NO3−-N and total dissolved N concentrations along the trial, two-way repeated measures ANOVA was used. Dietary effects at each time point were further tested by one-way ANOVA followed by pairwise t test for multiple comparisons. The relationship between dietary and water C:N ratios was examined with Pearson correlation test. The effect of dietary treatment and/or sample type (water and biofilter biofilm) on microbial community composition was examined with the permutational analysis of variance (PERMANOVA). The differences in alpha-diversity indices (Shannon diversity and Chao1 richness) between diets and sample types were examined with two-way ANOVA and Tukey post-hoc tests. The relationship with the relative abundance of sequences assigned to ammonia or nitrite oxidizing bacteria in the biofilters (AOB and NOB abundance) and dietary C:N ratio was examined with Pearson correlation test. Results are presented as mean ± standard deviation throughout, and the level of significance was set at 0.05.
Results
No feed spill was observed in any system during the 4-week trial period while two mortalities were recorded in two CN12.2 systems and one mortality was recorded in a CN10.0 system during the first 2 weeks.
Nitrogenous compounds
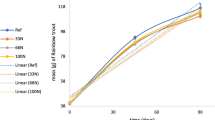
The development of NO3−-N in RAS water was significantly affected by the dietary C:N ratios (two-way ANOVA, F12,32 = 169.0, p < 0.001). NO3−-N concentrations measured in week 4 increased with the increasing dietary protein levels (i.e., the decreasing dietary C:N ratios; Pearson correlation, r = − 0.97, p < 0.001; Fig. 1). The dietary protein levels also affected the NO2−-N concentrations measured in week 4 (one-way ANOVA, F3,8 = 6.1, p = 0.018), with the average concentration being higher in the CN8.5 group than in the CN12.2 group (Tukey test, p = 0.013) (Table 3). Concomitantly, NO2−-N concentrations decreased with increasing dietary C:N ratios (Pearson correlation, r = − 0.83, p < 0.001). Urea-N and TAN concentrations, on the other hand, were not affected by dietary protein levels in week 4 (Table 3) (one-way ANOVA, F3,8 = 0.7, p > 0.05; F3,8 = 0.6, p > 0.05, respectively), averaging 30 ± 4 µg urea-N/l and 64 ± 22 µg TAN/l across all dietary treatments. As NO3−-N accounted for approximately 93% of total dissolved N (additionally including TAN, nitrite-N, and non-characterized N), total dissolved N in water was similarly correlated to dietary protein levels (i.e., negatively correlated to dietary C:N ratios; Pearson correlation, r = − 0.96, p < 0.001).
Weekly development of nitrate–N concentration in systems fed different diets: CN8.5, CN9.3, CN10.0, and CN12.2. Stars indicate the significant levels among treatment groups in a specific week (one-way ANOVA): * stands for p values ≥ 0.01 and ≤ 0.05, ** for p between ≥ 0.001 and < 0.01, while.*** for p < 0.001
Organic matter and C:N ratios
Total organic matter (BOD5 and COD) measured in water samples was not significantly affected by the dietary C:N ratios in week 4 (Table 3) (total BOD5: one-way ANOVA, F3,8 = 2.0, p > 0.05; total COD: F3,8 = 1.8, p > 0.05). Only the dissolved COD was found statistically lower in the CN12.2 group than in the CN8.5 group (Tukey test, p = 0.030). Although not significant, numerically the lowest organic matter content was all found in the CN12.2 group both for dissolved and particulate fractions. Molar C:N ratios in water samples did not differ between the treatments in week 4 (one-way ANOVA, F3,8 = 1.3, p > 0.05), ranging between 0.27 and 0.34 (Table 3) and did not correlate with the dietary C:N ratios (Pearson correlation, r = − 0.42, p > 0.05).
Microparticles and water clarity
Microparticle size distributions (β values) did not differ significantly between the treatments in week 4 (one-way ANOVA, F3,8 = 0.3, p > 0.05), averaging 3.03 ± 0.15 across all treatments by the end of the trial (Table 3). Consistent with this, the abundance of microparticles (both 1–5 µm and 5–100 µm) were not affected either (F3,8 = 2.3, p > 0.05; F3,8 = 1.0, p > 0.05, respectively). Water clarity was significantly affected by the dietary treatments with the highest UVT found in the CN12.2 group (F3,8 = 11.1, p = 0.003), whereas turbidity was not statistically different between the treatments (F3,8 = 1.6, p > 0.05) (Table 3).
Microbial activity and abundance
The dietary treatments significantly affected microbial catalase activities (one-way ANOVA, F3,8 = 4.2, p = 0.047), with the CN12.2 group having a 50% lower average value than the CN9.3 group in week 4 (Tukey test, p = 0.032). In comparison, microbial hydrolase activities measured by BactiQuant® were not significantly affected by the diets (one-way ANOVA, F3,8 = 1.6, p > 0.05). Similarly, different dietary C:N ratios did not affect the abundance of FL microbes (F3,8 = 3.0, p = 0.097), averaging 52 ± 16 million cells/ml across the treatments (Table 3).
Microbial communities in water and biofilms
In water, microbial community composition did not differ in the beginning of the trial (data not shown) but changed significantly from week 1 to week 4 (PERMANOVA, pseudo-F = 14.6, p = 0.001), whereas it was not affected by the dietary C:N ratios (pseudo-F = 1.43, p > 0.05) (Fig. 2). Similarly, the dietary C:N ratios did not cause changes in the microbial community composition in biofilter biofilm (pseudo-F = 1.39, p > 0.05). In contrast, microbial community compositions measured in RAS water samples differed significantly from those in RAS biofilter biofilm independently of the dietary C:N ratios in week 4 (pseudo-F = 30.0, p = 0.001). In water samples, the most abundant bacterial classes (relative abundance of > 20%) were Actinobacteria and Alpha- and Gammaproteobacteria, while in the biofilter biofilm samples the most abundant classes were only the two latter ones (Fig. 3). Neither OTU diversity (Shannon index) nor richness (Chao1) was affected by the diets or the sampling occasions (Shannon: two-way ANOVA, F3,28 = 0.08, p > 0.05, Chao1: F3,28 = 0.08, p > 0.05). Both diversity and richness were lower in the water than in the biofilter biofilm in week 4 across all dietary treatment groups (Shannon: two-way ANOVA, F1,16 = 561.9, p < 0.001, Chao1: F3,16 = 20.5, p < 0.001) (Fig. 4).
The relative abundance of ammonia-oxidizing bacteria (AOB abundance) decreased with increasing dietary C:N ratios (Pearson correlation, r = − 0.61, p = 0.036) (Table 4), while there was no relationship between the dietary C:N ratios and the relative abundance of nitrite oxidizing bacteria (NOB) (Table 4).
Discussion
In this study, we found that the dietary C:N ratios affected some physicochemical water quality parameters and the microbial activity in RAS water, but not microbial community composition in water or biofilter biofilm. The CN12.2 diet with the highest dietary energy content resulted in the best system performance in terms of low dissolved nitrogenous compounds, low dissolved organic matter, reduced microbial activity, and high water clarity.
In general, partially replacing protein with carbohydrate and fat in a diet based on the protein-sparing principle (Cho and Bureau 2001) generally leads to increases in dietary C:N ratios. Given that diets are optimized to fish requirements, such dietary macronutrient replacements reduce the excretion of ammonia and urea from fish, and subsequently reduce the accumulation of NO3−-N and total dissolved N in RAS (Cho et al. 1994; Godoy-Olmos et al. 2022). This explains why NO3−-N and total dissolved N concentrations in the current study correlated positively with dietary protein levels (i.e., were negatively related to the dietary C:N ratios).
In contrast to dissolved N, organic matter concentrations in RAS water were only moderately affected by the dietary treatments, probably due to the four diets having similar COD concentrations. Organic C in water and dietary COD did not correlate explaining why water C:N ratios and dietary C:N ratios also did not correlate.
Hence, based on the results obtained in this study and in previous RAS studies, there is not a strong relationship between dietary and water C:N ratios in RAS (Fig. 5), acknowledging that the different RAS compared varied in, e.g., system configuration, diet composition, and feed loading. It seems that molar C:N ratios in system water generally vary within a narrow range (0.11–0.63), a factor 10 or more below the ones in the associated fish feeds (8.2–14.2). Given that total BOD5 constitutes approximately 6–30% of total COD in RAS water (Fernandes et al. 2015, 2017; Pedersen et al. 2012; Rojas-Tirado et al. 2018), C:N ratios calculated by using only the readily biodegradable organic carbon (i.e., BOD5) instead of COD could be even below 0.01, indicating highly C-limited conditions for RAS microbes.
In previous ecological studies, the overall bacterial biomass has been found to be quite plastic and quickly adapt its C:nutrient ratios following changes in the environmental ratios (Tezuka 1990), while different specific strains of heterotrophic bacteria have been found to have individual and rather narrow ranges of optimal substrate C:nutrient ratios (Makino et al. 2003; Vrede et al. 2002). This means that increases in C:N ratios would cause a shift in the dominance of different heterotrophic microbial groups. While this is supported by both microbial catalase activity and COD concentrations being low in the CN12.2 group, the microbial community composition in RAS water and biofilter biofilms and the overall microbial abundance in RAS water remained largely unaffected. Previously, decreasing the water molar C:N ratios from 0.56 to 0.33 through particle removed by membrane filtration was found to induce significant changes in RAS water microbiome and decreases in the overall abundance (Bugten et al. 2022; Fossmark et al. 2020). However, this might have been due to the removal of microbial substrate (organic matter) or microbes being directly removed by membrane filtration (20–30 nm) rather than changes in water C:N ratio. As in this study the water molar C:N ratios were substantially lower across all the treatments (ranging from 0.27 to 0.34) than the previously suggested optimal range for exponential growth (C:N ratio of 4–6) (Vrede et al. 2002), it is most likely that the microbial biomass was significantly C-limited even with the highest dietary C:N ratio and that the microbial community in water and/or biofilm already initially consisted of taxa with their optimal C:nutrient ratios. However, we found that microbial community compositions in water differed from those in biofilter biofilm, which is in agreement with previous findings (Almeida et al. 2021; Bakke et al. 2017). This is probably explained by biofilters being an even more C-limited environment due to high microbial biomass receiving only the left-over organic matter from the rearing tanks, which should favour autotrophic nitrifiers but also heterotrophs adapted to C-limited environments.
Even though biofilter biofilm community compositions were generally not affected by the dietary C:N ratios, changes in element concentrations induced by the diets were still able to affect bacterial groups with specialized functions, as we found the relative AOB abundance decreasing with the decreasing dietary protein levels (i.e., the increasing dietary C:N ratios in this study). This was probably caused by the decreasing ammonia excretion from fish under higher dietary C:N ratios, which limited the growth of AOB and also explains the observed lower nitrite concentration in the CN12.2 treatment. The relative NOB abundance, on the other hand, did not differ cross the treatment groups. This is not surprising as NOBs can grow with other substrates than nitrite (Kuypers et al. 2018), e.g., the Nitrospira-group (considered as “NOB”) includes members capable of conducting a complete nitrification process from ammonia to nitrate (Daims et al. 2016), leading the NOB abundance being unrelated to nitrite availability.
Altogether, the results indicate that RAS microbial communities in both water and biofilm are rather insensitive to changes induced by dietary C:N ratios, at least when they span over the values of the normal commercial trout diets. Other measures would thus probably be more effective when aiming to manipulate or control RAS microbiology. A direct removal of organic matter with, e.g., membrane filtration or protein skimming has been shown to be effective in removing and/or suppressing the activity of living microbes (de Jesus Gregersen et al. 2021; Fossmark et al. 2020). Furthermore, manipulating dietary N:P ratios rather than C:N ratios may have a higher potential for influencing RAS microbiomes, as P is an essential component of nucleic acids (DNA and RNA) and lowering P availability (still meeting the minimum P requirement of fish) should favour slow growing bacterial taxa (Hessen et al. 2013; Scott et al. 2012). Recently, we found a positive relationship between dietary and water N:P ratios in RAS (Huang et al. 2023), suggesting an interesting way to influence RAS microbiomes.
Conclusion
In this study, we found that dietary C:N ratios affect some physicochemical water quality parameters and microbial activity in RAS. The highest dietary C:N ratio, achieved by replacing protein mainly with fat, resulted in the best system performance in terms of low concentrations of NO2−-N, NO3−-N, dissolved COD, and microbial activity in addition to high water clarity. In addition, dietary and water COD, and subsequently, C:N ratios were found to be uncorrelated. Water C:N ratios were below the optimum range for aquatic microbes across all the dietary treatments, demonstrating that the microbial biomass was significantly C-limited despite dietary C:N ratio. This phenomenon is likely to be observed in most RAS.
Data availability
Data will be made available on request.
References
Aalto SL, Syropoulou E, de Jesus Gregersen KJ, Tiirola M, Pedersen PB, Pedersen L-F (2022) Microbiome response to foam fractionation and ozonation in RAS. Aquaculture 550:737846. https://doi.org/10.1016/j.aquaculture.2021.737846
Almeida DB, Magalhães C, Sousa Z, Borges MT, Silva E, Blanquet I, Mucha AP (2021) Microbial community dynamics in a hatchery recirculating aquaculture system (RAS) of sole (Solea senegalensis). Aquaculture 539:736592. https://doi.org/10.1016/j.aquaculture.2021.736592
Avnimelech Y (1999) Carbon/nitrogen ratio as a control element in aquaculture systems. Aquaculture 176(3–4):227–235. https://doi.org/10.1016/S0044-8486(99)00085-X
Bakke I, Åm AL, Kolarevic J, Ytrestøyl T, Vadstein O, Attramadal KJK, Terjesen BF (2017) Microbial community dynamics in semi-commercial RAS for production of Atlantic salmon post-smolts at different salinities. Aquacult Eng 78:42–49. https://doi.org/10.1016/j.aquaeng.2016.10.002
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37(8):911–917. https://doi.org/10.1139/o59-099
Bugten AV, Attramadal KJK, Fossmark RO, Rosten TW, Vadstein O, Bakke I (2022) Changes in rearing water microbiomes in RAS induced by membrane filtration alters the hindgut microbiomes of Atlantic salmon (Salmo salar) parr. Aquaculture 548(P2):737661. https://doi.org/10.1016/j.aquaculture.2021.737661
Bureau DP, Hua K (2010) Towards effective nutritional management of waste outputs in aquaculture, with particular reference to salmonid aquaculture operations. Aquac Res 41(5):777–792. https://doi.org/10.1111/j.1365-2109.2009.02431.x
Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Lozupone CA, Turnbaugh PJ, Fierer N, Knight R (2011) Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc Natl Acad Sci U S A 108(supplement_1):4516–4522. https://doi.org/10.1073/pnas.1000080107
Cho CY, Bureau DP (2001) A review of diet formulation strategies and feeding systems to reduce excretory and feed wastes in aquaculture. Aquac Res 32:349–360. https://doi.org/10.1046/j.1355-557x.2001.00027.x
Cho CY, Hynes JD, Wood KR, Yoshida HK (1994) Development of high-nutrient-dense, low-pollution diets and prediction of aquaculture wastes using biological approaches. Aquaculture 124(1–4):293–305. https://doi.org/10.1016/0044-8486(94)90403-0
Daims H, Lücker S, Wagner M (2016) A new perspective on microbes formerly known as nitrite-oxidizing bacteria. Trends Microbiol 24(9):699–712. https://doi.org/10.1016/j.tim.2016.05.004
Dalsgaard J, Pedersen PB (2011) Solid and suspended/dissolved waste (N, P, O) from rainbow trout (Oncorynchus mykiss). Aquaculture 313(1–4):92–99. https://doi.org/10.1016/j.aquaculture.2011.01.037
Dalsgaard J, Pedersen PB (2016) Produktionsbidrag og dambrugsmodel: manual og modelforudsætninger (in Danish). DTU Aqua rapport nr. 309–2016. http://www.aqua.dtu.dk/Publikationer/Forskningsrapporter/Forskningsrapporter_siden_2008
de Jesus Gregersen KJ, Pedersen PB, Pedersen L-F, Dalsgaard J (2019) Micro particles and microbial activity in Danish recirculating rainbow trout (Oncorhynchus mykiss) farms. Aquacult Eng 84:60–66. https://doi.org/10.1016/j.aquaeng.2018.12.001
de Jesus Gregersen KJ, Pedersen L-F, Pedersen PB, Syropoulou E, Dalsgaard J (2021) Foam fractionation and ozonation in freshwater recirculation aquaculture systems. Aquacult Eng 95(August):102195. https://doi.org/10.1016/j.aquaeng.2021.102195
DS (1975) Water analysis – determination of ammonia-nitrogen, DS 224:1975. Danish Standards Foundation Denmark, 8
DS (1991) Water analysis – determination of the sum of nitrite- and nitrate-nitrogen, DS 223:1991. Danish Standards Foundation Denmark, 20
Fernandes PM, Pedersen L-F, Pedersen PB (2015) Microscreen effects on water quality in replicated recirculating aquaculture systems. Aquacult Eng 65:17–26. https://doi.org/10.1016/j.aquaeng.2014.10.007
Fernandes PM, Pedersen L-F, Pedersen PB (2017) Influence of fixed and moving bed biofilters on micro particle dynamics in a recirculating aquaculture system. Aquacult Eng 78:32–41. https://doi.org/10.1016/j.aquaeng.2016.09.002
Fossmark RO, Vadstein O, Rosten TW, Bakke I, Košeto D, Bugten AV, Helberg GA, Nesje J, Jørgensen NOG, Raspati G, Azrague K, Østerhus SW, Attramadal KJK (2020) Effects of reduced organic matter loading through membrane filtration on the microbial community dynamics in recirculating aquaculture systems (RAS) with Atlantic salmon parr (Salmo salar). Aquaculture 524:735268. https://doi.org/10.1016/j.aquaculture.2020.735268
Godoy-Olmos S, Jauralde I, Monge-Ortiz R, Milián-Sorribes MC, Jover-Cerdá M, Tomás-Vidal A, Martínez-Llorens S (2022) Influence of diet and feeding strategy on the performance of nitrifying trickling filter, oxygen consumption and ammonia excretion of gilthead sea bream (Sparus aurata) raised in recirculating aquaculture systems. Aquacult Int 30(2):581–606. https://doi.org/10.1007/s10499-021-00821-3
Goldman JC, Dennett MR (2000) Growth of marine bacteria in batch and continuous culture under carbon and nitrogen limitation. Limnol Oceanogr 45(4):789–800. https://doi.org/10.4319/lo.2000.45.4.0789
Hessen DO, Elser JJ, Sterner RW, Urabe J (2013) Ecological stoichiometry: an elementary approach using basic principles. Limnol Oceanogr 58(6):2219–2236. https://doi.org/10.4319/lo.2013.58.6.2219
Huang X, Dalsgaard J, Aalto SL, Lund I, Pedersen PB (2023) Influence of dietary phosphorus on orthophosphate accumulation in recirculating aquaculture systems with rainbow trout (Oncorhynchus mykiss). Aquacult Eng 103:102363. https://doi.org/10.1016/j.aquaeng.2023.102363
ISO (1986) Water quality – determination of nitrate – part 1: 2.6-dimethylphenol spectrometric method, ISO 7890–1:1986. International Organization for Standardization, Geneva
ISO (1989a) Water quality – determination of biochemical oxygen demand after n days (BODn) – part 2: method for undiluted samples, ISO 5815:1989, modified. International Organization for Standardization, Geneva
ISO (1989b) Water quality – determination of the chemical oxygen demand, ISO 6060:1989. International Organization for Standardization, Geneva
ISO (1997) Water quality – determination of nitrogen. Part 1:method using oxidative digestion with peroxodisulfate, ISO11905–1:1997. International Organization for Standardization, Geneva
ISO (1998) Animal feeding stuffs – determination of phosphorus content – spectrometric method, ISO 6491:1998. International Organization for Standardization, Geneva
ISO (2005) Animal feeding stuffs – determination of nitrogen content and calculation of crude protein content – part 2: block digestion/steam distillation method, ISO 5983–2:2005. International Organization for Standardization, Geneva
Jobling M (1995) Fish bioenergetics. Oceanogr Lit Rev 42(9):785
Kassambara A (2020) rstatix: pipe-friendly framework for basic statistical tests
Khanal SK, Huang J-C (2003) ORP-based oxygenation for sulfide control in anaerobic treatment of high-sulfate wastewater. Water Res 37(9):2053–2062. https://doi.org/10.1016/S0043-1354(02)00618-8
Kuypers MMM, Marchant HK, Kartal B (2018) The microbial nitrogen-cycling network. Nat Rev Microbiol 16(5):263–276. https://doi.org/10.1038/nrmicro.2018.9
Larsen BK, Dalsgaard J, Pedersen PB (2015) An optimized and simplified method for analysing urea and ammonia in freshwater aquaculture systems. Aquac Res 46(7):1608–1618. https://doi.org/10.1111/are.12314
Leonard N, Guiraud J, Gasset E, Cailleres J, Blancheton J (2002) Bacteria and nutrients—nitrogen and carbon—in a recirculating system for sea bass production. Aquacult Eng 26(2):111–127. https://doi.org/10.1016/S0144-8609(02)00008-0
Ling J, Chen S (2005) Impact of organic carbon on nitrification performance of different biofilters. Aquacult Eng 33(2):150–162. https://doi.org/10.1016/j.aquaeng.2004.12.002
Makino W, Cotner JB, Sterner RW, Elser JJ (2003) Are bacteria more like plants or animals? Growth rate and resource dependence of bacterial C : N : P stoichiometry. Funct Ecol 17(1):121–130. https://doi.org/10.1046/j.1365-2435.2003.00712.x
McMurdie PJ, Holmes S (2013) phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 8(4):e61217. https://doi.org/10.1371/journal.pone.0061217
NMKL (1991) Moisture and ash. Gravimetric determination in meat and meat products. Nordic Committee on Food Analysis, Oslo
Ogle DH (2018) Introductory fisheries analyses with R. Chapman and Hall/CRC. https://doi.org/10.1201/9781315371986
Oksanen J, Guillaume Blanchet F, Friendly M, Kindt R, Legendre P, McGlinn D, Wagner H (2019) Vegan: community ecology package. R Package Version 2:5–6
Parada AE, Needham DM, Fuhrman JA (2016) Every base matters: assessing small subunit rRNA primers for marine microbiomes with mock communities, time series and global field samples. Environ Microbiol 18(5):1403–1414. https://doi.org/10.1111/1462-2920.13023
Patterson RN, Watts KC, Timmons MB (1999) The power law in particle size analysis for aquacultural facilities. Aquacult Eng 19(4):259–273. https://doi.org/10.1016/S0144-8609(98)00054-5
Pedersen L-F, Suhr KI, Dalsgaard J, Pedersen PB, Arvin E (2012) Effects of feed loading on nitrogen balances and fish performance in replicated recirculating aquaculture systems. Aquaculture 338–341:237–245. https://doi.org/10.1016/j.aquaculture.2012.01.035
Pedersen PB, von Ahnen M, Fernandes PM, Naas C, Pedersen L-F, Dalsgaard J (2017) Particle surface area and bacterial activity in recirculating aquaculture systems. Aquacult Eng 78(April):18–23. https://doi.org/10.1016/j.aquaeng.2017.04.005
Pedersen L-F, Rojas-Tirado P, Arvin E, Pedersen PB (2019) Assessment of microbial activity in water based on hydrogen peroxide decomposition rates. Aquacult Eng 85:9–14. https://doi.org/10.1016/j.aquaeng.2019.01.001
Robertson A, Burnett D, McLeroy K (2016) Rapid, economic microbial monitoring methodologies for determining treated water stability. In: Proceedings of the 4th Unconventional Resources Technology Conference. https://doi.org/10.15530/urtec-2016-2459158
Rojas-Tirado P, Pedersen PB, Vadstein O, Pedersen L-F (2018) Changes in microbial water quality in RAS following altered feed loading. Aquacult Eng 81:80–88. https://doi.org/10.1016/j.aquaeng.2018.03.002
Scott JT, Cotner JB, LaPara TM (2012) Variable stoichiometry and homeostatic regulation of bacterial biomass elemental composition. Front Microbiol 3. https://doi.org/10.3389/fmicb.2012.00042
Tezuka Y (1990) Bacterial regeneration of ammonium and phosphate as affected by the carbon:nitrogen:phosphorus ratio of organic substrates. Microb Ecol 19(3):227–238. https://doi.org/10.1007/BF02017167
Vrede K, Heldal M, Norland S, Bratbak G (2002) Elemental composition (C, N, P) and cell volume of exponentially growing and nutrient-limited bacterioplankton. Appl Environ Microbiol 68(6):2965–2971. https://doi.org/10.1128/AEM.68.6.2965-2971.2002
Wickham H (2016) ggplot2: elegant graphics for data analysis. Springer-Verlag, New York
Xu W-J, Pan L-Q (2013) Dietary protein level and C/N ratio manipulation in zero-exchange culture of Litopenaeus vannamei: evaluation of inorganic nitrogen control, biofloc composition and shrimp performance. Aquacult Res 45(11). https://doi.org/10.1111/are.12126
Zhu S, Chen S (2001) Effects of organic carbon on nitrification rate in fixed film biofilters. Aquacult Eng 25(1):1–11. https://doi.org/10.1016/S0144-8609(01)00071-1
Acknowledgements
The authors appreciate the lab assistance from the lab technicians at DTU Aqua: Ulla Sproegel, Brian Møller, Signe Østergaard, and Nanna Henriksen. The authors also appreciate the assistance in fish husbandry from the technicians: Ole Larsen, Rasmus Jensen, and Jens Nedergaard.
Funding
Open access funding provided by Technical University of Denmark This work was part of the project RASbiome supported by the EU Horizon 2020 BlueBio Cofund (grant agreement no. 817992).
Author information
Authors and Affiliations
Contributions
Xiaoyu Huang: Conceptualization, Formal analysis, Investigation, Writing – Original Draft. Sanni L. Aalto: Formal analysis, Investigation, Writing – Review & Editing, Supervision. Johanne Dalsgaard: Writing – Review & Editing, Supervision. Per Bovbjerg Pedersen: Conceptualization, Writing – Review & Editing, Supervision, Funding acquisition.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Handling Editor: Brian Austin
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Huang, X., Aalto, S.L., Dalsgaard, J. et al. Can dietary C:N ratio influence water quality and microbiology in recirculating aquaculture systems?. Aquacult Int (2024). https://doi.org/10.1007/s10499-024-01541-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10499-024-01541-0