Abstract
Prebiotics are fibers that promote beneficial gut bacteria and play a pivotal role in enhancing host health. This study delves into the impact of various levels of prebiotics, specifically fructooligosaccharides and β-1,3 glucan (Fβ), on the growth performance, biochemical, hematological parameters, gene expression, histological variations in the internal organs, and disease resistance to Fusarium oxysporum in tilapia (Oreochromis niloticus). Two hundred forty Nile tilapia, initially weighing 34.0 ± 0.1 g, were distributed into four groups and given a commercial diet with varying Fβ treatments: control (0 g/kg) (C), T1 (0.5 g/kg), T2 (1.0 g/kg), and T3 (1.5 g/kg) for 70 days. The study revealed significant improvement of the biochemical, hematological, and digestive enzyme activities, as well as histological changes in hepatopancreatic, intestine, and spleen sections with the use of Fβ. Moreover, the expression of innate humoral genes significantly increased (P < 0.05) in tilapia at the 1.5 g/kg Fβ group compared to the control. Notably, challenging with F. oxysporum exhibited lower mortality rates in the three treatments supplemented with additive prebiotics (P < 0.05). Consequently, the feed additives utilized in this study emerge as a viable alternative to enhance growth performance, biochemical and hematological parameters, gene expression, histological variations in internal organs, and disease resistance in fish farming on a large scale.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aquaculture emerges as one of the most promising solutions to address global food shortages, providing a substantial source of dietary protein derived from aquatic animals (Idenyi et al. 2022; Mohan et al. 2022; Eissa et al. 2022a). Among the numerous fish species cultivated, tilapia stands out as one of the most significant fish species in terms of volume produced. Widely distributed across more than 100 countries, Egypt ranks among the top three nations, producing 875 thousand tonnes (Abdel-Latif et al. 2020; Eissa et al. 2023d; Hendam et al. 2023). According to the FAO Food and Agricultural Organization (2022), the global tilapia harvest from aquaculture was 4.4 million tonnes in 2020. The term “tilapia” serves as a general descriptor for various fish species, primarily those belonging to the genera Oreochromis and Sarotherodon. The popularity of this species can be attributed to several key traits, including its adaptability, resilience against various diseases, and proficiency in thriving under challenging farming conditions (Fishstat 2015).
Nile tilapia is known for its resilience, and the occurrence of infection outbreaks remains a significant challenge in tilapia production, particularly in intensive farming conditions (El-Sayed 2019). Fish infections often stem from primary stressful events, with numerous pathogens commonly identified in instances of pathogenic infection (Roon et al. 2015; Zhang et al. 2015; Kotob et al. 2017).
Nutritional supplements play a prominent role in increasing health status through increasing fish immunity against disease infection (Alagawany et al. 2016; Khafaga et al. 2019; El-Sayed et al. 2021; Abd El-Hack et al. 2022; Makled et al. 2022; Sayed et al. 2023; El-Sayed et al. 2023).
β-Glucans and fructooligosaccharides are two primary prebiotics frequently selected to complement probiotics in fish farming (Song et al. 2014; Huynh et al. 2017; Eissa et al. 2024). Extensive scholarly investigations have elucidated the positive impact of both compounds on the gut microbiome, unequivocally confirming their roles as growth stimulants within the fish farming sector (Mohammadian et al. 2019). The underlying mechanism of prebiotics involves the breakdown of these substances into their respective sugars within the fish’s digestive tract. Subsequently, these sugars serve as carbon sources for beneficial bacteria (Goh and Klaenhammer 2015). Beyond their role as growth stimulants, prebiotics have demonstrated the potential to enhance development productivity, fortify immune system function, and bolster resistance against diseases in fish aquaculture. Additionally, they play a crucial role in preventing the transmission of pathogenic diseases (Eissa et al. 2022b; Eissa et al. 2023d, Eissa et al. 2024).
To evaluate both the structure and physiological conditions of fish challenged to multiple toxins, blood test results have been extensively utilized as pathological markers (Panjvini et al. 2016; Baloch et al. 2022). The identification of specific organs and the overall health status of animals are significantly enhanced through the analysis of serum biochemical parameters. This approach is particularly valuable for its capability to signal various alterations in stressful conditions, as recommended by Usman et al. (2023). Histopathological methods are instrumental in assessing the sub-lethal effects of viruses or contaminants, with physiological changes serving as indicative markers. Moreover, structural alterations offer a swift and intermediate means of identifying stressors and evaluating their impact on tissues. (Figueiredo-Fernandes et al. 2007).
Various species of Fusarium are commonly found in ecosystems (Michielse and Rep 2009; Eissa et al. 2023a). Fusarium infections have been reported in amphibians and several aquatic animals, as reported by Salter et al. (2012), Eissa et al. (2023b), and Jastaniah et al. (2023). Fish experiencing fungal issues often exhibit concurrent health issues, such as microbial infections or fluctuations in water acidity or humidity. In addition to various aquatic fungi, Saprolegnia species pose challenges for both wild and cultivated freshwater fish. Aphanomyces and Fusarium are also recognized as significant contributors to fish diseases affecting both aquatic animals and crustaceans (Hatai 2012). Fish afflicted with microbiological infectious diseases not only manifest visible morphological changes and alterations but also experience substantial alterations in hemoglobin levels and hepatic enzyme function (Kulatunga et al. 2017; Ukwe and Oladapo-Akinfolarin 2019; Eissa et al. 2023c).
The objective of this study was to evaluate the possible application of Fβ at different levels in the diet of Oreochromis niloticus and to elucidate its effects on weight, body composition, histological changes, gene expression, and resistance against F. oxysporum infection.
Materials and methods
Setup and preparations for the study
The study was conducted during the period of August 2023 to November 2023 at a privately owned aquaculture farm in Egypt, utilizing 12 concrete ponds, each measuring 1 × 1 × 1.20 m with a water volume of 1 m3. The experiment involved four feed treatments: 0.0 g/kg Fβ, 0.5 g/kg Fβ, 1.0 g/kg Fβ, and 1.5 g/kg Fβ. The concrete ponds maintain a daily water change rate of 30%.
Management of experimental fish
Two hundred and forty normal O. niloticus were obtained from a commercial fish farm, with an initial body weight of 34.0 ± 0.1 g/fish. The fish were uniform in their body weights and in good health, as indicated by their regular eating habits and active surface swimming during feeding times. Fish were fed a commercial diet (30% protein) during acclimatization period of 15 days before the commencement of the trials. Twenty fish per m3/pond of tilapia were stocked, with each pond equipped with constant aeration. The feeding trial was conducted for 70 days.
Experimental feed management
The experimental fish diets comprised 30.22% crude protein, 8.16% crude lipid, and 7.22% ash. Fish were fed three times a day, and the feeding rate varied based on their biomass, maintaining it at 2.5%. The proximate composition of the ingredients and experimental diets is as outlined in Table 1. The formulation of the experimental diets was meticulously calculated to meet the optimal nutrient requirements for Nile tilapia (O. niloticus) requirement (NRC 2011).
Assessment of water quality
The assessment of water quality involved daily measurements of key indicators at 3 p.m., encompassing pH, temperature, dissolved oxygen, salinity, and ammonia. An Ecosenset DO330M MultiMeter (YSI, Brannum Lane, OH, USA) was utilized for measuring salinity, pH, and dissolved oxygen in the water. Ammonia (NH3) was measured using HI 34715-12A instrument (Table 2).
Growth performance
Randomly, sampling was carried out at fortnight intervals, and the final data were recorded for the calculation of growth efficiency variables, including final body weight (FW), weight gain (WG), average daily gain (ADG), specific growth rate (SGR), feed intake, and survival rate.
The calculation was performed using the following equations:
where Wt is its final mean weight in (g) and W0 is the fish’s beginning mean weight in (g).
where W0 and Wt are the starting and final mean weights of fish in grams, respectively, and n is the length of the period. This yields the mean daily gain (g/fish/day).
where W0 and Wt are the fish’s beginning and final average weights in (g), respectively.
Feed utilization
The feed utilization was calculated using the following formula:
Chemical analysis of fish body and feed
The proximate composition of fish tissue was analyzed including moisture content, crude protein, crude lipid, and ash levels. Ten fish and feed samples were collected randomly at the beginning and at the end of each experiment. Ten fish were chosen randomly on the loading day for body chemical analysis. The evaluation of moisture, crude protein, ether extract, and ash levels in the entire fish carcasses was conducted on a dry matter basis, following the methods outlined by AOAC Association of Official Analytical Chemists (1997).
Evaluationof blood samples
Blood samples were drawn from the distal vertebral vein in five randomly selected fish specimens by using heparinized syringes following the methods described by Feldman et al. (2000). Hemoglobin levels were assessed using Drabkin’s solution, the Natt-Herrick solution, and the cyanomet hemoglobin technique, along with the counting of erythrocytes and leukocyte numbers using a hemocytometer, as outlined by Stoskopf (1993). Data were collected using PCV% and differential count of leukocytes in accordance with the methods outlined by Dacie and Lewis (1991). Additionally, the differential leukocyte count (DLC) was calculated using the formula provided by Thrall (2004): total leukocyte count / 100 × the number of each white cell, yielding the absolute DLC. The formula for blood performance (BP) was calculated as Ln (Hb (g/dL) + Ln Ht (%) + Ln RBC (*105/mm3) + Ln WBC (*103/mm3) + Ln TP (g/L), as specified by Esmaeili (2021).
Biochemicalanalysis
The amount of globulins in the blood, total proteins, albumin, and creatinine was measured at 540 nm and 550 nm, respectively, with n = 5 samples per group (Doymas et al. 1981; Dumas and Biggs 1972). Colorimetric measurements of alkaline phosphatase (ALP), aspartate aminotransferase (AST), and alanine aminotransferase (ALT) activity were conducted with n = 5 samples per group at 540 nm, following the methods by Reitman and Frankel (1957). Cholesterol and triglycerides were measured using commercial clinical kit procedures from Bio-Merieux, France (Fynn-Aikins et al. 1992). Glucose levels were measured using kits from Bio-Merieux, France, in accordance with Trinder’s method (1969). The activity of amylase and lipase was tested in triplicates using Bio-Merieux kits (Diab et al. 2023). Red blood cells (RBCs), hemoglobin, and white blood cells (WBCs) were measured based on the method described by Makled et al. (2017).
Oxidative stress and immune-marker levels
Malondialdehyde (MDA), superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), lysozyme (LYZ), and immunoglobulin M (IGM) activities were quantified utilizing an ELISA kit from Thermo Fisher, USA, following the manufacturer’s protocol (Abdel-Tawwab et al. 2018; Hao et al. 2020).
Testing gene expression
Extraction of genetic material (DNA)
Total RNA was extracted from 50 mg of liver tissues (n = 5 samples per group) using the Trizol reagent (Invitrogen, Thermo Fisher, USA) following the manufacturer’s instructions. The RNA concentration was validated using a Nano-drop (Uv–Vis) spectrophotometer 1612 from Milton Roy, Tokyo, Japan. Complementary DNA (cDNA) was synthesized using the cDNA production kit from Thermo Fisher, USA, according to the supplier’s directions. Subsequently, the cDNA specimens were stored at − 20 °C until further use.
Real time qPCR (RT-PCR)
The specific primer sequences, product sizes, and NCBI GenBank accession numbers for the genes producing tumor necrosis factor-alpha (TNF-α), interleukin one beta (IL-1β), interleukin eight (IL-8), growth hormone (GH), and insulin-like growth factor (IGF-1) are listed in Table 3. Additionally, the housekeeping gene beta-actin was utilized as a reference for assessing mRNA expressions (Table 3). RT-PCR, to measure the mRNA expression folds of the target genes, was performed using the Thermo Fisher SYBR kit. The thermocycling parameters included an initial step at 95 °C for 10 min, followed by 40 cycles at 95 °C for 120 s, 60 °C for 1 min, and 72 °C for 20 s. The mRNA expression folds of each target gene were normalized and standardized to β-actin mRNA transcripts using the 2-ΔΔCT method as described by Schmittgen and Livak (2008).
Challenge testing for Fusarium oxysporum
Fusarium oxysporum isolated from O. niloticus was provided by the Microbiological Division of the Fish Infections, Department at the Veterinary Institute, Dokki, Giza, Egypt. Following the procedures outlined by Munir et al. (2018), F. oxysporum was cultured on Sabouraud dextrose agar (SDA, Thermo Fisher, USA) supplemented with penicillin (100 UI/mL) and streptomycin (100 µg/mL) to generate spore suspensions. The cultures were then incubated at 26 °C for 7 days. To collect conidial mass, each plate was filled with 25.0 mL of sterile distilled water, and the suspension was collected in sterile tubes, passing through a pair of layers of sterile gauze as filters. The conidial concentration in the suspension was determined using a hemocytometer’s erythrocyte counting chamber and adjusted to 4.0 × 103 conidia/mL in sterile distilled water.
Subsequently, twenty surviving fish from each group were injected with F. oxysporum (4.0 × 103 conidia/mL) using a sterile needle, and their condition was monitored daily for the next 15 days. Another twenty fish, also injected, received a baseline diet. The cumulative death percentage was calculated based on the outcomes of the challenge test.
Histological examination
Histological sections of hepatopancreas, intestine, and spleen from 3 fish were cut at 6 µ in thickness by rotatory microtome (Leica RM2125, Germany) and were stained with hematoxylin and eosin (H&E) dyes and examined using a light microscope (Nikon CX53, Japan) equipped with an ocular attachment (Carl Zeiss ERc 5s, Germany) and software (Zeiss, Germany). The ImageJ program (NIH, USA) was employed for the evaluation of histological characteristics, following the methods outlined by Matrosova et al. (2021) and Nikiforov-Nikishin et al. (2022).
Statistical analysis
GraphPad Prism (V5 San Francisco, CA, USA) was employed for all statistical analyses. Before conducting any statistical analysis, the assumptions of homoscedasticity and normality were checked. Analysis of variance (ANOVA) was utilized to assess all variables under consideration, determining whether Fβ levels had a significant (P < 0.05) impact on the observed outcomes. Additionally, a follow-up trend analysis using orthogonal polynomial analysis, as outlined by Wei et al. (2019), was performed to ascertain whether the effect was linear or quadratic. Differences between means were examined using Duncan’s multiple range test. The results are presented as the standard error of the mean, or Mean ± SE.
Results
Assessment of growth performance and feed utilization
The survival rate (%) of the 0.5 g/kg Fβ, 1.0 g/kg Fβ, and 1.5 g/kg Fβ treatments surpassed that of the control group (84.67 ± 1.67%) with the values of 95.33 ± 1.67%, 100.00 ± 0.00%, and 98.33 ± 1.67%, respectively, as shown in Table 4. Additionally, a significant improvement was observed in the final body weight, weight gain, and SGR of Nile tilapia fed on the 0.5 g/kg Fβ, 1.0 g/kg Fβ, and 1.5 g/kg Fβ groups (P < 0.05). The 1.5 g/kg Fβ group exhibited the best FCR. Moreover, the fish biomass in the 0.5 g/kg Fβ, 1.0 g/kg Fβ, and 1.5 g/kg Fβ groups exceed that of the control group (1276.36 ± 12.80 g per m3) with fish biomass recorded at 1422.19 ± 7.9 g, 1506.47 g, and 1509.51 ± 34.17 g per m3, respectively.
Chemical composition of the entire body
Ten fish were selected for analysis on the dry matter, protein, and ash content of their entire bodies. In comparison to the control group, all Fβ additive groups demonstrated a significant enhancement (P < 0.05) in the tested parameters including the dry matter, protein, ether extract, and ash (%) as illustrated in Table 5.
Hematological and biochemistry parameters
The hematological parameter findings for each of the four treatment groups are presented in Table 6. Fish treated with Fβ exhibited significantly (P < 0.05) lower levels of ALT, AST, ALP, albumin, cholesterol, and triglycerides compared to the control group. Conversely, there were notably higher values of globulin and total protein, along with increased activity of digestive enzymes (lipase and amylase) and enhanced hematological indices (P < 0.05). Remarkably, the level of creatinine remained consistent across all treatments.
Biomarkers for immunity and oxidative markers
The immunological and antioxidant activities observed in different groups of Nile tilapia are listed in Table 7. Antioxidant enzymes, including SOD, CAT, and GPx, showed significantly higher levels (P ≤ 0.05) in fish fed with Fβ compared to the control group, with the 1.5 g/kg Fβ (T3) group exhibiting the highest activity (P < 0.05). MDA levels were notably lower (P < 0.05) in all Fβ treatments than in the control group. Additionally, compared to the control group, the inclusion of Fβ in feed significantly increased (P < 0.05) IgM levels and stimulated serum LYZ activity across various treatments.
Gene expression outcomes
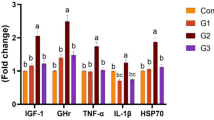
A pronounced upsurge in the mRNA expression levels of immunity-related cytokine genes (TNF-α, IL-1β, and IL-8) in proportion to the rising Fβ levels in the 0.5 g/kg Fβ, 1.0 g/kg Fβ, and 1.5 g/kg Fβ groups is compared to the control group. The relative mRNA expression of growth-correlated genes such as GH and IGF-1 genes significantly increased (P < 0.05) compared to the control group, with treatment 1.5 g/kg Fβ exhibiting the most robust growth-promoting effect in comparison to 0.5 g/kg Fβ and 1.0 g/kg Fβ levels (Fig. 1). The 1.5 g/kg Fβ treatment demonstrated the most potent immune-stimulant effect.
Challenge test using Fusarium oxysporum
On the third- and fourth-day post-challenge, the fish began to perish, exhibiting increased mucus discharges, scale separation, and hemorrhages in various areas of their external body. Postmortem examination revealed a pale, swollen liver with white nodules scattered throughout its outer layer and a distended gallbladder. The internal organs of the affected fish were utilized to re-isolate F. oxysporum.
In a dose-dependent manner, fish fed with any of the Fβ-supplemented treatments exhibited higher survival rates than those fed with the control treatment (Fig. 2). O. niloticus showed significant (P < 0.05) differences in survival rates between the treated groups and the control during the first and second weeks of the F. oxysporum challenge. Overall, fish fed with Fβ demonstrated superior survival rates in a dose-dependent manner across treatments compared to the control (Fig. 2).
Histopathological evaluation
Examination of hepatopancreatic sections
The liver maintained its treated histology throughout the entire set of trials in comparison to the infected control. Hepatocytes in the infected control group showed abnormalities in appearance caused by F. oxysporum infection (Fig. 3A), with easily recognizable nuclei, necrosis, and kupffer macrophage cells within enlarged sinusoid capillary, and central hepatic veins were engorged with blood. However, when utilizing prebiotic preparations based on Fβ (group T2), initial signs of treatment become apparent (Fig. 3B). The liver parenchyma’s structure in the T2 and T3 groups closely adhered to the normal structures: improving the hepatocytes with proper nuclei, clearly visible central veins, endothelium, and sinusoidal blood were observed (Fig. 3C, D).
Microscopic sections for hepatopancreatic O. niloticus after infection for the experimental groups (T0 group (infected control group)): (A), T1 group: (B), T2 group: (C), and T3 group: (D). Hepatocytes, hepatosinusoids (S), central vein (Cv), endothelium (Yellow arrows), firm adhesion of the leukocytes margination (Red stars), pancreas (P), inflammatory cells (Blue arrows), and Kupffer cells (Red stars). [H&E, Bar = 50 μm]
Examination of intestinal sections
The intestines maintained their typical histology throughout the entire series of trials in comparison to the control. After treatment of infected tilapia, an improvement in intestinal tissue structures was demonstrated (Fig. 4C, D). Treatments compared to infected control group (Fig. 4A) exhibited a significant increase in villi width and length (P < 0.05), and T3 was identified as the group with the longest and widest villi (Figs. 4 and 5).
Microscopic sections of intestine of O. niloticus after infection for the experimental groups (T0 group (infected control group): (A), T1 group: (B), T2 group: (C), and T3 group: (D). Intestinal lumen (IL), intestinal mucosa layer (ML), intestinal submucosa layer (SmL), intestinal muscular layer (MuL), and intestinal outer serosa layer (red stars). H&E, Bar = 50 μm
Graph for intestinal parameters (length and width); each column showed the M ± SE for four current experimental groups (n = 3 for each group), and the different subscript letters (a, b, and c) above each column illustrated the highly significant width villi parameter and non-significant villi length parameter
Examination of spleen sections
Spleen sections revealed some slight changes across the various tested groups. In all tested sections, the spleen sections displayed splenic red pulp, splenic white pulp, and the splenic capsule. Notably, lymphocyte colonies were discernible in both infected control group (Fig. 6A), and treatment groups (Fig. 6B–D).
Discussion
The Nile tilapia, O. niloticus, is highly recommended for its excellent meat quality, consumer appeal, and well-established rearing techniques, making it a valuable species for freshwater fish farming (Souza et al. 2019). However, infectious diseases and environmental stresses stand out as major and frequent challenges hindering the growth of the aquaculture industry (Baldissera et al. 2017; De Souza et al. 2017). Historically, the fish farming sector has heavily relied on chemotherapy treatments and antibiotics to combat infectious diseases. However, the overuse of these substances has raised concerns, prompting a shift toward incorporating probiotics, prebiotics, and synbiotics into aquaculture feed production (Fečkaninová et al. 2017; Morselli et al. 2019, 2020).
This study aimed to evaluate the effect of Fβ prebiotic supplementation in the feed of Nile tilapia and to examine the fish’s tolerance to F. oxysporum infection. The effects of Fβ were evaluated across various parameters, encompassing water quality, growth performance, blood biochemical parameters, internal organ histology, and the expression of growth and immune-related genes. Good water quality is pivotal for successful aquaculture production, and a thorough comprehension of the interplay between feed components and aquatic conditions is essential for achieving optimal growth, survival, and overall production (Soundarapandian and Babu 2010).
The growth outcomes of fish supplemented with Fβ exhibited a noticeable increase in weight gain, growth rate, and survival rate when compared to the control group. Research by Ebrahimi et al. (2012) suggested that dietary immunogens as prebiotic supplementation in the range of 1 to 1.5 g/kg have the potential to enhance feed efficiency, developmental outcomes, and immunity to infection in fingerling Cyprinus carpio, as well as in our study, the better range was 1 to 1.5 g/kg Fβ. Additionally, Xu et al. (2022) found that applying prebiotics to younger fish could enhance their effectiveness, and the use of multiple oligosaccharide prebiotic components might result in a more significant improvement in growth.
In the current study, β-1,3 glucan (β-1,3 GF) demonstrated an improvement in Nile tilapia growth and feed consumption. Furthermore, it exhibited a significantly lower FCR and higher WG compared to the control. These findings align with previous research indicating that feeding β-glucans enhances development and feed consumption in various species such as red seabream (Dawood et al. 2017), tilapia (Pilarski et al. 2017), and Pacific white shrimp (Boonanuntanasarn et al. 2016; Li et al. 2019).
Similarly, O. niloticus fed with a synbiotic rich in glucans and fructooligosaccharides demonstrated a significant increase in weight gain and SGRs compared to non-treated fish (Ismail et al. 2019). The growth-promoting effect of this molecule is believed to depend on its concentration, solubility, and structures, in addition to the species under investigation.
According to the present study’s body composition examination, fish in the 1.5 g/kg Fβ group, in particular, exhibited significantly higher levels of crude protein and dry matter contents compared to fish in the other control and experiment groups after probiotics were added to their water. Ash content, on the other hand, improved the most in the 1.5 g/kg Fβ and control groups. These findings align with the work of Munir et al. (2016), who demonstrated that prebiotic-based diets have a more favorable impact on C. striata fingerlings development, body composition, feed utilization, and survival.
The current results revealed a substantial impact of using Fβ in the regulation of biochemical and hematological parameters, as well as digestive enzymes in treated groups relative to the control. This is consistent with Anguiano et al. (2013), who illustrated that rather than being attributed to an increase in the functioning of digestive enzymes, the reported gains in digestible nutrients in fish after receiving prebiotic treatment seem to be mostly connected to modifications in the GIT structure. Additionally, Kumar et al. (2020) showed that laminari-oligosaccharides have the potential to be used as prebiotic ingredients in functional food items. Furthermore, Ziółkowska et al. (2020) demonstrated that the results of blood parameters showed that the addition of prebiotics had no detrimental effects on the common carp’s ability to grow or alter its homeostasis.
Prebiotics enhance Hb, Ht, and RBCs, leading to an increase in well-oxygenated blood reaching the tissue. Furthermore, fish with higher blood parameters, such as increased blood pressure, may exhibit stronger immune systems, indicated by higher WBC levels (Esmaeili 2021). These variables could explain the highly positive correlation observed between growth and the prebiotic substance utilized.
Liver enzymes serve as powerful indicators of the physiological condition of the liver, and monitoring enzyme activity is a biochemical approach to assess how food additives affect fish welfare and metabolism (Fadl et al. 2020). The present investigation revealed that the addition of prebiotic slightly decreased liver enzymes, reflecting the minimal burden of the applied prebiotic for O. niloticus.
Oxidative damage becomes more prevalent in stressful settings and infectious diseases that produce reactive oxygen species (ROS). This is attributed to differences in the production and disposal of free radicals (Martínez-Alvarez et al. 2005), and higher concentrations of ROS promote lipid peroxidation, as reflected in elevated concentrations of malondialdehyde (MDA) (Brewer 2011). The current investigation demonstrates a significant boost in oxidative enzymes, including CAT, GPx, and SOD, as well as notable reduction in MDA levels with the use of the prebiotic formula. This aligns with Wang et al. (2017), who showed that the addition of probiotics (Bacillus licheniformis) and 0.2% prebiotics (inulin) to water enhanced the development rate of juvenile groupers, along with increased activities of digestive enzymes and oxidative systems. Prebiotics can enhance fish’s immune and antioxidant systems, significantly increasing the ability of aquaculture-raised animals to survive adverse environments (Jin et al. 2018). Additionally, Zhu et al. (2023) demonstrated that dietary mannan-oligosaccharide and xylooligosaccharide as prebiotics can greatly increase vitality against stress.
In a study of Zhang et al. (2021) that was conducted on mice, they found that an indigestible fructooligosaccharide (FOS) can prevent the impairment of the intestinal barrier caused by dextran sulfate sodium. Galacto-oligosaccharide (GOS), another indigestible oligosaccharide when consumed together with FOS, can increase the concentration of acetic acid, a short-chain fatty acid (SCFA), and the mRNA expression of G-coupled protein receptors (GPR42 and GPR43), CLDN1, OCLN, and ZO1 in the colon of neonatal piglets (Zhang et al. 2020).
According to Uribe et al. (2011), LYZ, which is produced by leucocytes, is essential for the initiation of phagocytosis and has a bactericidal impact by lysis of the bacterial cell wall. Similarly, IgM plays a key role in the humoral immunological structure of fish (Tang et al. 2008). In this study, fish consuming prebiotics showed a substantial rise in lysozyme activity and IgM relative to the control. This is in line with Cavalcante et al. (2020), who demonstrated that probiotics, prebiotics, and synbiotics could be used to enhance growth performance and immune functions in Nile tilapia.
TNFα was initially discovered in fish as a single instance gene in the Japanese flounder’s activated leukocytes. It is now recognized as a crucial regulatory cytokine in antibacterial defense and inflammatory responses (Grayfer et al. 2008). It is well-established that the fish GH and IGF-I genes play essential roles in controlling cell functions and growth through various signaling pathways. IL-1β functions as a chemoattractant for fish leucocytes, with leucocytes movement being regulated by G protein-coupled receptor activation and a progressive gradient of chemokines. Inflammatory areas attract and activate neutrophils through IL-8 (Zou and Secombes 2016). In this study, the use of Fβ in various treatments resulted in the upregulation of TNF α, IL-1 β, IL-8, GH, and IGF-1, shifting the immune response toward a protective function. This may be one of the reasons for enhancing the tolerance of O. niloticus to F. oxysporum infection when using different levels of Fβ.
In this study, the histological investigations revealed improving the positive impacts of Fβ on the hepatopancreatic, intestinal, and splenic tissues of O. niloticus after the challenge test with F. oxysporum. This aligns with other studies reporting that the use of probiotics and prebiotics enhances the microscopic structures of digestive organs in fish (Ngamkala et al. 2020; Ruiz et al. 2020; Eissa et al. 2023c).
Conclusion
The study highlighted the efficacy of the fructooligosaccharides and β-1,3 glucan mixture addition, collectively referred to as Fβ, in improving fish health status. These improvements include enhanced growth performance, improved biochemical and hematological parameters, better organ health, and increased resistance against F. oxysporum infection. Moreover, it revealed a positive influence on the expression of developmental and defense genes, along with the histological structure of internal fish organs such as liver, intestine, and spleen. As a result, the inclusion of prebiotics into feed emerges as a secure and innovative approach for sustainable aquaculture, offering potential benefits for growth improvement, feed efficiency, and overall health in cultured Nile tilapia (O. niloticus). The author recommends incorporating Fβ at a level of 1–1.5g/kg diet to optimize fish health functions and growth.
Data availability
All data regarding this study are presented in the paper.
References
Abd El-Hack ME, El-Saadony MT, Elbestawy AR, Nahed A, Saad AM, Salem HM, El-Tarabily KA (2022) Necrotic enteritis in broiler chickens: disease characteristics and prevention using organic antibiotic alternatives–a comprehensive review. Poult Sci 101(2):101590
Abdel-Latif HMR, Mahmoud AO, Dawood MAO, Menanteau-Ledouble S, Mansour El-Matbouli M (2020) The nature and consequences of co-infections in tilapia: a review. J Fish Dis 43(6):651–664. https://doi.org/10.1111/jfd.13164
Abdel-Tawwab M, Samir F, Abd El-Naby AS, Monier MN (2018) Antioxidative and immunostimulatory effect of dietary cinnamon nanoparticles on the performance of Nile tilapia, Oreochromis niloticus (L.) and its susceptibility to hypoxia stress and Aeromonas hydrophila infection. Fish Shellfish Immunol 74:19–25. https://doi.org/10.1016/j.fsi.2017.12.033
Alagawany M, Abd El-Hack ME, El-Kholy MS (2016) Productive performance, egg quality, blood constituents, immune functions, and antioxidant parameters in laying hens fed diets with different levels of Yucca schidigera extract. Env Sci Poll Res 23:6774–6782
Anguiano M, Pohlenz C, Buentello A, Gatlin DM (2013) The effects of prebiotics on the digestive enzymes and gut histomorphology of red drum (Sciaenops ocellatus) and hybrid striped bass (Morone chrysops × M. saxatilis). Br J Nutr 109(4):623–9. https://doi.org/10.1017/S0007114512001754
AOAC, Association of Official Analytical Chemists (1997) Animal feed official methods of analysis, 16th Edition. Arlington, VA, USA. pp 30
Baldissera MD, Souza CF, Doleski PH, de Vargas AC, Duarte MM, Duarte T, Boligon AA, Leal DB, Baldisserotto B (2017) Melaleuca alternifolia essential oil prevents alterations to purinergic enzymes and ameliorates the innate immune response in silver catfish infected with Aeromonas hydrophila. Microb Pathog 109:61–66. https://doi.org/10.1016/j.micpath.2017.05.026
Baloch AA, Abdelsalam EEE, Piačková V (2022) Cytokines studied in carp (Cyprinus carpio L.) in response to important diseases. Fishes 7(1):3. https://doi.org/10.3390/fishes7010003
Boonanuntanasarn S, Wongsasak U, Pitaksong T, Chaijamrus S (2016) Effects of dietary supplementation with β-glucan and synbiotics on growth, haemolymph chemistry, and intestinal microbiota and morphology in the Pacific white shrimp. Aquac Nutr 22:837–845
Brewer MS (2011) Natural antioxidants: sources, compounds, mechanisms of action, and potential applications. Compr Rev Food Sci Food Saf 10:22. https://doi.org/10.1111/j.1541-4337.2011.00156.x
Cavalcante RB, Telli GS, Tachibana L (2020) Probiotics, prebiotics and synbiotics for Nile tilapia: growth performance and protection against Aeromonas hydrophila infection. Aquac Rep 17:100343. https://doi.org/10.1016/j.aqrep.2020.100343
Dacie S, Lewis S (1991) Practical hematology, 7th edn. Churchill Livingstone, London
Dawood MAO, Koshio S, Ishikawa M, Yokoyama S, El Basuini MF, Hossain MS, Nhu TH, Moss AS, Dossou S, Wei H (2017) Dietary supplementation of β-glucan improves growth performance, the innate immune response and stress resistance of red sea bream, Pagrus major. Aquac Nutr 23:148–159. https://doi.org/10.1111/anu.12376
De Souza RC, da Costa MM, Baldisserotto B, Heinzmann BM, Schmidt D, Caron BO, Copatti CE (2017) Antimicrobial and synergistic activity of essential oils of Aloysia triphylla and Lippia alba against Aeromonas spp. Microb Pathog 113:29–33. https://doi.org/10.1016/j.micpath.2017.10.013
Diab AM, Eldeghaidy EE, Abo-Raya MH, Shukry M, Abdeen A, Ibrahim SF, Fericean L, Abdo M, Khalafalla MM (2023) Assessment of growth-related parameters, immune-biochemical profile, and expression of selected genes of red tilapia fed with Roselle calyces (Hibiscus sabdariffa) extract. Fishes 8(4):172. https://doi.org/10.3390/fishes8040172
Doymas BT, Bay DD, Carter RJ, Schaffer R (1981) A candidate reference method for determination of total serum protein I. Development and validation. Clin Chem 27:1642–1643
Dumas BT, Biggs HG (1972) Standard methods of clinical chemistry. Academic Press, New York
Ebrahimi G, Ouraji H, Khalesi MK, Sudagar M, Barari A, Zarei Dangesaraki M, Jani Khalili KH (2012) Effects of a prebiotic, Immunogen®, on feed utilization, body composition, immunity and resistance to Aeromonas hydrophila infection in the common carp Cyprinus carpio (Linnaeus) fingerlings. J Anim Physiol Anim Nutr (berl) 96(4):591–599. https://doi.org/10.1111/j.1439-0396.2011.01182.x
Eissa ESH, Elsayed SB, Alkhateib YG, Ashraf AE, Walaa KB, Omayma MA, Nadia NBA (2022a) Assessing the influence of dietary Pediococcus acidilactici probiotic supplementation in the feed of European sea bass (Dicentrarchus labrax L.) (Linnaeus, 1758) on Farm water quality, growth, feed utilization, survival rate, body composition, blood biochemical parameters, and intestinal histology. Aquacu Nutr 2022(5841220):11. https://doi.org/10.1155/2022/5841220
Eissa ESH, Ahmed NH, El-Badawi AA, Munir MB, Abd Al-Kareem OM, Eissa MEH, Hussien EHM, Sakr SS (2022b) Assessing the influence of the inclusion of Bacillus subtilis AQUA-GROW® as feed additive on the growth performance, feed utilization, immunological responses and body composition of the Pacific white shrimp, Litopenaeus vannamei. Aquac Res 53:6606–6615. https://doi.org/10.1111/are.16129
Eissa ESH, Ahmed RA, Abd Elghany NA, Elfeky A, Saadony S, Ahmed NH, Sakr SS, Dayrit GB, Tolenada CPS, Atienza AAC (2023a) Potential symbiotic effects of β-1,3 glucan, and fructooligosaccharides on the growth performance, immune response, redox status, and resistance of Pacific white shrimp, Litopenaeus vannamei to Fusarium solani Infection. Fishes 8(2):105. https://doi.org/10.3390/fishes8020105
Eissa ESH, Elbahnaswy S, El-Baz AH, El-Haroun E, Ashour M, Mansour AT, Eldessouki EA (2023b). Effects of dietary commercial phytobiotic “Sanacore® GM” on Pacific white shrimp (Litopenaeus vannamei) growth, immune response, redux status, intestinal health, and disease resistance against Fusarium solani. Aquac Int 1–20. https://doi.org/10.1007/s10499-023-01310-5
Eissa ESH, Bazina WK, Abd El-Aziz YM, Abd Elghany NA, Tawfik WA, Mossa MI, Abd El Megeed OH, Abd El-Hamed NB, El-Saeed AF, El-Haroun E (2023c). Nano-selenium impacts on growth performance, digestive enzymes, antioxidant, immune resistance and histopathological scores of Nile tilapia, Oreochromis niloticus against Aspergillus flavus infection. Aquac Int1–25. https://doi.org/10.1007/s10499-023-01230-4
Eissa MEH, Alaryani FS, Elbahnaswy S, Khattab MS, Elfeky A, AbouelFadl KY, Eissa EH, Ahmed RA, Van Doan H, El-Haroun E (2023d) Dietary inclusion of Pediococcus acidilactici probiotic promoted the growth indices, hemato-biochemical indices, enzymatic profile, intestinal and liver histomorphology, and resistance of Nile tilapia against Aspergillus flavus. Anim Feed Sci and Tech 306:115814. https://doi.org/10.1016/j.anifeedsci.2023.115814
Eissa ESH, El-Sayed AM, Ghanem SF, Dighiesh HS, Abd Elnabi HE, Hendam BM, Elleithy AA, Eissa MEH, Abd El-Aziz YM (2024) Dietary mannan-oligosaccharides enhance hematological and biochemical parameters, reproductive physiology, and gene expression of hybrid red tilapia (Oreochromis niloticus x O. mossambicus). Aquaculture 740453. https://doi.org/10.1016/j.aquaculture.2023.740453
El-Sayed AFM (2019) Tilapia culture, 2nd edn. Academic Press, Cambridge, MA
El-Sayed AFM, Tammam MS, Makled SO (2021) Lecithin-containing bioemulsifier boosts growth performance, feed digestion and absorption and immune response of adult Nile tilapia (Oreochromis niloticus). Aquac Nutr 27:757–770. https://doi.org/10.1111/anu.13221
El-Sayed AF, Figueiredo-Silva C, Zeid SMS, Makled SO (2023) Metal–amino acid complexes (Zn, Se, Cu, Fe, and Mn) enhance immune response, antioxidant capacity, liver function enzymes, and expression of cytokine genes in Nile tilapia reared under field conditions. J Aquat Anim Heath 35:248–251. https://doi.org/10.1002/aah.10194
Esmaeili M (2021) Blood performance: a new formula for fish growth and health. Biology 10:1236. https://doi.org/10.3390/biology10121236
Fadl SE, El-Gammal GA, Abdo WS (2020) Evaluation of dietary chitosan effects on growth performance, immunity, body composition and histopathology of Nile tilapia (Oreochromis niloticus) as well as the resistance to Streptococcus agalactiae infection. Aquac Res 51(3):1120–1132. https://doi.org/10.1111/are.14458
FAO, Food and Agricultural Organization (2022) The state of world fisheries and aquaculture towards blue transformation, Rome. https://doi.org/10.4060/cc0461en
Fečkaninová A, Koščová J, Mudroňová D, Popelka P, Toropilová J (2017) The use of probiotic bacteria against Aeromonas infections in salmonid aquaculture. Aquaculture 469:1–8. https://doi.org/10.1016/j.aquaculture.2016.11.042
Feldman BF, Zinkl JG, Jain NC (eds) (2000) Schalm’s veterinary hematology, 5th edn. Narayana Press, Denmark, p 1380
Figueiredo-Fernandes A, Ferreira-Cardoso JV, Garcia-Santos S, Monteiro SM (2007) Histopathological changes in liver and gill epithelium of Nile tilapia, Oreochromis niloticus, exposed to water borne copper. Pesq Vet Bras 27:103–109. https://doi.org/10.1590/S0100-736X2007000300004
Fishstat J (2015) Fisheries and aquaculture software. FishStatJ-software for fishery statistical time series. Rome: FAO Fisheries and Aquaculture Department.
Fynn-Aikins K, Hung SSO, Liu W, Li H (1992) Growth, lipogenesis and liver composition of juvenile white sturgeon fed different levels of D-glucose. Aquaculture 105:61–72. https://doi.org/10.1016/0044-8486(92)90162-E
Goh YJ, Klaenhammer TR (2015) Genetic mechanisms of prebiotic oligosaccharide metabolism in probiotic microbes. Annu Rev Food Sci Technol 6:137–156. https://doi.org/10.1146/annurev-food-022814-015706
Grayfer L, Walsh JG, Belosevic M (2008) Characterization and functional analysis of goldfish (Carassius auratus L.) tumor necrosis factor-alpha. Dev Comp Immunol 32(5):532–543. https://doi.org/10.1016/j.dci.2007.09.009
Hao K, Ullah H, Jarwar AR, Nong X, Tu X, Zhang Z (2020) Functional identification of an FMRFamide-related peptide gene on diapause induction of the migratory locust, Locusta migratoria L. Genomics 112:1821–1828. https://doi.org/10.1016/j.ygeno.2019.10.015
Hatai K (2012) Diseases of fish and shellfish caused by marine fungi. In Biology of marine fungi. Springer, Berlin, Heidelberg 15–52
Hendam BM, Munir MB, Moaheda E.H.E, El-Haroun E, van Doan H, Chung TH, Eissa ESH (2023) Effects of water additive probiotic, Pediococcus acidilactici on growth performance, feed utilization, hematology, gene expression and disease resistance against Aspergillus flavus of Nile tilapia (Oreochromis niloticus). Anim Feed Sci Tech 303:115696. https://doi.org/10.1016/j.anifeedsci.2023.115696
Huynh TG, Shiu YL, Nguyen TP, Truong QP, Chen JC, Liu CH (2017) Current applications, selection, and possible mechanisms of actions of synbiotics in improving the growth and health status in aquaculture: a review. Fish Shellfish Immunol 64:367–382. https://doi.org/10.1016/j.fsi.2017.03.035
Idenyi JN, Eya JC, Nwankwegu AS, Nwoba EG (2022) Aquaculture sustainability through alternative dietary ingredients: microalgal value-added products. Eng Microbiol 2:100049. https://doi.org/10.1016/j.engmic.2022.100049
Ismail M, Wahdan A, Yusuf MS, Metwally E, Mabrok M (2019) Effect of dietary supplementation with a synbiotic (Lacto Forte) on growth performance, haematological and histological profiles, the innate immune response and resistance to bacterial disease in Oreochromis niloticus. Aquac Res 50:2545–2562. https://doi.org/10.1111/are.14212
Jastaniah SD, Alaidaroos BA, Shafi ME, Aljarari RM, Abd El-Aziz YM, Munir MB, Moaheda EHE, Al- Farga A, Eissa EH, Said RM (2023) Dietary Pediococcus acidilactici improved the growth performance, feed utilization, gut microbiota, and disease resistance against Fusarium solani in Pacific white shrimp, Litopenaeus vannamei. Aquac Int. https://doi.org/10.1007/s10499-023-01318-x
Jin M, Xiong J, Zhou QC, Yuan Y, Wang XX, Sun P (2018) Dietary yeast hydrolysate and brewer’s yeast supplementation could enhance growth performance, innate immunity capacity and ammonia nitrogen stress resistance ability of Pacific white shrimp (Litopenaeus vannamei). Fish Shellfish Immunol 82:121–129. https://doi.org/10.1016/j.fsi.2018.08.020
Khafaga AF, Abd El-Hack ME, Taha AE, Elnesr SS, Alagawany M (2019) The potential modulatory role of herbal additives against Cd toxicity in human, animal, and poultry: a review. Env Sci Poll Res 26:4588–4604
Kotob MH, Menanteau-Ledouble S, Kumar G, Abdelzaher M, El-Matbouli M (2017) The impact of co-infections on fish: a review. Vet Res 47(1):98. https://doi.org/10.1186/s13567-016-0383-4
Kulatunga DCM, Dananjaya SHS, Park BK, Kim CH, Lee J, De Zoysa M (2017) First report of Fusarium oxysporum species complex infection in zebrafish culturing system. J Fish Dis 40:485–494. https://doi.org/10.1111/jfd.12529
Kumar K, Rajulapati V, Goyal A (2020) In vitro prebiotic potential, digestibility and biocompatibility properties of laminari-oligosaccharides produced from curdlan by β-1,3-endoglucanase from Clostridium thermocellum. 3 Biotech 10(6):241. https://doi.org/10.1007/s13205-020-02234-0
Li H, Xu C, Zhou L, Dong Y, Su Y, Wang X, Qin JG, Chen L, Li E (2019) Beneficial effects of dietary β-glucan on growth and health status of Pacific white shrimp Litopenaeus vannamei at low salinity. Fish Shellfish Immunol 91:315–324
Makled SO, Hamdan AM, El-Sayed AM, Hafez EE (2017) Evaluation of marine psychrophile, Psychrobacter namhaensis SO89, as a probiotic in Nile tilapia (Oreochromis niloticus) diets. Fish Shellfish Immunol 61:194–200. https://doi.org/10.1016/j.fsi.2017.01.001
Makled SO, Almisherfi HM, Mahmoud ME, Nabil MG (2022) Dietary surface-modified zirconium silicate nanoparticles enhance immune response, antioxidant activity and cytokine genes expression of the white shrimp Litopenaeus vannamei against Vibrio harveyi. Aquaculture 557:738325. https://doi.org/10.1016/j.aquaculture.2022.738325
Martínez-Álvarez RM, Morales AE, Sanz A (2005) Antioxidant defenses in fish: biotic and abiotic factors. Rev Fish Biol Fisheries 15:75–88. https://doi.org/10.1007/s11160-005-7846-4
Matrosova SV, Kuchko TY, Sidorova NA (2021) Biotechnological potential of Lactobacillus brevis 2k. Gv for aquaculture purposes. IOP Conf Ser: Earth Environ Sci 689(1):120–125
Michielse CB, Rep M (2009) Pathogen profile update: Fusarium oxysporum. Mol Plant Pathol 10(3):311–324. https://doi.org/10.1111/j.1364-3703.2009.00538.x
Mohammadian T, Nasirpour M, Tabandeh MR, Mesbah M (2019) Synbiotic effects of β-glucan, mannan oligosaccharide and Lactobacillus casei on growth performance, intestine enzymes activities, immune-hematological parameters and immune-related gene expression in common carp, Cyprinus carpio: an experimental infection with Aeromonas hydrophila. Aquaculture 511:634197. https://doi.org/10.1016/j.aquaculture.2019.06.011
Mohan K, Rajan DK, Muralisankar T, Ganesan AR, Sathishkumar P, Revathi N (2022) Use of black soldier fly (Hermetia illucens L.) larvae meal in aquafeeds for a sustainable aquaculture industry: a review of past and future needs. Aquaculture 553:738095. https://doi.org/10.1016/j.aquaculture.2022.738095
Morselli MB, Reis JH, Baldissera MD, Souza CF, Baldisserotto B, Petrolli TG, Paiano D, Lopes DL, Da Silva AS (2019) Benefits of thymol supplementation on performance, the hepatic antioxidant system, and energetic metabolism in grass carp. Fish Physiol Biochem 46:305–314. https://doi.org/10.1007/s10695-019-00718-2
Morselli MB, Baldissera MD, Souza CF, Reis JH, Baldisserotto B, Sousa AA, Zimmer F, Lopes DL, Petrolli TG, Da Silva AS (2020) Effects of thymol supplementation on performance, mortality and branchial energetic metabolism in grass carp experimentally infected by Aeromonas hydrophila. Microb Pathog 139:103915. https://doi.org/10.1016/j.micpath.2019.103915
Munir MB, Hashim R, Abdul Manaf MS, Nor SA (2016) Dietary prebiotics and probiotics influence the growth performance, feed utilisation, and body indices of snakehead (Channa striata) fingerlings. Trop Life Sci Res 2:111–25. https://doi.org/10.21315/tlsr2016.27.2.9
Munir MB, Roshada H, Azizah S, Terence LM (2018) Effect of dietary prebiotics and probiotics on snakehead (Channa striata) health: hematology and disease resistance parameters against Aeromonas hydrophila. Fish Shellfish Immunol 75:99–108. https://doi.org/10.1016/j.fsi.2018.02.005
Ngamkala S, Satchasataporn K, Setthawongsin C, Raksajit W (2020) Histopathological study and intestinal mucous cell responses against Aeromonas hydrophila in Nile tilapia administered with Lactobacillus rhamnosus GG. Vet World 13(5):967–974. https://doi.org/10.14202/vetworld.2020.967-974
Nikiforov-Nikishin A, Nikiforov-Nikishin D, Kochetkov N, Smorodinskaya S, Klimov V (2022) The influence of probiotics of different microbiological composition on histology of the gastrointestinal tract of juvenile Oncorhynchus mykiss. Microsc Res Tech 85(2):538–547. https://doi.org/10.1002/jemt.23927
NRC (2011) National. Committee on Animal Nutrition. Board on Agriculture. National Research Council. National Academy Press, Washington DC., USA
Panjvini F, Safoura A, Hossein K, Hossein MP (2016) Parasitic infection alters hematology and immunity parameters of common carp, Cyprinus carpio. J Parasit Dis 40:1540–1543. https://doi.org/10.1007/s12639-015-0723-8
Pilarski F, de Oliveira CAF, de Souza FPBD, Zanuzzo FS (2017) Different β-glucans improve the growth performance and bacterial resistance in Nile tilapia. Fish Shellfish Immunol 70:25–29
Reitman S, Frankel SA (1957) Colorimetric method for the determination of serum glutamic oxaloacetic and glutamic pyruvic transaminases. Amer J Clin Pathol 28:56–63. https://doi.org/10.1093/ajcp/28.1.56
Roon SR, Alexander JD, Jacobson KC, Bartholomew JL (2015) Effect of Nanophyetus salmincola and bacterial co-infection on mortality of juvenile Chinook salmon. J Aquat Anim Health 27(4):209–216. https://doi.org/10.1080/08997659.2015.1094
Ruiz ML, Owatari MS, Yamashita MM, Ferrarezi JVS, Garcia P, Cardoso L, Martins ML, Mouriño JLP (2020) Histological effects on the kidney, spleen, and liver of Nile tilapia Oreochromis niloticus fed different concentrations of probiotic Lactobacillus plantarum. Trop Anim Health Prod 52(1):167–176. https://doi.org/10.1007/s11250-019-02001-1
Salter CE, O’Donnell K, Sutton DA, Marancik DP, Knowles S, Clauss TM, Berliner AL, Camus A (2012) Dermatitis and systemic mycosis in lined seahorses Hippocampus erectus associated with a marine adapted Fusarium solani species complex pathogen. Dis Aquat Org 101:23–31. https://doi.org/10.3354/dao02506
Sayed AEDH, Idriss SK, Abdel-Ghaffar SK (2023) Hussein AAA (2023) Haemato-biochemical, mutagenic, and histopathological changes in Oreochromis niloticus exposed to BTX. Environ Sci Pollut Res 30:59301–59315. https://doi.org/10.1007/s11356-023-26604-2
Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative CT method. Nat Protoc 3:1101–1108. https://doi.org/10.1038/nprot.2008.73
Song SK, Beck BR, Kim D, Park J, Kim J, Kim HD, Ringø E (2014) Prebiotics as immunostimulants in aquaculture: a review. Fish Shellfish Immunol 40:40–48. https://doi.org/10.1016/j.fsi.2014.06.016
Soundarapandian P, Babu R (2010) Effect of probiotics on the hatchery seed production of black tiger shrimp, Penaeus monodon (Fabricius). Int J Anim Vet Adv 2(1):9–15
Souza CDF, Baldissera MD, Verdi CM, Santos RC, Da Rocha MIU, da Veiga ML, da Silva AS, Baldisserotto B (2019) Oxidative stress and antioxidant responses in Nile tilapia Oreochromis niloticus experimentally infected by Providencia rettgeri. Microb Pathog 131:164–169. https://doi.org/10.1016/j.micpath.2019.04.007
Stoskopf MK (1993) Fish medicine. Harcourt Brace Jovanovich Inc, Philadelphia. London. Toronto. Montreal. Sydney. Tokyo. WB Saunders company
Tang HG, Wu TX, Zhao ZY, Pan XD (2008) Effects of fish protein hydrolysate on growth performance and humoral immune response in large yellow croaker (Pseudosciaena crocea R.). J Zhejiang Univ Sci B 9:684–690. https://doi.org/10.1631/jzus.B0820088
Trinder P (1969) Determination of glucose in blood using glucose oxidase with an alternative oxygen acceptor. Ann Clin Biochem Int J Lab Med 6(1):24–27. https://doi.org/10.1136/jcp.22.2.158
Ukwe O, Oladapo-Akinfolarin T (2019) Alternations in enzyme activities of Clarias gariepinus infected with Aeromonas hydrophila and Pseudomonas aeruginosa. Asian J Fish Aquat Res 4:1–9. https://doi.org/10.9734/ajfar/2019/v4i230053
Uribe H, Folch R, Enríquez G (2011) Moran. Innate and adaptive immunity in teleost fish: a review. Vet Med Sci 56:486–503. https://doi.org/10.17221/3294-VETMED
Usman Z, Kanwal Z, Tayyeb A, Noshair I, Haider I, Ahmad N, Alomar SY (2023) A comparative analysis on the innate immune responses of Cirrhinus mrigala challenged with Pseudomonas aeruginosa and Fusarium oxysporum. Int J Mol Sci 24(15):12392. https://doi.org/10.3390/ijms241512392
Wang T, Cheng Y, Chen X (2017) Effects of small peptides, probiotics, prebiotics, and synbiotics on growth performance, digestive enzymes, and oxidative stress in orange-spotted grouper, Epinephelus coioides, juveniles reared in artificial seawater. Chin J Ocean Limnol 35:89–97. https://doi.org/10.1007/s00343-016-5130-1
Wei Y, Chen H, Jia M, Zhou H, Zhang Y, Xu W, Zhang W, Mai K (2019) Effects of dietary Antarctic krill Euphausia superba meal on growth performance and muscle quality of triploid rainbow trout Oncorhynchus mykiss farmed in sea water. Aquaculture 509:72–84. https://doi.org/10.1016/j.aquaculture.2019.05.013
Xu W, Lutz CG, Taylor CM, Ortega MC (2022) Improvement of fish growth and metabolism by oligosaccharide prebiotic supplement. Aquac Nutr 5715649. https://doi.org/10.1155/2022/5715649
Zhang C, Li DL, Chi C, Ling F, Wang GX (2015) Dactylogyrus intermedius parasitism enhances Flavobacterium columnare invasion and alters immune-related gene expression in Carassius auratus. Dis of Aqua Organ 116(1):11–21. https://doi.org/10.3354/dao02902
Zhang LMY, Qiu Y, Wu Z, Zhong Z, Zeng X, Zeng Y, Xiong L, Wen Y, Liu R (2021) Fructooligosaccharide supplementation alleviated the pathological immune response and prevented the impairment of intestinal barrier in DSS-induced acute colitis mice. Food Func 12:984454. https://doi.org/10.1039/D1FO01147B
Zhu L, Wang S, Cai Y, Shi H, Zhou Y, Zhang D, Guo W, Wang S (2023) Effects of five prebiotics on growth, antioxidant capacity, non-specific immunity, stress resistance, and disease resistance of juvenile hybrid grouper (Epinephelus fuscoguttatus ♀ × Epinephelus lanceolatus ♂). Animals (basel) 13(4):754. https://doi.org/10.3390/ani13040754
Ziółkowska E, Bogucka J, Dankowiakowska A, Rawski M, Mazurkiewicz J, Stanek M (2020) Effects of a trans-galactooligosaccharide on biochemical blood parameters and intestine morphometric parameters of common carp (Cyprinus carpio L.). Animals (Basel) 10(4):723. https://doi.org/10.3390/ani10040723
Zou J, Secombes CJ (2016) The function of fish cytokines. Biology 5(2):23. https://doi.org/10.3390/biology5020023
Acknowledgements
All authors extend their appreciation to their universities.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Methodology, software: E.H.E., H.S.D.; conceptualization, visualization, methodology: E.H.E., N.M.N., F.A.J., O.F.A., and W.F.A.; software, validation, formal analysis: A.H.Q., F.A.J., O.F.A.; investigation, data curation: E.H.E., H.S.D.; histological processing, examination, and histological data interpretation: Y.M.S, H.S.D.; writing original draft preparation: Y.M.S, E.H.E.; writing review and editing: H.S.D., E.H.E.; supervision: A.R.; revised manuscript: E.H.E; project administration: E.H.E.; All authors have read and approved to the published version of the manuscript.
Corresponding authors
Ethics declarations
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of fish were followed by the authors and according to Suez University protocol (SUEZ Sci_IRB:21/04/2024/7)
Consent for publication
All authors review and approve the manuscript for publication.
Competing interests
The authors declare no competing interests.
Informed consent
Not applicable.
Additional information
Handling Editor: Brian Austin
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abd El-Aziz, Y.M., Jaber, F.A., Nass, N.M. et al. Strengthening growth, digestion, body composition, haemato-biochemical indices, gene expression, and resistance to Fusarium oxysporum infection and histological structure in Oreochromis niloticus by using fructooligosaccharides and β-1,3 glucan mixture. Aquacult Int (2024). https://doi.org/10.1007/s10499-024-01526-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10499-024-01526-z