Abstract
Recently, an increasing disparity has emerged in the need for raw fish meal (FM) and its supply, along with the environmental and financial obstacles associated with its use. Therefore, increasing and elevating the diversity of alternative protein sources for aquaculture nutrition is imperative. The study investigated the impact of substituting FM protein with EW meal on the growth, haemato-biochemical response and intestinal histomorphology of Nile tilapia. Up to 20% fishmeal replacement with EW meal had no adverse effects on fish growth performance. A quadratic analysis of the final body weight and EW meal level indicated the best growth performance at 17.5% replacement. Fish fed the 40% replacement level had significantly decreased height and width of intestinal folds and number of mucosal goblet cells compared to the control. Although red and white blood cell counts were found unchanged between the control, the 10% and 20% replacement groups, a significantly higher number of white blood cells and a lower number of red blood cells were found in the 40% group compared to the control. Blood glucose level was the highest, while haemoglobin level was the lowest in the 40% group. In the same group, significantly higher frequencies of erythrocyte cellular and nuclear abnormalities were noted. Lipid droplet accumulation in the liver was significantly higher in the 40% group, whilst the 10% and 20% groups showed no significant difference compared to the control.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aquaculture production has expanded dramatically over the last decades as a sustainable and profitable economic activity. It aims to meet the high protein demand in the human diet (Madsen et al. 2022). However, the continuous increase in aquafeed prices has become a significant challenge influencing the sector's potential. Protein content and quality are the most critical factors affecting farmed fish's production cost and health (Younis et al. 2018). Fishmeal (FM) has excellent amino and fatty acid profiles and improved digestibility. With the increasing production trend of aquaculture, it is predicted that the demand for FM will increase, which will also put pressure on the abundance of the low-cost pelagic fish used as raw materials for FM production (Tacon and Metian 2008). Therefore, aquaculture has started exploring alternative, more sustainable protein sources to partially or totally replace FM (Albrektsen et al. 2022).
The research for alternative protein sources has focused on plant and animal protein sources (Siddik et al. 2018; Van Vo et al. 2020; Hender et al. 2021). The results so far indicate that plant ingredients are less effective as aquafeed ingredients due to their endogenous anti-nutritional components (e.g., protease inhibitors, phytate, alkaloids, gossypols, cyanogens, mimosine, etc.) and imbalanced amino acid profiles. They also contain high levels of ash and fibre, which reduce the palatability and digestibility of the feeds (Macias-Sancho et al. 2014). On the other hand, animal protein sources have issues with improper amino acid profiles, such as lack of methionine in meat and bone meal, lysine deficiency in poultry by-products meal (Chaklader et al. 2020), reduced isoleucine content and digestibility in blood meal (Njieassam 2016). The issues of microbial contamination and potential disease transmission (from livestock to fish and humans) are additional concerns regarding animal-based protein sources (Ogello et al. 2014). All these challenges have directed the research to other unconventional sources of low-cost proteins. One of these sources is the earthworm (EW) meal, which has recently attracted attention (Parolini et al. 2020).
Several EW species, including Lumbricus rubellus, Eisenia Andrei, and Dendrobaena veneta, are very rich in protein and beneficial amino acids and have high lipid levels, naturally rich in ω-6 and ω-3 fatty acids (Garczyńska et al. 2023). Extensive biochemical analysis of EW by several researchers revealed that the protein and lipid content of EW vary from 50 to 70% and 5 to 10%, respectively, which are close to that of FM (Parolini et al. 2020). In addition, the mineral composition of EW is also ideal for fish (Sogbesan and Ugwumba 2008). Many researchers have tested several EW species for aquafeed production and found that their nutritional compositions could meet the nutritional requirements of most fish species. The use of red EW, Perionyx excavatus in particular, can boost reproductivity, improve feed digestibility, increase survival rate and reduce stress levels (Musyoka et al. 2019). Olele (2011) suggested including up to 50% of EW in the feed for hybrid catfish Heteroclarias spp. fingerlings, whereas Sogbesan and Ugwumba (2008) recommended 7.5%—25% inclusion of EW in the diet of vundu catfish, H. longifilis. An inclusion level of up to 35% of EW was suggested for the diet of Nile tilapia, Oreochromis niloticus and, carp post larvae, Cyprinus carpio for increased growth performance (Musyoka et al. 2019). According to Ahmed et al. (2020), the blood analysis of Nile tilapia fed with FM also revealed that with increasing levels of EW in the diet, the amylase activity was increased in the fish, while the cholesterol in the blood serum was decreased, which suggested that EW could improve liver function and facilitate digestion.
EWs, however, may contain anti-nutritional factors, demonstrating that coelomic fluid in EW may reduce the absorption of nutrients, inhibit the digestive enzyme secretion and reduce the nutrient bioavailability in fish. Also, this fluid may contain lysenin that binds to sphingomyelin with toxic effects. However, these toxic effects appear to depend on the technique used to preserve them (Nhi et al. 2010). Lysenin and other haemolytic factors present in EW may be heat-labile proteins, as evidenced by the fact that heat treatment of coelomic fluid reduced the toxic effects (Kauschke et al. 2007). EW pre-treatment methods recommended by Tacon et al. (1983) included removing the coelomic fluid, heating during drying, or blanching with hot water.
Nile tilapia is a high-profit aquaculture fish due to its excellent flesh quality, rapid growth, and resilience (El‐Sayed and Fitzsimmons 2023). Aquaculture's fast growth has also accelerated the Nile tilapia's global expansion (Khanjani and Sharifinia 2021). Nile tilapia, being an ideal choice for fish farming, has now become the second most cultured species after carps. The estimated market value of farmed Nile tilapia in 2020 was 12 billion US dollars, representing a vital part of all cultured aquatic animals (FAO 2022). Tilapia require 30–45% protein daily for optimum growth (FAO 2021). Since the protein content of EW is about 45%, it can be utilized as a potential protein source in Nile tilapia diet.
This study aimed to assess the effectiveness of replacing FM with EW protein in the diet of Nile tilapia and to determine its potential impacts on the species' growth, intestinal health, haemato-biochemical response, and erythrocyte-metric parameters.
Materials and method
Experimental diets
Four isonitrogenous (~ 31%) and isolipidic (~ 7%) diets were prepared based on EW's analysed crude protein and lipid levels. FM protein in the prepared diet was replaced by red EW protein at 0, 10, 20, and 40%. The diets were labelled as control, D1, D2 and D3, respectively (Table 1). Experimental diets were formulated using the Pearson’s Square Method (Wagner and Stanton 2012). Firstly, the EW were purchased from the local farmers. To prepare the experimental feed, all feed ingredients were crushed into fine particles using a hammer mill. Once ground, all fine particles' weights were measured. Subsequently, these fine particles were carefully placed in a clean drum mixer for thorough mixing with binders, where molasses served as the binder. To prepare the dough, water was added to the bowl containing all other components. Following dough preparation, a pellet machine was utilized to carry out the extrusion process, producing pellets of the desired size (2 mm). The moist pellets obtained were then left to air dry for a short period at room temperature. To prevent microbial and fungal contamination, the pellets were quickly moved to a hot air oven and dried for 72 h at 60 °C. Once dried in the oven, the pellets were allowed to cool down to room temperature. Finally, the prepared floating pelleted diets were meticulously packed into clean, plastic-sealed, and airtight containers for further use and stored in a refrigerator at 4 °C. The proximate compositions of the prepared feeds were examined using the standard method outlined in the "Official Methods of Analysis" (Horwitz and Latimer Jr 2006). Crude protein was determined by using the Kjeldahl apparatus (Bloc Digest 12, JP Selecta, Spain) and a 6.25 conversion factor was applied. Lipid content was measured using the Soxhlet apparatus (J-SH3, JISICO, Korea) with ether extraction methods. To determine the moisture, a hot air oven (HAS/50/TDIG/SS, Genlab, UK) was run at 105 0C. Ash was determined by operating a muffle furnace (HM-9MP, Raypa, Spain) for four hours at 550 0C. Finally, the nitrogen-free extract (NFE) was measured by subtracting from the 100 the total sum of crude protein, crude lipid, moisture, ash, and fibre. The ingredients and proximate composition of the four experimental feeds are detailed in Table 1.
Experimental fish
Three hundred fingerlings of Nile tilapia were procured from Bhola Monosex tilapia hatchery, Bhola, Barishal. Before giving the experimental diet, the fish were acclimated for one week in the laboratory with a commercial tilapia feed (crude protein 30.97%, lipid 6.43%, ash 12.13%, and crude fibre 5.89%). The feeding trial was conducted in a hapa (4ft × 3ft) setting, out in a fish pond at the university. Groups of 25 healthy and uniformly sized tilapia fingerlings with initial weight and length of 3.47 ± 0.02 g and 5.45 ± 0.13 cm, respectively, were randomly distributed in triplicate hapas for each dietary treatment following a complete randomised design. Initially, feeding was done at a rate of 5% of body weight; however, this rate was later adjusted by the body weight gain during the trial. Fish were hand-fed twice daily for 56 days, at 8:00 and 16:00. Water quality parameters (dissolved oxygen, temperature, total ammonia, pH) were measured consistently by employing a multimeter (AD800), and these parameters were within the acceptable range during the experimental period (Table 2).
Sample collection
At the end of the feeding trial, all fish were euthanised with an overdose of anaesthesia (clove oil, 5.0 mg/L) and various samples were collected for analysis. For each treatment’s replication, six fish in total were randomly chosen for further analysis. For the histomorphometric evaluation, the distal intestine, liver, and dorsal muscles were collected, immediately transferred into 10% buffered formalin for fixation, and stored at 4 ºC until further processing. Blood was immediately collected and processed according to standard protocols for the biochemical assessment, and the blood cell counts and the assessment of the erythrocytes' morphology.
Evaluation of growth performance and feed utilisation efficiency
At the end of the 56-day of feeding trial, the bulk weight of each replicate tank as well as individual fish weight and length were measured after fasting about 24 h. Growth parameters, such as final body weight (FBW), weight gain (WG), specific growth rate (SGR), feed conversion ratio (FCR), survival rate (SR%), and condition factor (CF) were calculated using the following equations:
Histological analysis
Histomorphological assessment of intestine
All histomorphometric procedures and quantitative assessment of intestinal indices were measured and assessed as described in previous publications (Islam et al. 2024). The collected tissue samples were immediately transferred into 10% buffered formalin for fixation and stored at 4 ºC until further processing. The samples were then dehydrated in a series of ethanol concentrations before being cleaned with pure chloroform. The sample blocks for sectioning were produced by embedding the tissues in paraffin and sectioned at a thickness of 5 µm. The sections were placed on slides and dried overnight. Subsequently, the slides were stained using Haematoxylin and Eosin (H&E) and covered with coverslips. The stained sections were photographed using a digital camera (Leica DM1000, Wetzlar, Germany) affixed to a digital light microscope (Leica DM1000, Wetzlar, Germany). Ten undistorted mucosal folds per section were randomly selected to assess and quantify the intestinal mucosal morphology in terms of height of mucosal folds (hMF), (µm); width of mucosal folds (wMF), (µm); lamina propria (LP), (µm); and number of goblet cells (GCs). GCs were counted in multiple fields of view to ensure a representative count, and the number of GCs in each field of view was recorded. Finally, the density of GCs (number of cells per unit area) was calculated.
Cryosectioning and oil red o staining of liver and muscle tissue
Oil-Red O (ORO) staining demonstrated lipid accumulation in the experimental fish in liver and muscle tissues. For this, fish liver and muscle samples were first kept in cryoprotectant solution for a few hours. Then, both tissue samples were removed and kept overnight in 30% sucrose. Then, the tissue samples were collected from sucrose and covered with aluminium foil to protect the sample’s quality. The frozen samples were then immersed in isopentane (stored in a stainless jar in liquid nitrogen) for 45 s and attached to particle cardboard using Shandon Cryomatrix (Thermo Scientific, Runcorn, UK). Then, all the processed samples were cryosectioned (15 µm), and the sections were fixed gently on microscope slides. Three different slides from each sample were prepared.
The prepared slides were then placed in propylene glycol and ORO (lipid stain) solution for 2 and 6 min, respectively. All the incubated slides were then placed into 85% propylene glycol for 1 min and then rinsed twice in distilled water. After that, all slides were incubated in haematoxylin for 1–2 min and then rinsed thoroughly with tap water. Subsequently, all slides were rinsed twice with distilled water and then covered with a coverslip using an aqueous mounting medium.
Blood sampling and biochemical analysis
Blood was collected by caudal venipuncture using a 1 ml syringe with a 25-gauge needle after anaesthetising the fish with clove oil. Blood samples were immediately collected in a BD vacutainer containing an anticoagulant (K2 EDTA). Haemoglobin and glucose content in blood were then determined by a digital EasyMate® GHb (Model: ET 232, Hb/Glu double monitoring system, Bioptic technology Inc. Taiwan 35,057) using haemoglobin (Hb) and glucose (Glu) strips, respectively. The Neubauer hemacytometer was used to count red blood cells (RBC) and white blood cells (WBC) in blood smears.
Erythrocyte morphology assessment
The blood was smeared on a glass microscope slide soon after being collected. The slides were fixed with methanol and stained with 5% Giemsa solution. Stained slides were subsequently rinsed with distilled water and subsequently air-dried. DPX was used to mount the slides. Then ENA (erythrocyte nuclear abnormalities) and ECA (erythrocyte cellular abnormalities) were examined under a digital light microscope (Leica DM1000, Wetzlar, Germany) coupled to a digital camera (Leica MC 170HD, Heersbrugg, Switzerland) at 20 × magnification. To reduce technical variation, ENA and ECA were scored blindly using randomised coded slides.
Different ENAs were scored following the criteria set by Islam et al. (2020a). Different ENA were defined as: i) micronuclei separated by small circular chromatin bodies with the same staining shapes of the central nucleus, ii) blebbed nuclei with nuclear membrane evagination and euchromatin iii) notched nuclei with nuclear materials-free vacuoles. Different ECA were distinguished: i) cells having a teardrop shape, ii) distorted erythrocytes tugged with a nipple at one end, iii) two twin cells connected together by their cell surface, and iv) elongated cells with an unusual length.
Economic analysis
For the economic analysis, the economic conversion ratio (ECR, USD/Kg of fish) and economic profit index (EPI, USD/Kg of fish) were calculated using the following formula (Martínez‐Llorens et al. 2007):
The price was calculated based on USD/Kg unit (1 USD = 109.21 BDT). Each ingredient price was as follows: FM = 1.0072, EW = 0.915, soybean meal = 0.5494, wheat flour = 0.5951, wheat gluten = 2.7470, rice bran = 0.4578, vegetable oil = 3.6626, fish oil = 6.8675, mineral premix = 3.7359, vitamin premix = 2.4265.
Statistical analysis
The open-source software R (version 4.2.2) and R Studio (2022.12.0 + 353) for Windows 10 (Version 22H2; OS Build 19,045.3208) were used to perform the statistical analyses. All measured data were expressed as mean ± SD (standard deviation). One-way analysis of variance (ANOVA) was used to evaluate the differences between the various dietary groups, and post hoc Tukey's test was used to determine significant differences (HSD) in multiple comparison tests. Orthogonal polynomial (linear and quadratic) regression was used to examine the effects of EW inclusion increasing in the diets. The datasets derived from (a) growth and haematology and (b) histomorphometry were subjected to two PCA (principal component analysis) respectively to evaluate the collective covariation of their respective variables. For PCA analysis “ggfortity” R package was used (Zhang et al. 2020). The first principal component (PC1) scores were gathered in all cases, and then a mixed model was applied. A mixed regression model was applied to correlate PC1 values and CF. All mixed model analyses were carried out using the ‘R’ package “lme4” (Bates et al. 2015). The figures expressed different statistical significance as: '*', p < 0.05, or as '**', p < 0.01.
Results
Growth performance
The fish growth, feed intake, FCR and survival rate of juvenile tilapia are shown in Table 3. After the 56-day feeding trial, Nile tilapia supplemented with D3 appeared to have lower growth performance. The FBW, WG, and SGR of fish fed D3 were found to be significantly lower (p < 0.05), while fish fed D1 and D2 were similar to those fed the control diet. However, FI and FCR showed no significant variation between control and test diets (p > 0.05). The survival rate of fish in the culture units ranged from 76.88 ± 1.21 to 85.33 ± 1.09% and was not affected by the dietary supplementation of EW. Condition factor (CF) was decreased in the D3 dietary group, whilst for the D1 and D2 groups, CF showed no difference compared to the control diet. Based on the quadratic regression analysis between FBW and dietary EW meal level (R2 = 0.877; y = 31.57—0.1550*X -0.006740*X2), Nile tilapia showed to have the best growth performance when replacing 17.5% of FM in the diet with EW meal (Fig. 1). No evidence of disease was observed during the experimental period.
Economic analysis
Although increasing the level of EM in the diets decreased the cost of the experimental feeds, no significant difference (p > 0.05) was observed among the dietary groups for ECR and EPI (Table 3).
Histology
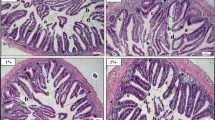
Histomorphological assessment of intestine
The intestinal tissues of Nile tilapia in group D3 exhibited mild to severe alternation. Significantly decreased hMF and wMF were noted in fish fed the D3 diet, whilst fish from the D1 and D2 groups showed non-significant differences (p > 0.05) compared to control (Fig. 2a & b). The LP was significantly increased (p < 0.01) in the D3 group, whilst the lamina propria was decreased in the D2 group (Fig. 2c). The GC index exhibited no significant (p > 0.05) differences between the dietary groups (Fig. 2d). The assessment of different indices of intestinal tissue is presented in the supplementary file (Fig. S.1).
Histomorphological assessment of the intestine of Nile tilapia after 56 days of feeding EW at various levels. a. mucosal fold height (µm), b. mucosal fold width (µm), c. lamina propria (µm) and d. number of mucosal goblet cells/fold (GC). Significant levels are expressed as '*' p < 0.05; '**' p < 0.01
Lipid droplet deposition
Images of ORO staining of neutral lipid droplets in hepatocytes are presented in Fig. 3a. The measurement of neutral lipid droplets is elaborated in the supplementary file (Fig. S.2). The level of neutral lipid droplets increased significantly (p < 0.05) in the D3 dietary group. In contrast, the D1 and D2 groups did not show any significant differences compared to the control diet (Fig. 3b).
Assessment of neutral lipid droplets in the liver tissue of Nile tilapia. Cryosectioning was performed at -20 °C stained with ORO and haematoxylin solution. All images were captured using 40X magnification. Scale bar = 50 µm. a. neutral lipid droplets in liver tissue. b. area of neutral lipid droplets (%) measured in liver tissues using ImageJ software (version 1.53). The significant difference among the experimental diets is expressed as '*' p < 0.05
Muscle quality
Lipid droplet deposition in dorsal muscle is presented in Fig. 4a. No significant differences were observed between the D1, D2 and the control groups. However, a significantly higher lipid droplet deposition was observed in the D3 dietary group compared to control (Fig. 4b).
Lipid droplet analysis of muscle samples of Nile tilapia tissue stained with ORO and solution H&E. All images were captured at 40X magnification. a. lipid droplets in muscle tissue. b. area of lipid droplets (%) in muscle tissues. All measurements were performed using ImageJ software (version 1.53). A significant difference is expressed as '*' p < 0.05
Haematological parameters
FM replacement with EW at different levels significantly (p < 0.05) influenced the haematological parameters in Nile tilapia. The WBC counts exhibited no significant differences in the D1 and D2 dietary groups compared to the control. In contrast, the count was increased in the D3 group (Fig. 5a). The RBC count decreased significantly in the D3 group but exhibited no significant differences between the D1, D2 groups and the control (Fig. 5b). The glucose level was significantly higher in the D3 and D2 groups compared to control, whilst the value of the D1 group was similar to that of the control (Fig. 5c). The Hb level was increased significantly in the D1 group, while the level of Hb was markedly decreased in the D3 group, compared to control diet (Fig. 5d).
Erythrocytic nuclear and cellular abnormalities
The frequency distributions of various ENAs and ECAs in fish fed different levels of EW are shown in Fig. 6. The observed ENAs included regular cells (Fig. S.3a), notched nuclei (Fig. S.3b), blebbed nuclei and micronuclei (Fig. S.3c). Various ECAs were also observed in the erythrocytes of the experimental fish, including twin (Fig. S.3d), elongated (Fig. S.3e) and tear‐drop shaped cells (Fig. S.3f).
Frequencies of erythrocyte nuclear and cellular abnormalities of Nile tilapia after the 56-day feeding trial. a. frequency of notched nuclei; b. frequency of micronucleus; c. frequency of twin cells; d. frequency of elongated cells, e. frequency of tear-drop cells. '*' indicates a significant difference (p < 0.05)
A significant increase (p < 0.05) of notched cells has been detected in the blood of fish fed the D3 diet compared to the control. In contrast, the D1 and D2 groups showed no significant differences compared to the control (Fig. 6a). Regarding micronuclei, there were no significant differences in the D2 group compared to the control diet. However, the D1 and D3 groups showed significant differences compared to the control diet (Fig. 6b).
The frequencies of teardrop-shaped cells appeared significantly lower in the group D1 than those in the fish fed the D3 diet (Fig. 6c). The frequency of twin cells did not show any significant differences between groups (Fig. 6d). However, the frequency of elongated cells was significantly lower in the D1 group, whilst groups D2 and D3 were not influenced by the EW diets (Fig. 6e).
PCA analysis
Regarding the PCA, the loading of all the variables, including FCR, FBW, RBC, WBC, Glu, Hb, MN, NN, TD, EG, and TW, are presented in Fig. 7a. Among all the loading variables, FBW, Hb and RBC were positively correlated with each other in PC1. On the other hand, FCR, WBC, Glu, MN, NN, TD, EG, and TW showed a negative correlation with each other, whilst FCR and Glu overlapped. Among all the loading variables, FBW showed the highest positive loading on PC1 (Table S.1). This orientation of the variables reveals that the first principal component (hence referred to as ‘PC1’) explained 64.31% of the variance in the data and reflected a composite perspective of most of the loading variables. At the same time, the second principal component (PC2) explained 10.39% of the variance in the data.
PCA biplot representing (a) growth and haematological parameters in fish. Different colours indicate the different dietary treatments; (b) intestinal histomorphometric indices (PC1_intestinal health) of the distal intestine; and (c) mixed-effects model of ‘PC1_intestinal health’ and condition factor of fish
Mixed model analysis
The loadings of all the histomorphometric variables in the intestine are presented in Fig. 7b. Loading of all variables, such as the height of hMF, wMF, LP and GC, were correlated with each other. Among all the variables, hMF, wMF and GC were positively correlated with each other in PC1, while LP showed a negative correlation with others. Among all the loading variables, wMF showed the highest loading on PC1 (Table S.2). The first principal component explained 56.86% of the variance and was used to represent the overall status of the intestine. The second principal component ‘PC2’ explained 21.13% of the variance in the data. The nested ANOVA on ‘PC1 intestinal health’ demonstrated that the D2 group significantly differed from the control group. Finally, ‘PC1 intestinal health positively correlated with condition factor (Fig. 7c).
Discussion
A significant concern in aquaculture production is the reliance on FM, so identifying more environmentally acceptable, economically sustainable feed ingredients is important. The excellent nutritional properties of EW, including its high protein and amino acid profile, make it a promising aquafeed ingredient (Mohanta et al. 2016). However, over the past, most research on EW focused mainly on the nutritional status of the prepared diets and the possible influences they could have on growth performance and proximate composition of the cultured fish (Chaves et al. 2015; Beg et al. 2016). The effects of EW on the haematological and histological indices in fish have not yet been thoroughly studied. Therefore, the present study gives an insight into the potential application of EW in Nile tilapia, focusing on some aspects of fish physiology. Our initial results suggested that substituting up to 20% of FM with EW in Nile tilapia diets has no negative impact on growth performance compared to the control diet. Similar findings were noted in earlier studies. Russio et al. (2022), after supplementing up to 20% of EW meal in the experimental diets, did not observe any negative impacts on Nile tilapia's growth. Similarly, replacing FM with EW meal up to 20% in the feed of Nile tilapia did not negatively affect the growth and physiological performance of fish (El-Ouny et al. 2023). In another study, Sogbesan et al. (2007) investigated the potential beneficial effects of EW meal in the diet of vundu catfish, Heterobranchus longifilis. In that study, the authors observed the suitability of EW meals for this species' diet, which agrees with our findings. Similar growth performances were also observed in the earlier study when a moderate substitution was made (up to 32.2%-40%), which could be explained by the comparable protein and lipid content of EW to FM, which could satisfy the nutritional needs of Nile tilapia (Bag et al. 2012; Mohanta et al. 2016).
However, the retardation of growth performance was observed when EW replaced 40% of the FM in the diet. This indicates that low FM diets may not provide enough nutrition or digestibility for Nile tilapia. Additionally, the high coelomic fluid content in EW can be toxic to fish (Kobayashi et al. 2001) and reduce feed palatability (Ngoc et al. 2016; Musyoka et al. 2019), which likely contributed to the poor growth performance of fish in the D3 group. EW, in particular, have a poisonous coelomic fluid that contains lysenin, a defense protein that can harm vertebrates (Yamaji et al. 1998). Since the EW used in our investigation was not pre-treated (heated), it is possible that the lysenin facilitated these adverse effects. Furthermore, the higher chitin content in whole EW at higher meal levels can reduce feed efficiency and cause growth depression in fish due to the glucosamine polymer. Nile and blue tilapia are especially intolerant to chitin and exhibit suppressed growth, regardless of the inclusion level (Shiau and Yu 1999). A shortage of specific amino acids like lysine, methionine, and cysteine could also contribute to poor performance, but this varies depending on the species of fish (Pereira and Gomes 1995). For example, efficient utilisation of EW was observed in butter catfish (Ompok pabda) (Chakraborty et al. 2021), hybrid catfish fingerlings (Monebi and Ugwumba 2013) and Rohu (Labeo rohita) at more than 40% FM replacement (Mohanta et al. 2016). EW's nutritional composition and digestibility also vary depending on the worm species and the EW culture system (Ding et al. 2019).
The microscopic structure and function of the intestine can be assessed by various indices, such as absorption surface area, height and width of the intestinal fold, and goblet cell density (Siddik et al. 2022; Khojasteh 2012). Multiple factors can impact the intestinal structure, reduce nutrient absorption, and ultimately impact the growth performance (Rašković et al. 2011). Among all the factors, diet is one of the most important factors that regulates the gut microbiota and affects local and general immune responses (Dawood 2021). In the present study, we explored the effects of EW meal on the microscopic structure of fish intestine. The results showed that up to 20% FM substitution, no morphological alterations of the intestine were observed between the treatments and the FM-based control group. These observations do not agree with an earlier study on hybrid snakehead (Channa argus ♂ × Channa maculate ♀) fed different levels of EW. In that study, the author reported that including 10% EW in the diet enhanced the intestinal fold height, width and muscular structure (Chao et al. 2013). The variation in the findings could be due to the study duration or other experimental conditions, such as the ages and nature of EW.
The histomorphological assessment of the intestine, substituting FM up to 20% with EW, exhibited no morphological alterations. On the other hand, intestines from the D3 dietary group showed a reduction in intestinal fold height, width and GC index. Such alternations may be associated with a decrease in the nutrient absorption area of the intestine in fish fed the highest inclusion level of EW. Similar results were observed in rainbow trout, where the authors identified an adverse effect on intestinal fold length when the fish were fed a high inclusion (50%) of full-fat insect meal (Cardinaletti et al. 2019). These changes could be associated with excessive antinutrient factors or undigested material, though the role of altered gut microbiota cannot be excluded (Mi et al. 2022). In this study, GC increased slightly in the D2 dietary group, indicating the positive impacts of EW on the digestion and absorption of nutrients, leading to enhanced intestinal immunity of fish (Alesci et al. 2022).
Condition factor (CF) is considered indicative of the fish's physiological state and is also connected to the fish's welfare during different life stages (Nash et al. 2006). The principle based on which conditions factors are generally used to assess the fitness of the fish is that the heavier a fish of a certain length is, the better its condition (Froese 2006). In the current study, we evaluated the overall intestine wellness using the association of PC1 with CF. This approach has also been used before to analyse the histomorphology of the intestine in lumpfish (Willora et al. 2022). The present study measured several intestinal histological indices such as hMF, wMF, LP and GC. These histological indices have been used as biomarkers to assess the effect of the diets (Hernández et al. 2012). In the PCA biplot, PC1 explained most of the variance of intestinal histology indices, indicating the significant nutritional effects in the dataset. Regarding the overall fitness of the intestine, a linear regression mixed effects model was employed between PC1 intestinal health and CF, and the result revealed a positive correlation. Although the positive scoring of the PC1 intestinal indices (hMF, wMF, LP and GC) with the condition factor demonstrated the beneficial role of the dietary EW, further study is needed to examine the mechanisms and interactions that lead to the improvement of the overall health status of fish.
The haemato-biochemical parameters routinely assessed include RBC and WBC count, as well as Hb and Glu concentration (Burgos-Aceves et al. 2019). The differential blood cell counts can often be indicators of infectious diseases (Francesco et al. 2012). In the current study, EW inclusion of up to 20% in the diets showed no significant impact on the values of the different haematological parameters. However, we observed significantly lower values of RBC and Hb in fish fed the highest inclusion levels of EW. Similar findings were also observed in earlier studies, where RBC and Hb were also decreased at high inclusion levels of black soldier fly meal in the diet of juvenile goldfish (Kamalii et al. 2022). As a high level of FM replacement with EW may not fulfil all the nutritional requirements of Nile tilapia, deficiencies of nutrients necessary for erythropoiesis may be responsible for reducing RBC and Hb, as Witeska (2015) has suggested. Such alterations in the blood parameters can substantially reduce the oxygen transfer to tissues and compromise multiple physiological functions.
Glucose is the primary energy source for various cell functions, and its blood levels are under hormonal regulation (Islam et al. 2020a). As plasma glucose is highly responsive to stress, variation in the blood glucose is often strongly connected with stress factors rather than with the nutritional state of fish (Wagner and Congleton 2004). Moreover, poor nutrition is frequently regarded as a stressor for fish and chronic stress resulting from poor diet can also increase glucose levels in fish blood. In our study, fish fed the D3 diet showed increased blood glucose levels, which may be explained by the enhanced gluconeogenesis of a fish stressed from improper nutrition in response to their unsatisfied energy demand.
In the present study, various erythrocytic morphological changes (cellular and nuclear) were noticed in the blood of Nile tilapia. These alternations may cause physiological complications at higher inclusion levels of EW. The nucleus structure and morphology of blood cells might be altered in response to stressors or malnutrition (Sharaf et al. 2010). Though many studies demonstrated the potential of EW for growth promotion in Nile tilapia, none of these studies investigated any possible morphological alternations of erythrocytes in this species in response to EW. However, several studies reported nuclear and cellular erythrocytic alterations in different fish species due to many stressors other than malnutrition (Shahjahan et al. 2020; Al-Emran et al. 2022). For instance, according to a study by Islam et al. (2020a), high temperatures affected the morphology of blood cells and the haemato-biochemical parameters of Nile tilapia. Similarly, multiple ECA, elongated forms, fusion, tear-drop shape, and twin cells were detected in fish blood exposed to various salinities (Jahan et al. 2019). In addition, hazardous compounds alter cell metabolism, ion permeability, and membrane shape, causing structural damage to erythrocytes (Islam et al. 2020b; Ritu et al. 2022; Sadiqul et al. 2016). Therefore, we believe that the cellular alternations observed here indicate a stress response due to the high levels of EW (Zafalon-Silva et al. 2017).
In the current study, the haematological analysis indicated that replacing fishmeal (FM) with earthworm (EW) at more than 20% can cause some toxic effects on Nile tilapia. However, in contrast to this, (Chakraborty et al. 2021) found that the haematology of the butter catfish, Ompok pabda was not affected when EW substituted the FM in the diet. According to their study, replacing FM protein with EW protein at any level had no toxic effect on the butter catfish. One possible reason for this difference could be that carnivorous butter catfish are more resistant to the toxins of such organisms, which could be part of their diets.
Fish fed a high-lipid diet have generally been shown to accumulate excess lipids in various organs of fish. For instance. Ruyter et al. (2016) have reported a higher occurrence of hepatic lipid accumulation in fish liver fed diets containing < 1% of EPA and DHA. However, contradictory results were reported in the case of Atlantic salmon, where 100% replacement of FM with insect meal (Black soldier fly meal) in the diet did not show any significant changes in lipid accumulation in the liver or size distribution of the lipid droplets in the liver (Belghit et al. 2019). A study by Li et al. (2017) reported histological alternations in the liver of Jian carp using defatted black soldier fly larvae meal in the diet and notably lower lipid content in the liver. It might be because of the cuticular chitin content of the insects that inhibited fatty acid synthesis (Li et al. 2017). Lipid deposition is also regulated by hepatic lipogenesis, increased adipocyte lipolysis rates and the absorption of the released fatty acids from the adipose tissue (Vyas et al. 2012). Generally, excess lipid droplets accumulated in the liver of fish can lead to a condition known as hepatic steatosis or fatty liver disease. This excess accumulation of lipid droplets can interfere with normal cellular functions. Moreover, fatty acids are metabolised in the liver through various enzymatic pathways, including beta-oxidation and lipogenesis. Imbalances in these pathways can lead to altered production and accumulation of intermediate products that may influence cholesterol and triglyceride synthesis (Dai et al. 2015). Furthermore, these imbalances can lead to metabolic alterations in the circulatory system in different body parts and ultimately compromise the fish's growth performance (Li et al. 2019). However, the current study assumes that a higher inclusion level of EW might have produced excess caloric in the body and created stress and indigestibility that altered the normal physiological condition. The present study suggests that including up to 20% of EW in the diet has no apparent negative impact and significant variation in lipid deposition in the liver.
Muscle structure is an important parameter that reflects the nutritional condition but also the agility of fish. This muscle structure is also connected to the growth and quality of fish and can be significantly influenced by dietary ingredients (Alami-Durante et al. 2018). Thus, various nutritional deficiencies can alter the muscle structure of Atlantic salmon, causing myodegeneration (Rodger et al. 1991). In the present study, lipid droplets in the muscle increased significantly in the D3 dietary group compared to the control group. Although lipid droplets are essential sources of energy for muscle fibres, excessive lipid droplets may produce lipotoxicity stress and irrigate the structure of muscle fibre (Brøns and Grunnet 2017; Morales et al. 2017). Excessive lipids result in fatty infiltrations in the intermuscular adipose tissue. This adipose tissue can regulate inflammation by producing fatty acid-binding proteins, adipokines, resistin leptin and lipid droplet proteins (Wu et al. 2014; Konigorski et al. 2019). This change is related to the ageing process and can result in loss of muscle strength and a decrease in muscle insulin sensitivity (Brioche et al. 2016). So, our study suggests that including up to 20% of EW in the diet does not negatively impact lipid droplet deposition, muscle fibre strength and lipid metabolism pathways.
The simple economic analysis in the present study revealed that the diet price decreased while the ECR increased with the increasing levels of EW meal in the diets (Table 4). As the profit level is mainly affected by various factors, such as the prices of feed, the feeding rate, the fish size, fish yield, the fish sales etc. (Hebicha et al. 2013), the net profit will therefore be increased if the fish selling price increases, or one of the main costly factors, like diet cost, decreases (Abdel-Tawwab et al. 2020). However, based on the obtained results, including EW as a replacer of FM in the diets has a positive economic outcome and reduces the feeding cost.
Conclusion
Based on the findings, it can be concluded that EW is a suitable alternative source of protein to replace up to 20% of FM in the diet of Nile tilapia. This conclusion is supported by the lack of histological alternations in the intestine or changes in blood parameters and lipid accumulation in the liver or muscle. Higher inclusion levels (40%) of EW affected the growth and the biochemical parameters while altering the erythrocyte morphology. In addition, it resulted in increased accumulation of lipid droplets in the liver and muscle tissue. The current study also indicates a reduction in the cost of the diet with an increasing replacement level of EW in the diets.
Data availability
Data is provided within the manuscript or supplementary information files.
References
Abdel-Tawwab M, Khalil RH, Metwally AA, Shakweer MS, Khallaf MA, Abdel-Latif HMR (2020) Effects of black soldier fly (Hermetia illucens L.) larvae meal on growth performance, organs-somatic indices, body composition, and hemato-biochemical variables of European sea bass, Dicentrarchus labrax. Aquaculture 522:735136
Ahmed R, Eissa H, Shafi M, Aly M, Al-Kareem O (2020) Influence of replacement of fish meal with the earthworm Eisenia fetida on growth performance, feed utilization and blood parameters of Nile tilapia (Oreochromis niloticus). J Aquac Mar Biol 9(3):7–42
Alami-Durante H, Bazin D, Cluzeaud M, Fontagné-Dicharry S, Kaushik S, Geurden I (2018) Effect of dietary methionine level on muscle growth mechanisms in juvenile rainbow trout (Oncorhynchus mykiss). Aquaculture 483:273–285
Albrektsen S, Kortet R, Skov PV, Ytteborg E, Gitlesen S, Kleinegris D, Mydland LT, Hansen JØ, Lock EJ, Mørkøre T (2022) Future feed resources in sustainable salmonid production: A review. Rev Aquac 14(4):1790–1812
Al-Emran M, Hasan NA, Khan MP, Islam SM, Bashar A, Zulfahmi I, Shahjahan M, Sumon KA (2022) Alterations in hematological parameters and the structure of peripheral erythrocytes in Nile tilapia (Oreochromis niloticus) exposed to profenofos. Environ Sci Pollut Res 29(19):29049–29061
Alesci A, Pergolizzi S, Savoca S, Fumia A, Mangano A, Albano M, Messina E, Aragona M, Lo Cascio P, Capillo G (2022) Detecting intestinal goblet cells of the broadgilled hagfish Eptatretus cirrhatus (forster, 1801): A confocal microscopy evaluation. Biology 11(9):1366
Bag MP, Mahapatra SC, Rao PS, Debajyoti C, Himadri P (2012) Nutritive potential of earthworm (Eisenia foetida) meal in the diet for Nile tilapia (Oreochromis niloticus) fingerlings. Int Res J Pharm Appl Sci 2(5):117–123
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67(1):1–48
Beg M, Mandal B, Moulick S (2016) Potential of earthworm meal as a replacement of fish meal for Indian major carps. Int J Fish Aquat Stud 4(3):357–361
Belghit I, Liland NS, Gjesdal P, Biancarosa I, Menchetti E, Li Y, Waagbø R, Krogdahl Å, Lock E-J (2019) Black soldier fly larvae meal can replace fish meal in diets of sea-water phase Atlantic salmon (Salmo salar). Aquaculture 503:609–619
Brioche T, Pagano AF, Py G, Chopard A (2016) Muscle wasting and aging: experimental models, fatty infiltrations, and prevention. Mol Aspects Med 50:56–87
Brøns C, Grunnet LG (2017) Mechanisms in endocrinology: skeletal muscle lipotoxicity in insulin resistance and type 2 diabetes: a causal mechanism or an innocent bystander? Eur J Endocrinol 176(2):R67–R78
Burgos-Aceves MA, Lionetti L, Faggio C (2019) Multidisciplinary haematology as prognostic device in environmental and xenobiotic stress-induced response in fish. Sci Total Environ 670:1170–1183
Cardinaletti G, Randazzo B, Messina M, Zarantoniello M, Giorgini E, Zimbelli A, Bruni L, Parisi G, Olivotto I, Tulli F (2019) Effects of graded dietary inclusion level of full-fat Hermetia illucens prepupae meal in practical diets for rainbow trout (Oncorhynchus mykiss). Animals 9(5):251
Chao W, Mao S, Xie S, Zhou A, Liang R, Zou J (2013) Effects of fresh diet Eisenia foetida on the intestinal tissue structure of hybrid snakehead (in chinese). Guangxi Agric Sci 22:131–136. https://doi.org/10.16768/j.issn.1004-874x.2013.22.052
Chaklader MR, Siddik MA, Fotedar R (2020) Total replacement of fishmeal with poultry by-product meal affected the growth, muscle quality, histological structure, antioxidant capacity and immune response of juvenile barramundi, Lates calcarifer. PLoS One 15(11):e0242079
Chakraborty P, Islam MR, Hossain MA, Fatema UK, Shaha DC, Sarker MSA, Akter T (2021) Earthworm meal (Perionyx excavatus) as an alternative protein source to fish meal in feed for juvenile butter catfish (Ompok pabda). Aquac Int 29:2119–2129
Chaves RC, de Paula RQ, Gücker B, Marriel IE, Teixeira AO, Boëchat IG (2015) An alternative fish feed based on earthworm and fruit meals for tilapia and carp postlarvae. Rev Bras Biol 13(1):15–24
Dai W, Wang K, Zheng X, Chen X, Zhang W, Zhang Y, Hou J, Liu L (2015) High fat plus high cholesterol diet lead to hepatic steatosis in zebrafish larvae: a novel model for screening anti-hepatic steatosis drugs. Nutr Metab (Lond) 12:1–11
Dawood MA (2021) Nutritional immunity of fish intestines: Important insights for sustainable aquaculture. Rev Aquac 13(1):642–663
Ding S, Lin X, He S (2019) Earthworms: A source of protein. J Food Sci Eng 9:159–170
El-Ouny YM, Maulu S, Zaki MAA, Helaly AA, Nour AAM, ElBasuini MF, Labib EMH, Khalil RH, Gouda AH, Hessein AAA, Verdegem M, Khalil HS (2023) Effect of fishmeal replacement with dried red wigglers (Eisenia fetida) worm meal on growth and feed utilization, production efficiency, and serum biochemistry in Nile tilapia (Oreochromis niloticus) fingerlings. Aquac Rep 29:101518
El-Sayed AFM, Fitzsimmons K (2023) From Africa to the world—the journey of Nile tilapia. Rev Aquac 15:6–21
FAO (2021) Atlantic salmon-nutritional requirements. Food and Agriculture Organization. Available: http://www.fao.org/fishery/affris/species-profiles/atlantic-salmon/nutritionalrequirements/en/. Accessed 13 Apr 2021
FAO (2022) The state of world fisheries and aquaculture 2022. In: Towards blue transformation. FAO, Rome. https://doi.org/10.4060/cc0461en
Francesco F, Satheeshkumar P, Senthil Kumar D, Caterina F, Giuseppe P (2012) A comparative study of hematological and blood chemistry of Indian and Italian Grey Mullet (Mugil cephalus Linneaus 1758). HOAJ Biol 1(1):5
Froese R (2006) Cube law, condition factor and weight–length relationships: history, meta-analysis and recommendations. J Appl Ichthyol 22(4):241–253
Garczyńska M, Kostecka J, Pączka G, Mazur-Pączka A, Cebulak T, Butt KR (2023) Chemical composition of earthworm (Dendrobaena veneta Rosa) biomass is suitable as an alternative protein source. Int J Environ Res Public Health 20(4):3108
Hebicha HA, El Naggar GO, Nasr-Allah AM (2013) Production economics of Nile tilapia (Oreochromis niloticus) pond culture in El-Fayum Governorate. Egypt J Appl Aquacult 25(3):227–238
Hender A, Siddik MA, Howieson J, Fotedar R (2021) Black soldier fly, Hermetia illucens as an alternative to fishmeal protein and fish oil: impact on growth, immune response, mucosal barrier status, and flesh quality of juvenile barramundi, Lates calcarifer (Bloch, 1790). Biology 10(6):505
Hernández DR, Santinón JJ, Sánchez S, Domitrovic HA (2012) Dietary soybean meal on growth and intestinal morphology of South American catfish, Rhamdia quelen, larvae. Ciênc Rural 42:1662–1668
Horwitz W, Latimer G Jr (2006) Association of official analytical chemists (AOAC). In: Official methods of analysis of AOAC International, 18th edn. AOAC International, Gaithersburg
Islam SM, Sultana R, Imran M, Jannat MFT, Ashaf-Ud-Doulah M, Rohani MF, Brown C, Shahjahan M (2020a) Elevated temperature affects growth and hemato-biochemical parameters, inducing morphological abnormalities of erythrocytes in Nile tilapia. Oreochromis Niloticus Aquac Res 51(10):4361–4371
Islam SM, Rohani MF, Zabed SA, Islam MT, Jannat R, Akter Y, Shahjahan M (2020b) Acute effects of chromium on hemato-biochemical parameters and morphology of erythrocytes in striped catfish Pangasianodon hypophthalmus. Toxicol Rep 7:664–670
Islam SMM, Willora FP, Sørensen M, Rbbani G, Siddik MAB, Zatti K, Gupta S, Carr I, Santigosa E, Brinchmann MF, Thompson KD, Vatsos IN (2024) Mucosal barrier status in Atlantic salmon fed rapeseed oil and Schizochytrium oil partly or fully replacing fish oil through winter depression. Fish Shellfish Immunol 8(149):109549
Jahan A, Nipa TT, Islam SM, Uddin MH, Islam MS, Shahjahan M (2019) Striped catfish (Pangasianodon hypophthalmus) could be suitable for coastal aquaculture. J Appl Ichthyol 35(4):994–1003
Kamalii A, Antony C, Ahilan B, Uma A, Prabu E (2022) Dietary protein replacement of fish meal with black soldier fly larvae meal: effects on growth, whole-body composition, digestive enzyme activity, muscle-growth-related gene expression and haemato-biochemical responses of juvenile goldfish, Carassius auratus. Turk J Fish Aquat 23(2):TRJFAS21837
Kauschke E, Mohrig W, Cooper EL (2007) Coelomic fluid proteins as basic components of innate immunity in earthworms. Eur J Soil Biol 43:S110–S115
Khanjani MH, Sharifinia M (2021) Production of Nile tilapia Oreochromis niloticus reared in a limited water exchange system: The effect of different light levels. Aquaculture 542:736912
Khojasteh SB (2012) The morphology of the post-gastric alimentary canal in teleost fishes: a brief review. Int J Aqua Sci 3(2):71–88
Kobayashi H, Ohtomi M, Sekizawa Y, Ohta N (2001) Toxicity of coelomic fluid of the earthworm Eisenia foetida to vertebrates but not invertebrates: probable role of sphingomyelin. Comp Biochem Physiol C Toxicol Pharmacol 128(3):401–411
Konigorski S, Janke J, Drogan D, Bergmann MM, Hierholzer J, Kaaks R, Boeing H, Pischon T (2019) Prediction of circulating adipokine levels based on body fat compartments and adipose tissue gene expression. Obes Facts 12(6):590–605
Li S, Ji H, Zhang B, Zhou J, Yu H (2017) Defatted black soldier fly (Hermetia illucens) larvae meal in diets for juvenile Jian carp (Cyprinus carpio var. Jian): Growth performance, antioxidant enzyme activities, digestive enzyme activities, intestine and hepatopancreas histological structure. Aquaculture 477:62–70
Li H, Xu W, Jin J, Zhu X, Yang Y, Han D, Liu H, Xie S (2019) Effects of dietary carbohydrate and lipid concentrations on growth performance, feed utilization, glucose, and lipid metabolism in two strains of gibel carp. Front Vet Sci 6:165
Macias-Sancho J, Poersch LH, Bauer W, Romano LA, Wasielesky W, Tesser MB (2014) Fishmeal substitution with Arthrospira (Spirulina platensis) in a practical diet for Litopenaeus vannamei: effects on growth and immunological parameters. Aquaculture 426:120–125
Madsen H, Nguyen HM, Lanza GR, Stauffer JR Jr (2022) A one health approach relative to trematode-caused diseases of people and animals associated with aquaculture. Rev Fish Sci Aquac 30(4):542–566
Martínez-Llorens S, Moñino AV, Tomás Vidal A, Salvador VJM, Pla Torres M, Jover Cerdá M (2007) Soybean meal as a protein source in gilthead sea bream (Sparus aurata L.) diets: effects on growth and nutrient utilization. Aquac Res 38(1):82–90
Mi J, Lu R, Yan X, Song D, Yang L, Qin C, Yang G, Zhang H, Lin M, Nie G (2022) Evaluating the mixture of earthworm meal and wormcast as a protein source for common carp (Cyprinus carpio L.) based on growth performance, antioxidant, immune capacity, lipid metabolism and intestinal health. Aquac Rep 24:101118
Mohanta KN, Subramanian S, Korikanthimath VS (2016) Potential of earthworm (Eisenia foetida) as dietary protein source for rohu (Labeo rohita) advanced fry. Cogent Food Agric 2(1):1138594
Monebi C, Ugwumba A (2013) Utilization of the earthworm, Eudrilus eugeniae in the diet of Heteroclarias fingerlings. Int J Fish Aquac 5(2):19–25
Morales PE, Bucarey JL, Espinosa A (2017) Muscle lipid metabolism: role of lipid droplets and perilipins. J Diabetes Res 2017:1789395
Musyoka SN, Liti DM, Ogello E, Waidbacher H (2019) Utilization of the earthworm, Eisenia fetida (Savigny, 1826) as an alternative protein source in fish feeds processing: A review. Aquac Res 50(9):2301–2315
Nash RD, Valencia AH, Geffen AJ (2006) The origin of Fulton’s condition factor—setting the record straight. Fisheries 31(5):236–238
Ngoc TN, Pucher J, Becker K, Focken U (2016) Earthworm powder as an alternative protein source in diets for common carp (Cyprinus carpio L.). Aquac Res 47(9):2917–2927
Nhi NHY, Preston T, Ogle B, Lundh T (2010) Effect of earthworms as replacement for trash fish and rice field prawns on growth and survival rate of marble goby (Oxyeleotris marmoratus) and Tra catfish (Pangasius hypophthalmus). Livest Res Rural Dev 22:204
Njieassam E (2016) Effects of using blood meal on the growth and mortality of catfish. J Ecosyst Ecography 6:204
Ogello EO, Munguti JM, Sakakura Y, Hagiwara A et al (2014) Complete replacement of fish meal in the diet of nile tilapia (Oreochromis niloticus L.) grow-out with alternative protein sources. A review. Int J Adv Res 2(8):962–978
Olele N (2011) Growth response of Heteroclarias fingerlings fed on earthworm meal in hatchery tanks. J Life Sci 3(2):131–136
Parolini M, Ganzaroli A, Bacenetti J (2020) Earthworm as an alternative protein source in poultry and fish farming: Current applications and future perspectives. Sci Total Environ 734:139460
Pereira JÓ, Gomes EF (1995) Growth of rainbow trout fed a diet supplemented with earthworms, after chemical treatment. Aquac Int 3:36–42
Rašković B, Stanković M, Marković Z, Poleksić V (2011) Histological methods in the assessment of different feed effects on liver and intestine of fish. J Agric Sci (Belgrade) 56(1):87–100
Ritu RF, Islam SM, Rashid H, Haque SM, Zulfahmi I, Sumon KA (2022) Application of fenitrothion on Heteropneustes fossilis causes alteration in morphology of erythrocytes via modifying hematological parameters. Toxicol Rep 9:895–904
Rodger H, Murphy T, Drinan E, Rice D (1991) Acute skeletal myopathy in farmed Atlantic salmon Salmo salar. Dis Aquat Org 12(1):17–23
Russio SCP, Cardoso IL, Ferri GH, Silva LC, de Queiroz JF, Ivo MA, Ishikawa MM (2022) Use of earthworm (Eisenia fetida) meal as a supplement for feeding tilapia juveniles (Oreochromis niloticus). Int J Sci Res 4(2):86–91
Ruyter B, Bou M, Bæverfjord G, Østbye T-KK, Ytrestøy T, Bjerke MT, Sigholt T, Berge GM (2016) Long-term effects of low omega-3 levels in feed on salmon health. Nofima report series [1061]. https://nofima.brage.unit.no/nofima-xmlui/handle/11250/2394844
Sadiqul IM, Ferdous Z, Nannu MTA, Mostakim GM, Rahman MK (2016) Acute exposure to a quinalphos containing insecticide (convoy) causes genetic damage and nuclear changes in peripheral erythrocytes of silver barb, Barbonymus gonionotus. Environ Pollut 219:949–956
Shahjahan M, Khatun MS, Mun MM, Islam SM, Uddin MH, Badruzzaman M, Khan S (2020) Nuclear and cellular abnormalities of erythrocytes in response to thermal stress in common carp Cyprinus carpio. Front Physiol 11:543
Sharaf S, Khan A, Khan MZ, Aslam F, Saleemi MK, Mahmood F (2010) Clinico-hematological and micronuclear changes induced by cypermethrin in broiler chicks: Their attenuation with vitamin E and selenium. Exp Toxicol Pathol 62(4):333–341
Shiau S-Y, Yu Y-P (1999) Dietary supplementation of chitin and chitosan depresses growth in tilapia, Oreochromis niloticus× O. aureus. Aquaculture 179(1–4):439–446
Siddik MA, Howieson J, Partridge GJ, Fotedar R, Gholipourkanani H (2018) Dietary tuna hydrolysate modulates growth performance, immune response, intestinal morphology and resistance to Streptococcus iniae in juvenile barramundi, Lates Calcarifer. Sci Rep 8(1):15942
Siddik MAB, Vatsos IN, Rahman MA, Pham HD (2022) Selenium-enriched spirulina (SeE-SP) enhance antioxidant response, immunity, and disease resistance in juvenile Asian seabass, Lates calcarifer. Antioxidants 11(8):1572
Sogbesan A, Ugwumba A (2008) Nutritional values of some non-conventional animal protein feedstuffs used as fishmeal supplement in aquaculture practices in Nigeria. Turkish J Fish Aquat Sci 8(1):159–164
Sogbesan OA, Ugwumba AAA, Madu CT, Eze SS, Isa J (2007) Culture and utilization of earthworm as animal protein supplement in the diet of Heterobranchus longifilis fingerlings. J Fish Aquat Sci 2(6):375–386
Tacon AG, Metian M (2008) Global overview on the use of fish meal and fish oil in industrially compounded aquafeeds: Trends and future prospects. Aquaculture 285(1–4):146–158
Tacon A, Stafford E, Edwards C (1983) A preliminary investigation of the nutritive value of three terrestrial lumbricid worms for rainbow trout. Aquaculture 35:187–199
Van Vo B, Siddik MA, Fotedar R, Chaklader MR, Hanif MA, Foysal MJ, Nguyen HQ (2020) Progressive replacement of fishmeal by raw and enzyme-treated alga, Spirulina platensis influences growth, intestinal micromorphology and stress response in juvenile barramundi, Lates Calcarifer. Aquaculture 529:735741
Vyas D, Kadegowda AK, Erdman RA (2012) Dietary conjugated linoleic Acid and hepatic steatosis: species-specific effects on liver and adipose lipid metabolism and gene expression. J Nutr Metab 2012:932928
Wagner T, Congleton JL (2004) Blood chemistry correlates of nutritional condition, tissue damage, and stress in migrating juvenile chinook salmon (Oncorhynchus tshawytscha). Can J Fish Aquat Sci 61(7):1066–1074
Wagner JJ, Stanton TL (2012) Formulating rations with the Pearson square, vol 01. Colorado State University Extension, p 618
Willora FP, Vatsos IN, Mallioris P, Bordignon F, Keizer S, Martınez-Llorens S, Sørensen M, Hagen Ø (2022) Replacement of fishmeal with plant protein in the diets of juvenile lumpfish (Cyclopterus lumpus, L. 1758): effects on digestive enzymes and microscopic structure of the digestive tract. Aquaculture 561:738601
Witeska M (2015) Anemia in teleost fishes. Bull Eur Ass Fish Pathol 35(4):148–160
Wu LE, Samocha-Bonet D, Whitworth PT, Fazakerley DJ, Turner N, Biden TJ, James DE, Cantley J (2014) Identification of fatty acid binding protein 4 as an adipokine that regulates insulin secretion during obesity. Mol Metab 3(4):465–473
Yamaji A, Sekizawa Y, Emoto K, Sakuraba H, Inoue K, Kobayashi H, Umeda M (1998) Lysenin, a novel sphingomyelin-specific binding protein. J Biol Chem 273(9):5300–5306
Younis E-SM, Al-Quffail AS, Al-Asgah NA, Abdel-Warith A-WA, Al-Hafedh YS (2018) Effect of dietary fish meal replacement by red algae, Gracilaria arcuata, on growth performance and body composition of Nile tilapia Oreochromis niloticus. Saudi J Biol Sci 25(2):198–203
Zafalon-Silva B, Zebral YD, Bianchini A, Da Rosa CE, Marins LF, Colares EP, Martinez PE, Bobrowski VL, Robaldo RB (2017) Erythrocyte nuclear abnormalities and leukocyte profile in the Antarctic fish Notothenia coriiceps after exposure to short-and long-term heat stress. Polar Biol 40:1755–1760
Zhang Z, Lin E, Zhuang H, Xie L, Feng X, Liu J, Yu Y (2020) Construction of a novel gene-based model for prognosis prediction of clear cell renal cell carcinoma. Cancer Cell Int 20(1):1–18
Acknowledgements
This study was undertaken by the funding of Patuakhali Science and Technology University, Bangladesh. The authors would like to thank Md Arifur Rahman for helping to purchase juvenile tilapia and MS students Kawser, Tanveer for assisting with sample collection.
Funding
Open access funding provided by Nord University. This research was supported by the Research and Training Centre (RTC) (Project no. Fish-2021-52), Patuakhali Science and Technology University, Bangladesh.
Author information
Authors and Affiliations
Contributions
B.D.: Investigation, Data curation, Formal analysis, Writing – original draft. S.M.M.I.: Data curation, Formal analysis, Writing – original draft. S.K.N.: Data curation, Investigation. A.A.: Data curation, Investigation. I.N.V.: Writing – review & editing, Funding acquisition, Supervision. M.A.B.S.: Conceptualization, Formal analysis, Writing – review & editing, Funding acquisition, Supervision.
Corresponding authors
Ethics declarations
Ethical approval
The experiment procedures and protocol followed the guidelines for the Care and Use of Laboratory Animals. This study was approved by the Ethical Committee for Laboratory Animals Care and Use of Patuakhali Science and Technology University, Patuakhali, Bangladesh (Approval no: PSTU.FoF/2019/04).
Competing interests
The authors declare no competing interests.
Additional information
Handling Editor: Brian Austin
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Clinical trial number
Not applicable.
Clinical trial number
Not applicable.
Bethi Das and S. M. Majharul Islam combined first author and equal contribution.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Das, B., Islam, S.M.M., Nag, S.K. et al. Earthworm, Perionyx excavatus as an alternate protein source for Nile tilapia: Effects on growth performance, blood biochemistry, erythrocyte morphology and intestinal health. Aquacult Int (2024). https://doi.org/10.1007/s10499-024-01533-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10499-024-01533-0