Abstract
Legal restrictions and consumer trends are contributing to the growing movement for fish welfare. Therefore, the aquaculture sector is a means of reducing animal stress and enhancing physiological state, which raises financial profits. Feed additives have therefore been suggested as possible dietary stress alleviation. In this trend, a 75-day feeding trial was conducted to evaluate the effects of three organic acids (formic acid (FA), lactic acid (LA), and commercial organic acids mix (COM)) as dietary additives in Nile tilapia (Oreochromis niloticus) reared under a stressful condition (unchanged water). Fish (weighing 3.95 ± 0.05 g) were randomly housed into eight groups (n = 210 fish/group; 70 fish/replicate; 3 replicates/group). The first (negative control) and second (positive control) groups were fed a basal diet without additives and with water exchange at 20% of the water volume every 2 days for the first group and without water exchange for the second. The other six groups (FA1, FA2, LA1, LA2, COM1, and COM2) were fed basal diets supplemented with OAs at two doses (1 and 2%) without water exchange during the trial. The results revealed that fish kept without water exchange (positive control) had the highest level of water-unionized ammonia and nitrite and a marked decline in growth performance (weight gain and specific growth rate). The unchanged water induced substantial decreases in the hematological profile (white and red blood cell count, hemoglobin value, and hematocrit %), protein profile indices (total protein, albumin, and globulin), and antioxidant-immune response (superoxide dismutase, catalase, lysozyme, and total immunoglobulin M). In addition, the biochemical indices (glucose, creatinine, urea, alanine aminotransferase, and aspartate aminotransferase) and lipid peroxide (malondialdehyde) were substantially increased in the positive control group. In addition to that, marked and severe histopathological alteration in the gills, liver, and intestine including hyperplasia, hemorrhage, and degenerative and necrotic changes that were associated with desquamation and sloughing were obvious in the PC group. Dietary LA1 significantly enhanced (P < 0.05) the overall measured indices; meanwhile, the dietary FA and COM recorded the worst results. Overall, our findings demonstrate that the unchanged water negatively affected the growth and physiological functions of Nile tilapia. Dietary incorporation of organic acids did not protect the fish from these hazards except the LA (1%). The latter can reduce nitrogenous compounds, enhance immune response, and alleviate these alterations for the sustainable aquaculture industry.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aquaculture is a rapidly growing industry that satisfies the global demand for reliable, protein-rich food sources, particularly in developing countries (FAO et al. 2023). Oreochromis niloticus (L.), also known as the Nile tilapia, is one of the most popular fish species in the world. This fish species can utilize various protein sources, adapt to different environmental conditions, grow quickly, and have high consumer acceptability (El-Sayed 2019). In the last decade, the aquaculture industry in Egypt has greatly progressed, especially tilapia production, which represents more than 88% of the total aquaculture production (GAFRD 2019). Achieving the maximum economic return requires the following: keeping high water quality for as long as possible, stocking fish at a high rate, raising feed efficiency, and controlling pathogens (Kaleem and Bio Singou Sabi 2021; FAO et al. 2023). On the other hand, due to high stress levels brought on by high stocking density and the resulting poor water quality, tilapias maintained in intensive production systems may be more vulnerable to diseases which leads to a reduction in fish production (Mahmoud et al. 2021; Maulu et al. 2021). This occurred because of declining water quality and rising ammonia levels combined with inadequate fish pond management (Mehrim and Refaey 2023). Because stress can lead to oxidative responses, immune suppression, cell damage, and growth decline, it is critical to develop methods for reducing these detrimental effects on fish (Rajabiesterabadi et al. 2020; Lee et al. 2022).
Elevating the feed efficiency is one method for decreasing these hazards, thereby decreasing wastes in culture water and preventing the deterioration of water quality (Takase 2023). Many substances have been used as feed additives to improve the health status of fish, which achieved positive results (Bilen et al. 2020; Busti et al. 2020; Marimuthu et al. 2022; Vijayaram et al. 2023). One of these additives in aquafeeds is organic acids (acidifiers) that can be very significant in the future of aquaculture diets (Agouz et al. 2015). Organic acids (OAs) are classified into short-chain fatty acids, volatile fatty acids, and weak carboxylic acids. OAs are produced through the microbial fermentation of carbohydrates in the digestive system of animals (Kim et al. 2005). Two main pathways are involved in the potential modes of action of OAs in the digestive tract. Firstly, OAs lower the stomach and small intestine pH by delivering H+ ions. Secondly, OAs prevent the growth of Gram-negative bacteria by causing the acids to dissociate and generating anions inside bacterial cells (Lückstädt 2008; Asriqah et al. 2018). Many studies have confirmed that dietary supplementation of OAs plays a great role in controlling pathogenic bacteria, stimulating growth, fish gut health, and immune system function (Tran-Ngoc et al. 2019; das Neves et al. 2021; da Silva et al. 2023). Additionally, OAs are promising alternatives for growth-promoting antibiotics (Ng and Koh 2011; Lim et al. 2015).
Most studies have estimated the feed additives as health promoters under suitable conditions for tilapia rearing. For example, previous research recorded that feed or water additives presented the in pond water reduced levels of nitrogenous wastes and enhanced water quality (Abdel-Tawwab et al. 2022; El-Kady et al. 2022). Moreover, the stress condition (high stocking density or unchanged water) badly altered the water quality and fish health condition, and these impacts were improved by dietary herbal and watery probiotics supplements (Ayyat et al. 2022; Kord et al. 2022). However, the data on the palliative role of OAs against unchanged water-induced stress is still scarce in fish. Consequently, this study was designed to evaluate the potential functional interaction of feed additives [formic acid (FA), lactic acid (LA), and commercial organic acids mix (COM)] on growth performances, biochemistry, and pathology under a stressful condition (water exchange).
Materials and methods
Ethical statement
All experimental procedures, management conditions, handling, and sampling were approved by the National Institute of Oceanography and Fisheries (NIOF, Egypt) Committee for Ethical Care and Use of Animals/Aquatic Animals (Code: NIOF-AQ1-F-23-R-050).
Diets preparation
FA (molecular formula: CHOOH; molecular weight: 46.03) and LA (molecular formula: C3H6O3: molecular weight: 90.08) were obtained from Finar (ACETO), India. Meanwhile, COM (1,2-propanediol, E200 sorbic acid, E236 FA, E260 acetic acid, E270 LA, E280 propionic acid, and E284 ammonium propionate) was obtained from Selko®, Trouw Nutrition, a Nutrico Company (Fylax Forte-HC liquid). The FA, LA, and COM were added at 1% (10 mL/kg diet) and 2% (20 mL/kg diet) of the basal diet (FA1, FA2, LA1, LA2, COM1, and COM2), respectively. The doses of FA and LA were according to Reda et al. (2022) and El-Dakar et al. (2022) approaches, but no recorded trial on the dietary addition of COM in Nile tilapia.
The basal diet was formulated to cover the nutrient requirements of Nile tilapia, according to NRC (2011). The proximate composition of the basal diet used in the feeding trial was shown in Table 1. All ingredients were mixed well for 15 min before adding oil and water to make a moist, doughy mass for fish feeding. Then, the dough mass was pelleted without steam, yielding 2-mm-diameter sinking pellets. The pellets were then dried at room temperature and stored in clean, sterile plastic bags at −4 °C until use. During the acclimation period and 75 days of the trial, the fish were manually fed every other day (alternating-day feeding) three times daily at 8:00, 12:00, and 17:00 (four days/a week) with a feeding rate of 4% of the total fish biomass. The remains of uneaten feed and fish wastes were removed from the pond bottom using a small suction pump. Every 2 weeks, a sample of fish per replicate was weighed to adjust feed intake.
Fish rearing and experimental design
This study was conducted at a fish-feeding laboratory of the Fish Research Station, National Institute of Oceanography and Fisheries, Fayoum Governorate, Egypt. The fish were obtained from a commercial fish farm on the south coast of Qarun Lake (29°30°N, 30°40°E). They were transferred in aerated plastic bags and acclimatized to laboratory conditions for 15 days, where the fish were randomly distributed into indoor concrete ponds of 1 m3 water capacity (70 juveniles/pond). The average initial body weight (IBW) of the fish was 3.95 ± 0.05 g (with an age of 2 months).
For 75 days, the fish were randomly housed into eight groups (n = 210 mono-sex male tilapia juveniles; 70 fish/replicate; 3 replicates/group) and fed on the tested diets (control, FA1, FA2, LA1, LA2, COM1, and COM2). The first (negative control, NC) and second (positive control, PC) groups were fed on a basal diet without additives with water exchange at 20% of the water volume every 2 days for the first group and without water exchange for the second. The other six groups (FA1, FA1, LA1, LA2, COM1, and COM2) were fed on OAs-supplemented diets without water exchange throughout the trial. The clinical observation of the fish was performed to record any mortality and clinical signs during the 75 days. The loss of water volume due to evaporation or by taking fish samples every 15 days to adjust the feeding rate was compensated.
Water quality analysis
Water samples were collected from fish ponds, and the water quality criteria were measured as water temperature, pH, dissolved oxygen (DO) concentration, unionized ammonia (NH3), and nitrite (NO2) levels using chemical methods (APHA 2005).
Determination of growth indices and survival rate
After 30 and 75 days of the feeding trial, the final body weight (FBW) was estimated. The growth metrics in terms of weight gain (WG, g), specific growth rate (SGR, %/day), and feed conversion rate (FCR), as well as fish survival rate (SR, %), were determined using the following formulas:
Proximate chemical composition
The chemical composition of the whole fish body (15 fish/group) was estimated according to standard methods (AOAC 2005). Moisture was estimated using a hot air oven, while crude protein (N × 6.25) was analyzed using the Kjeldahl method. The crude fat was measured using the Soxhlet method with ether extraction. Ash content was determined by adding ash at 550 °C for 16 h in a muffle Furance 6000 (Thermolyne, USA).
Sampling
At the end of the trial period (75 days), five fish from each replicate were anesthetized using 100 mg/L benzocaine solution (Neiffer and Stamper 2009). Two blood samples (15 fish/group) were withdrawn using 3-mL syringes from the caudal blood vessels and emptied into two tubes containing anticoagulants for hematological assays. The other tubes did not contain anticoagulants for serum separation and measuring the biochemical, oxidant/antioxidant, and immunological assays. Moreover, fish were euthanized by decapitation, and representative tissue specimens were collected from the gills, liver, and intestine for histopathological analysis.
Hematological and biochemical assays
The hematological indices, such as white blood cell count (WBCs, 103/mm3), hemoglobin (Hb, g/dl), red blood cell count (RBCs, 106/mm3), and hematocrit (Hct, %), were determined according to the standard methods as described by Blaxhall and Daisley (1973). Serum levels of creatinine (catalog No.:7D64-20), urea (catalog No.:319-001), glucose (catalog No.:250-007), alanine aminotransferase (ALT, catalog No.:292-007), aspartate aminotransferase (AST, catalog No.:291-007), albumin (catalog No.:7D53-20), and total protein (catalog No.: 7D73-20) were measured spectrophotometrically using commercial kits of Egyptian CO. for Biotechnology with a semi-automated analyzer (3000 Evolution), Biochemical System International, Arezzo, Italy. The globulin level was calculated by subtraction of the albumin value from the total protein value.
Oxidant/antioxidant and immunological assays
The level of malondialdehyde (MDA; catalog No.:MBS268427) and the activity of superoxide dismutase (SOD; catalog No.: CSB-E08555r) and catalase (CAT; catalog No.:MB2600683), as well as the total immunoglobulin M level (IgM; catalog No.:CSB-E07978r), were measured using ELISA kits available from Biosource Inc. (San Diego, CA, USA) that were manufactured by CUSABIO BIOTECH CO., Ltd. In addition, lysozyme activity was assayed using the protocol of Ellis (1990).
Histopathological analysis
Specimens from the gills, liver, and intestine of tilapia fish per group (15 fish/group) were taken and instantly fixed in 10% neutral buffered formalin solution for 48 h and dehydrated in a series of graded ethyl alcohol (70%, 80%, 90%, and 100%; 1 h each). The dehydrated specimens were cleared in two changes of xylene (1 h each), impregnated and embedded in melted paraffin wax, microtomed at 4–5-μm sections, stained with hematoxylin and eosin (H&E) following standard procedures (Suvarna et al. 2018). Moreover, the intestinal morphometric indices (villus width, villus heights, and goblet cell hyperplasia) were scored according to Bernet et al. (1999) approach. Concisely, five non-overlapping randomly selected microscopic fields per intestine per fish were photographed, and the images were then analyzed.
Statistical analysis
To check the norm homogeneity of the data, Bartlett and Kolmogorov–Smirnov analyses were performed. The data were statistically examined by one-way analysis of variance (ANOVA) using Statistical Package for the Social Sciences (SPSS, version 20, IBM Corp.) following the approach of Dytham (1999). The differences among the treatment groups were determined using the Tukey test at the significance level of P < 0.05.
Results
Water quality indices
Table 2 shows the means of the water quality parameters assessed during 75 days of the trial. The water temperature showed no significant alteration among all groups. There was a marked decline (P < 0.05) in the DO level in the PC group compared to the control group (NC). No significant changes were observed in the DO level between the PC group and other groups that fed-OAs incorporated diets. The highest significant (P < 0.05) levels of pH, NH3, and NO2 were observable in the PC group compared to the NC group. In addition, these variables declined in the other experimental groups without significant variations.
Growth performance, SR (%), and clinical observations
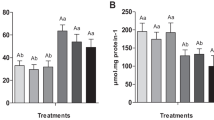
Figs. 1 and 2 show the growth metrics regarding FBW, WG, SGR, and FCR of Nile tilapia after 30 and 75 days of the trial. After 30 days, there were significant (P < 0.05) declines in the FBW, WG, and SGR and an elevation in the FCR of the fish reared without water exchange (PC group) compared to the NC fish. Dietary OAs significantly enhanced (P < 0.05) the FBW, WG, and SGR and improved the FCR compared to the PC group, except for the FA1 and COM1. The highest values were recorded in the LA1 group.
After 75 days, the FBW, WG, and SGR recorded marked decreases with an increase in the FCR of the PC group compared to the NC group. Dietary LA addition significantly enhanced (P < 0.05) the FBW, WG, and SGR and improved the FCR compared to the PC group, and the dietary LA1 recorded the best results. In contrast, dietary FA1 and COM1 lowered the growth performance compared to the PC group.
Table 3 reveals that the PC group had a significantly (P < 0.05) lower SR (%) than the NC group. The SR (%) was decreased in the OAs groups (FA1, FA2, and COM1) compared to the PC group. Meanwhile, the other OAs groups (LA1, COM2, and LA2) revealed a significant (P < 0.05) increase. The LA1 group recorded the highest SR (%) among OAs groups. Moreover, the fish of PC and OAs (FA and COM) exhibited a decreased activity, skin darkness, and fin rot, and some fish showed moderate body hemorrhages. These observations were recorded from the second month of experiment till the end (75 days) and also were ameliorated in the fish of LA group.
Proximate body composition indices
Table 3 exhibits the results of body composition indices, where no significant differences were observed between the PC and NC groups except for a decline in the moisture level. Marked elevations in the moisture, crude protein, and ash levels along with a lowering in the crude fat value were observed in the fish-fed diets containing OAs compared to the PC group, except for the ash content of the FA1 group. The highest level of crude protein was obvious in the LA1 group. Meanwhile, the COM2 group recorded the highest content of Ash. In contrast, the lowest value of crude fat was noted in the LA1 group.
Hematological and protein profile indices
As shown in Table 4, there were substantial decreases (P < 0.05) in the WBCs count, Hb value, RBCs count, and Hct (%) as well as protein profile indices (total protein, albumin, and globulin) of the PC group compared to the NC group. The highest count of WBCs was obvious in the LA groups. Meanwhile, the lowest count was in FA groups. No significant alterations were observed in the Hb value, RBCs count, Hct (%), and albumin level between the OAs and PC groups, and the LA1 group recorded the highest values.
The total protein and globulin values were substantially increased (P < 0.05) in the LA1 group, followed by the LA2, COM1, and COM2 groups relative to the PC group. The FA groups showed no marked alteration in the total protein and globulin levels with the PC group.
Biochemical indices
Table 5 reveals that serum biochemical indices (creatinine, urea, ALT, AST, and glucose) were significantly elevated (P < 0.05) in the PC group compared to the NC group. Dietary OAs did not significantly alter the creatinine and urea relative to the PC group. However, the lowest values were obvious in the LA1 group. The highest levels of ALT and AST were obvious in the FA2 group, while the LA1 group recorded the lowest value. Dietary OAs significantly reduced (P < 0.05) blood glucose compared to the PC group except for FA and COM2 groups, with the lowest level noted in the LA1 group.
Oxidant/antioxidant and immunological indices
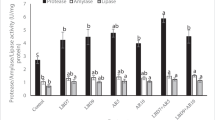
Table 6 shows a substantial increase in the MDA level and a decrease in the activity of SOD, CAT, and lysozyme, as well as the total IgM level of the PC group relative to the NC group. Dietary FA1 and COM1 significantly increased the MDA (P < 0.05) value compared to the PC group, while the lowest value was noted in the LA1 group. Values of SOD, CAT, lysozyme, and total IgM were markedly enhanced (P < 0.05) in LA groups relative to the PC group. Meanwhile, the other groups (FA and COM) did not significantly differ from the PC group.
Histopathological investigation
Fig. 3 reveals the histopathological alterations in the gills of O. niloticus fed diets containing different levels of OAs and with/without water exchange for 75 days. The control fish (NC) showed normal histological architectures with no morphological alterations. The gills of the fish kept without water exchange (PC group) were severely affected and displayed numerous lesions, including epithelial hyperplasia with a fusion of secondary lamellar and capillary congestion. Dietary OAs addition did not protect the gills from the impact of stress (non-water exchange) except for moderate changes attained by dietary LA1. The gills of the FA groups revealed an interlamellar hemorrhage, lamellar necrosis, and epithelial desquamation (FA1 group), as well as capillary telangiectasia, lamellar sloughing, and epithelial hyperplasia (FA2 group). The gills of the LA groups displayed inflammatory cell infiltrate, and capillary telangiectasia (LA1 group), as well as epithelial hyperplasia with lamellar fusion, capillary dilatation, and hemorrhage with hemosiderosis (LA2 group). The gills of the COM groups exhibited necrosis of lamellar epithelial, epithelial uplifting as well as congested capillaries (COM1 group), capillary telangiectasia, and epithelial uplifting (COM2 group).
Photomicrograph (H&E) of gills sections of O. niloticus. Fish of the NC group show a normal histological picture. Fish of the PC group show epithelial hyperplasia with lamellar fusion (arrowheads) and capillary congestion (arrows). Fish of the FA1 group show interlamellar hemorrhage (arrows), lamellar necrosis (red arrowhead), and epithelial desquamation (black arrowhead). Fish of the FA2 group show capillary telangiectasia (arrow), lamellar sloughing (red arrowhead), and epithelial hyperplasia (black arrowhead). Fish of the LA1 group inflammatory cell infiltrate (red arrow), capillary telangiectasia (black arrow), lamellar necrosis (red arrowhead), and epithelial uplifting (black arrowhead). Fish of the LA2 group show epithelial hyperplasia with lamellar fusion (arrowhead), capillary dilatation (black arrow), and hemorrhage with hemosiderosis (red arrow). Fish of the COM1 group show congested capillaries (black arrow), necrosis of lamellar epithelial (black arrowheads), and epithelial uplifting (red arrowhead). Fish of the COM2 group showing capillary telangiectasia (arrow) and epithelial uplifting (red arrowhead). Scale bar: 30 and 100 μm
The histopathological alterations in the hepatic tissues were presented in Fig. 4, where the liver of the NC group displayed normal histological architectures with no histopathological changes. The liver of the PC group exhibited a wide array of hepato-pathic histological alterations, including focal coagulative necrosis. Dietary addition of OAs worsens the hepato-pathic changes as more severe lesions were noted. However, moderate alterations were obvious by LA1. The livers of the FA groups showed individualization of hepatic cells, vascular congestion, sinusoidal dilatation, hyperplastic melanomacrophages (FA1 group), and thrombosis in a central vein (FA2 group). The livers of the LA groups showed vascular congestion and focal mononuclear-cell aggregation (LA1 group), and extensive vacuolation with single-cell necrosis (LA2 group). The livers of COM groups exhibited vascular congestion, focal mononuclear-cell aggregation (COM1 group), and focal coagulative necrosis (COM2 group).
Photomicrograph (H&E) of hepatic tissue sections of O. niloticus. Fish of the NC group show a normal histological picture. Fish of the PC group show focal coagulative necrosis (ellipse). Fish of the FA1 group show individualization of hepatic cells, vascular congestion (black arrows), sinusoidal dilatation (black arrowhead), and hyperplastic melanomacrophages (red arrow). Fish of the FA2 group showing thrombosis in a central vein (arrowhead). Fish of the LA1 group showing vascular congestion (arrow) and focal mononuclear-cell aggregation (arrowhead). Fish of the LA2 group show extensive vacuolation with single-cell necrosis (arrowheads). Fish of the COM1 group showing vascular congestion (arrow) and focal mononuclear-cell aggregation (arrowhead). Fish of the COM2 group showing focal coagulative necrosis (ellipse). Scale bar: 30 μm
Fig. 5 displays the histopathological alterations in the intestinal tissues, where the intestine of the NC group displayed normal histological architectures with no histopathological alterations. The intestine of the fish kept without water exchange (PC group) was severely affected and displayed numerous lesions, including necrotic enterocytes and epithelial desquamation. Dietary OAs additions exhibited no significant protective effects on intestinal histology except in the LA1 group, which showed mild lesions. The intestine of the FA groups revealed hyperplastic goblet cells (FA1 group) and coagulative necrosis of the villous tips (FA2 group). The intestine of the LA groups displayed leucocytic infiltration at the villous tip (LA1 group) and enterocytes necrosis and desquamation at the villous tips (LA2 group). The intestine of the COM groups exhibited villous curling (COM1 group) and villous necrosis with epithelial desquamation (COM2 group).
Photomicrograph (H&E) of intestinal tissue sections of O. niloticus. Fish of the NC group show a normal histological picture. Fish of the PC group show necrotic enterocytes (arrow) and epithelial desquamation (arrowhead). Fish of the FA1 group showing hyperplastic goblet cells (arrowheads). Fish of the FA2 group showing coagulative necrosis of the villous tips (arrowheads). Fish of the LA1 group show leucocytic infiltration at the villous tip (arrowhead). Fish of the LA2 group show enterocytes necrosis and desquamation at the villous tips (arrowheads). Fish of the COM1 group showing villous curling (arrowhead). Fish of the COM2 group showing villous necrosis (arrowhead) with epithelial desquamation (arrow). Scale bar: 100 μm
Moreover, there were marked reductions in the villous width and length with marked increases in goblet cell hyperplasia frequency of the PC compared with the NC group as shown in Table 7. Dietary LA significantly elevated (P < 0.05) villous width and length and decreased the goblet cell hyperplasia frequency relative to the PC group. However, the other groups (FA and COM) did not significantly differ from the PC group (Table 7).
Discussion
The effectiveness of employing OAs on fish performance is variable and based on various variables, including fish species, the types, concentrations of OAs employed, water quality, and culture management (Paul et al. 2024). This study intended to evaluate how Nile tilapia reared without water renewal responded to dietary OAs in terms of growth rate and physiological functions.
Water quality variables have acceptable limits in which aquatic organisms function optimally (Verma et al. 2022). In our study, the level of DO declined and NH3 and NO2 increased in the PC group. Reduced DO levels could have been caused by the microbial decomposition of accumulated organic wastes in unchanged water (Obirikorang et al. 2019). The low DO levels resulted in ion loss in freshwater fish, negatively affected osmoregulation, and can lead to fish mortalities (Samaras et al. 2023). In the OAs-fed groups, NH3 and NO2 declined, but their values were not acceptable for the optimum Nile tilapia growth (Makori et al. 2017). The considerable decreases in the release of excreted nitrogen compounds caused by the increased feed digestion and nutrient absorption may cause these outcomes (Fabay et al. 2022). OAs markedly improved water quality by reducing nitrogen compounds excretion (NH3 and NO2). According to earlier research, adding OAs to the diets of gilthead seabream (Sparus aurata) and Nile tilapia decreased nitrogen compound excretion and improved water quality in pond water (Abdel-Tawwab et al. 2022; El-Dakar et al. 2022).
The growth performance decreased in the PC group after 30 and 75 days of the trial compared to the control group with a lower SR (%). The higher deposition of fish wastes and metabolites and the reduced oxygen level from unchanged water tanks are the causes of the slower growth (Katsika et al. 2021). Since oxygen is essential for the production of energy in aerobic metabolic activities, a decrease in DO availability may, over time, limit the use of dietary energy for growth and metabolic processes. This finding follows a prior study (Obirikorang et al. 2019), which found that fish in culture can experience persistent hypoxia, impairing their ability to consume food and grow.
Dietary OAs (LA) improved these indices and augmented SR except for the FA and COM1. The highest values were obvious in the LA1 group. The positive effect of dietary LA can be attributed to their ability to reduce pH in the fish gastrointestinal tract, stimulating pepsin activity, thus increasing protein metabolism and mineral intake (Asriqah et al. 2018). The lowered pH inhibits the pathogenic bacteria and reduces the immune system’s involvement in consuming dietary proteins to form antibodies to combat the pathogenic bacteria, allowing this protein to contribute to muscle development (Fabay et al. 2022). Furthermore, OAs can promote the colonization of beneficial microflora in the gastrointestinal tract and nutrient utilization and absorption (Busti et al. 2020). Moreover, the improved intestinal histomorphometric indices as observed in our findings by dietary LA which indicated the improved absorption capacity of nutrients can be another rationale. Therefore, employing OAs especially LA in this circumstance will undoubtedly increase muscle mass and growth in the fish (Sobhy et al. 2018). Similar outcomes reported the growth and survival-enhancing effects of the dietary OAs, including LA in fishes (Pelusio et al. 2020; da Silva et al. 2023).
Concerning body composition, the crude protein and ash were positively affected by adding dietary OAs (LA and COM). This may be attributed to that OAs improve the fish digestive system to utilize dietary protein and mineral absorption (Chen et al. 2024). In the same trend, Soltan et al. (2017) and Omosowone et al. (2018) observed an enhancement in the crude protein value of Nile tilapia by dietary OAs.
Hemato-biochemical measurements are significant biomarkers for improper environmental circumstances (Hastuti and Subandiyono 2018). The findings revealed that hematological variables of the PC group and OAs-fed groups exhibited a decline. These variables are vital indicators of fish’s ability to breathe adequately and reflect the presence of anemia (Seibel et al. 2021). According to Hastuti and Subandiyono (2018), the hematological indices decrease when fish are exposed to stress. In contrast, the LA1 group recorded the highest values among these groups, demonstrating a significant improvement in general health. The enhancement could be related to LA supplementation that maximizes the liberation of vital minerals from the diet ingredients (Shafique et al. 2018). In addition, because of the entrance of LA into the gastrointestinal tract, LA may undergo dissociation, releasing lactate, an important substance for supplying energy to all body cells, including blood cells. This will elevate the number of blood cells and the Hb content (Lall 2022). Similar outcomes were retrieved by Hoseini et al. (2022) and Mirghaed et al. (2023).
Liver and renal function activities are considered crucial diagnostic indicators as they reflect the overall nutritional state and exposure to water pollutants in aquaculture (Shahid et al. 2022). It is observed that the fish kept without water exchange had lower levels of protein fractions and higher renal-hepatic biomarkers (creatinine, urea, ALT, and AST) levels. This outcome reflected a protein deficiency, altered hepato-renal function, and lowered immunity (Ghelichpour et al. 2017). The histopathological alterations observed in the liver in our study induced by the unchanged water confirmed the hepatic dysfunction. In addition, the increase in gluconeogenesis in response to the increased demand for energy under the stress situation may cause elevated hepatic enzymes (Rasal et al. 2020). Concurrently, Abd Elnabi et al. (2018) and Elshopakey et al. (2023) reported elevating in the hepato-renal indices of red tilapia (Oreochromis sp) and Nile tilapia exposed to stress (ammonia toxicity). Moreover, the OAs-fed fish exhibited decreases in these variables except for the FA group. The decrease in liver and kidney function indices implies improved health status, confirming the protective function of LA. Similarly, Sobhy et al. (2018) and Hussein et al. (2023) observed a reduction in the ALT and AST values of Nile tilapia and gilthead sea bream-fed OAs-supplemented diets.
Blood glucose levels are crucial physiological markers for evaluating fish health (Jeong et al. 2021). Significant elevation in the level of blood glucose was noted in the PC group and OAs-fed groups except in the LA and COM1 groups. This observation may be related to the rise in glucose oxidation during chronic stress to fulfill greater energy needs, as reported by Raposo de Magalhães et al. (2020). Furthermore, fish must spend more energy on homeostatic processes, which decreases growth performance (Goodrich and Clark 2023).
Nonspecific immunity in fish is a crucial defense mechanism (Mokhtar et al. 2023). In this study, the fish kept under unchanged water had lower immune response manifested by a decline in WBCs count, globulin, lysozyme, and total IgM. The minimal drops in these variables are commonly associated with stress (Elshopakey et al. 2023). These results were harmonious with the findings of Obirikorang et al. (2019) and Rajabiesterabadi et al. (2020). In contrast, dietary LA improved these variables, indicating the augmented immune, which could enhance a better SR (%). OAs can affect indigenous intestinal flora, which is required to develop the gut immune system (Busti et al. 2020). Similarly, Torrecillas et al. (2021) reported a modulated immune response of European sea bass (Dicentrarchus labrax) by dietary OAs.
Antioxidant enzymes are crucial in reducing oxidative stress in fish by preserving redox equilibrium and regulating imbalances in the antioxidant system (Ighodaro and Akinloye 2018). Our finding cleared a substantial increase in the MDA level and a decrease in the SOD and CAT activity of the PC group. This result may be due to the elevated NH3 from the unchanged water that could accumulate in aquatic animals’ tissues, causing the release of reactive oxygen species (ROS), which in turn could disrupt cell membranes and produce lipid peroxides (Zhao et al. 2021). Dietary LA improved these variables, indicating a reduction of oxidative stress and augmenting the antioxidant capacity that enhances fish health and welfare. Previous reports confirmed these results (Hoseini et al. 2022; Hoseini et al. 2023).
Investigating the health status and stress exposure requires a thorough evaluation of the histopathological changes in fish tissues (Georgieva et al. 2021). Here, we reported numerous histopathological lesions in the gills, liver, and intestine of the fish kept without water exchange. These lesions may be induced by the high NH3 level resulting from unchanged water, which causes intracellular ROS, disrupts intracellular balance, and ultimately results in DNA damage and cell death (Liu et al. 2021). Furthermore, dietary OAs additions did not protect the tissues from the impact of stress (non-water exchange) except for LA1. Romano et al. (2016) and Ebrahimi et al. (2017) observed a hepatic injury of Nile tilapia and red hybrid tilapia fed on a high dietary level of OAs. Similarly, moderate histopathological alterations in the liver, intestine, and kidney of Nile tilapia-fed OAs diets were reported (Rabea et al. 2023).
However, the dietary LA1 revealed moderate lesions that resulted from the enhanced immune-antioxidant parameters and reduction in the release of ROS which shielded the tissues from the impacts of stress. Similar histopathological impacts were exerted by dietary OAs as reported by da Silva et al. (2023) and Nimalan et al. (2023).
Conclusion
Based on our findings, the unchanged water has a number of major negative consequences on the health status of Nile tilapia. Among these include a decline in growth performance, body composition, antioxidant-immune response as well as alterations in the physiological and histopathological pictures. Dietary FA and COM did not protect fish from these hazards and gave worse results. In contrast, dietary additions of LA at a dose of 1% could alleviate these alterations and promote the health status of fish. As long as the water quality is within allowable bounds, OAs generally work well; however, when the quality of the water drops, they constitute another source of stress. In low-water situations, LA is thought to be less damaging than OAs. Future research is necessary to examine the impact on a molecular level and in different fish species.
Data availability
All data generated or analyzed during this study are included in this article.
References
Abd Elnabi HE, Abdel-Baky AMM, Hassanen GDI (2018) Impact of total ammonia on growth, physiological status and histological examination of red tilapia (Oreochromis sp.). J Ani Poult Prod 9(12):515–524
Abdel-Tawwab M, Abo Selema TAM, Soliman AM, Shady SHH, Khalil RH (2022) Effects of a commercial feed additive (Sanacore® GM) on water quality, productive performance and blood profile of gilthead seabream (Sparus aurata). Aquac Res 53(17):6310–6323
Agouz HM, Soltan MA, Meshrf RN (2015) Effect of some organic acids and organic salt blends on growth performance and feed utilization of Nile tilapia, (Oreochromis niloticus). Egypt J Nutr Feed 18(2):443–450
AOAC (2005) Determination of moisture, ash, protein and fat in animal feed 4.1. Official Method of Analysis of the Association of Analytical Chemists, 18th edn. Washington DC
APHA (2005) Standard methods for examination of water and wastewater. American Public Health Association, American Water Works Association, 21th edn. Water Pollution Control Federation, New York
Asriqah L, Nugroho RA, Aryani R (2018) Effect of various organic acid supplementation diets on Clarias gariepinus Burchell, 1822: Evaluation of growth, survival and feed utilization. F1000Res 7:1465
Ayyat MS, Al-Sagheer AA, Ayyat AMN, Abdelrhman AM, Ahmed NH, Naiel MAE (2022) Effects of a blend of herbal feed supplements on growth, associated blood indices and body chemical analysis in Nile tilapia reared under high stocking density. Aquac. Res. 53(16):5475–5485
Bernet D, Schmidt H, Meier W, Burkhardt-Holm P, Wahli T (1999) Histopathology in fish: proposal for a protocol to assess aquatic pollution. J. Fish Dis. 22:25–34
Bilen S, Altief TAS, Özdemir KY, Salem MOA, Terzi E, Güney K (2020) Effect of lemon balm (Melissa officinalis) extract on growth performance, digestive and antioxidant enzyme activities, and immune responses in rainbow trout (Oncorhynchus mykiss). Fish Physiol Bioch 46:471–481
Blaxhall PC, Daisley KW (1973) Routine haematological methods for use with fish blood. J Fish Biol 5:771–781
Busti S, Rossi B, Volpe E, Ciulli S, Piva A, D’Amico F et al (2020) Effects of dietary organic acids and nature identical compounds on growth, immune parameters and gut microbiota of European sea bass. Sci Rep 10(1):21321
Chen J, He S, Zhang Z, Li J, Zhang X, Li J et al (2024) Application of organic acid salts as feed additives in some aquatic organisms: potassium diformate. Fishes 9(3):85
da Silva VG, Favero LM, Mainardi RM, Ferrari NA, Chideroli RT, Di Santis GW et al (2023) Effect of an organic acid blend in Nile tilapia growth performance, immunity, gut microbiota, and resistance to challenge against francisellosis. Res Vet Sci 159:214–224
das Neves SCV, da Silva SMBC, GKA C, Correia ES, Santos AL, da Silva LCR et al (2021) Dietary supplementation with fumaric acid improves growth performance in Nile Tilapia juveniles. Animals 12:1–10
Dytham C (1999) Choosing and using statistics: a biologist’s guide. Blackwell Science Ltd., London, pp 320
Ebrahimi M, Daeman NH, Chong CM, Karami A, Kumar V, Hoseinifar SH et al (2017) Comparing the effects of different dietary organic acids on the growth, intestinal short-chain fatty acids, and liver histopathology of red hybrid tilapia (Oreochromis sp.) and potential use of these as preservatives. Fish Physiol Biochem 43(4):1195–1207
El-Dakar AY, Shalaby SM, Mohamed BK, Abdel-Aziz MFA (2022) Improving the growth, feed efficiency and hematological indicators of Nile tilapia fingerlings Oreochromis niloticus using dietary lactic acid supplementation with different feeding routines. Mediterr Aquac J 9:25–37
El-Kady AA, Magouz FI, Mahmoud SA, Abdel-Rahim MM (2022) The effects of some commercial probiotics as water additive on water quality, fish performance, blood biochemical parameters, expression of growth and immune-related genes, and histology of Nile tilapia (Oreochromis niloticus). Aquaculture 546:737249
El-Sayed AFM (2019) Tilapia culture. In: El-Sayed AFM. TilapiaCulture, 2nd ed. CABI, Cambridge, MA, USA. Academic Press, 2006
Ellis AE (1990) Lysozyme assays. In: Stolen JS, Fletcher TC, Anderson DP, Roberson BS, Van Muiswinkel WB (eds) Techniques in fish immunology. SOS Publications, Fair Haven, pp 101–103
Elshopakey GE, Mahboub HH, Sheraiba NI, Abduljabbar MH, Mahmoud YK, Abomughaid MM et al (2023) Ammonia toxicity in Nile tilapia: potential role of dietary baicalin on biochemical profile, antioxidant status and inflammatory gene expression. Aquac Rep 28:101434
Fabay R, Serrano JA, Alejos M, Fabay J (2022) Effects of dietary acidification and acid source on fish growth and feed efficiency (Review). World Acad Sci J 4:21
FAO, IFAD, UNICEF, WFP, WHO (2023) The state of food security and nutrition in the world 2023. In: Urbanization, agrifood systems transformation and healthy diets across the rural–urban continuum. FAO, Rome
GAFRD (2019) General Authority For Fish Resources Development. Agriculture Ministry Press, Cairo, Fish statistics year book
Georgieva E, Yancheva V, Stoyanova S, Velcheva I, Iliev I, Vasileva T et al (2021) Which is more toxic? Evaluation of the short-term toxic effects of Chlorpyrifos and Cypermethrin on selected biomarkers in common carp (Cyprinus carpio), Linnaeus 1758). Toxics 9(6):125
Ghelichpour M, Taheri Mirghaed A, Mirzargar SS, Joshaghani H, Ebrahimzadeh Mousavi H (2017) Plasma proteins, hepatic enzymes, thyroid hormones and liver histopathology of Cyprinus carpio (Linnaeus, 1758) exposed to an oxadiazin pesticide, indoxacarb. Aquac Res 48(11):5666–5676
Goodrich HR, Clark TD (2023) Why do some fish grow faster than others? Fish Fish 24(5):796–811
Hastuti SD, Subandiyono S (2018) Haematological parameters of the North African catfish Clarias gariepinus farmed using biofloc technology. AACL Bioflux 11(4):1415–1424
Hoseini SM, Rajabiesterabadi H, Abbasi M, Khosraviani K, Hoseinifar SH, Doan HV (2022) Modulation of humoral immunological and antioxidant responses and gut bacterial community and gene expression in rainbow trout, Oncorhynchus mykiss, by dietary lactic acid supplementation. Fish Shellfish Immunol 125:26–34
Hoseini SM, Yousefi M, Afzali-Kordmahalleh A, Pagheh E, Taheri MA (2023) Effects of Dietary lactic acid supplementation on the activity of digestive and antioxidant enzymes, gene expressions, and bacterial communities in the intestine of common carp, Cyprinus carpio. Animals 13(12):1934
Hussein EE, Habiba MM, Ashry AM, Al-Zayat AM, Teiba II, Shehata AI et al (2023) Effects of dietary supplementation with organic acids mixture on growth, feed efficiency, hematobiochemical parameters, immunity, and intestinal microbiota of Gilthead seabream (Sparus aurata) juveniles. Aquac Rep 33:101846
Ighodaro OM, Akinloye OA (2018) First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): their fundamental role in the entire antioxidant defence grid. Alex Med J 54(4):287–293
Jeong H, Kang M, Cha S-Y, Byun J, Kim J, Baek JW et al (2021) Usefulness of clustering blood biochemical markers to assess thermal stress and acclimation in red seabream Pagrus major. Aquaculture 545:737197
Kaleem O, Bio Singou Sabi A-F (2021) Overview of aquaculture systems in Egypt and Nigeria, prospects, potentials, and constraints. Aquac Fish 6(6):535–547
Katsika L, Huesca Flores M, Kotzamanis Y, Estevez A, Chatzifotis S (2021) Understanding the interaction effects between dietary lipid content and rearing temperature on growth performance, feed utilization, and fat deposition of sea bass (Dicentrarchus labrax). Animals 11(2):392
Kim YY, Kil DY, Oh HK, Han IK (2005) Acidifier as an alternative material to antibiotics in animal feed. Asian-Australas J. Anim. Sci. 18:1048–1060
Kord MI, Maulu S, Srour TM, Omar EA, Farag AA, Nour AAM et al (2022) Impacts of water additives on water quality, production efficiency, intestinal morphology, gut microbiota, and immunological responses of Nile tilapia fingerlings under a zero-water-exchange system. Aquaculture 547:737503
Lall SP (2022) Chapter 6—The minerals. In: Hardy RW, Kaushik SJ (eds) Fish nutrition (Fourth Edition). Academic Press, pp 469–554
Lee JW, Kim JH, Lee D, Lim H, Kang J (2022) Toxic effects on oxidative stress, neurotoxicity, stress, and immune responses in juvenile olive flounder, Paralichthys olivaceus, exposed to waterborne hexavalent chromium. Biology 11(5):766
Lim C, Lückstädt C, Webster CD, Kesius P (2015) Organic acids and their salts. In: Dietary nutrients, additives, and fish health. pp 305-319
Liu M-J, Guo H-Y, Zhu K-C, Liu B-S, Liu B, Guo L et al (2021) Effects of acute ammonia exposure and recovery on the antioxidant response and expression of genes in the Nrf2-Keap1 signaling pathway in the juvenile golden pompano (Trachinotus ovatus). Aquat Toxicol 240:105969
Lückstädt C (2008) The use of acidifiers in fish nutrition. CAB Rev.: Perspect. Agric Vet Sci Nutr Nat Resour 3:1–8
Mahmoud HK, Reda FM, Alagawany M, Farag MR (2021) Ameliorating deleterious effects of high stocking density on Oreochromis niloticus using natural and biological feed additives. Aquaculture 531:735900
Makori AJ, Abuom PO, Kapiyo R, Anyona DN, Dida GO (2017) Effects of water physico-chemical parameters on tilapia (Oreochromis niloticus) growth in earthen ponds in Teso North Sub-County Busia County. Fish Aquat Sci 20(1):30
Marimuthu V, Shanmugam S, Sarawagi AD, Kumar A, Kim IH, Balasubramanian B (2022) A glimpse on influences of feed additives in aquaculture. eFood 3(1-2):1–10
Maulu S, Hasimuna OJ, Mphande J, Munang’andu HM (2021) Prevention and control of streptococcosis in tilapia culture: a systematic review. J Aquat Anim Health 33:162–177
Mehrim AI, Refaey MM (2023) An overview of the implication of climate change on fish farming in Egypt. Sustainability 15(2):1679
Mirghaed AT, Mirzargar SS, Ghelichpour M, Moghaddam AA, El-Haroun E, Hoseini SM (2023) Effects of dietary lactic acid supplementation on growth performance, hemato-immunological parameters, and calcium and phosphorus status of common carp Cyprinus carpio. Aquac Rep 29:101499
Mokhtar DM, Zaccone G, Alesci A, Kuciel M, Hussein MT, Sayed RKA (2023) Main components of fish immunity: an overview of the fish immune system. Fishes 8(2):93
Neiffer DL, Stamper MA (2009) Fish sedation, anesthesia, analgesia, and euthanasia: considerations, methods, and types of drugs. ILAR J. 50:343–360
Ng WK, Koh CB (2011) Application of organic acids in aquafeeds: impacts on fish growth, nutrient utilization and disease resistance. In: Luckstadt C (ed) Standards for acidifiers, principles for the use of organic acids in animal nutrition. Nottingham University Press, Nottingham, UK, pp 49–58
Nimalan N, Sørensen SL, Fečkaninová A, Koščová J, Mudroňová D, Gancarčíková S et al (2023) Supplementation of lactic acid bacteria has positive effects on the mucosal health of Atlantic salmon (Salmo salar) fed soybean meal. Aquac Rep 28:101461
NRC (2011) Nutrient requirements of fish and shrimp. The National Academies Press, Washington, DC
Obirikorang KA, Agbo NW, Obirikorang C, Adjei-Boateng D, Ahiave SE, Skov PV (2019) Effects of water flow rates on growth and welfare of Nile tilapia (Oreochromis niloticus) reared in a recirculating aquaculture system. Aquac Inter 27(2):449–462
Omosowone OO, Dada AA, Adeparusi EO (2018) Comparison of dietary butyric acid supplementation effect on growth performance and body composition of CLarias gariepinus and Oreochromis niloticus fingerlings. Iran J Fish Sci 17:403–412
Paul P, Islam MS, Hasanuzzaman AFM (2024) Water quality, nutritional, hematological, and growth profiles of Ompok pabda fish fry reared in biofloc technology and traditional culture system with different stocking densities. Animals 14(1):90
Pelusio NF, Rossi B, Parma L, Volpe E, Ciulli S, Piva A et al (2020) Effects of increasing dietary level of organic acids and nature-identical compounds on growth, intestinal cytokine gene expression and gut microbiota of rainbow trout (Oncorhynchus mykiss) reared at normal and high temperature. Fish Shellfish Immunol 107:324–335
Rabea AM, Ibrahim I, Mahgoub AH, El-Shaieb A (2023) Pathological, ultrastructural and hematological studies on the single and combined effect of dietary supplementation with lauric and benzoic acids on Nile tilapia. Mansoura Vet Med J 24(3). https://doi.org/10.21608/mvmj.2023.190788.1134
Rajabiesterabadi H, Yousefi M, Hoseini SM (2020) Enhanced haematological and immune responses in common carp Cyprinus carpio fed with olive leaf extract-supplemented diets and subjected to ambient ammonia. Aquac Nutr 26(3):763–771
Raposo de Magalhães C, Schrama D, Farinha AP, Revets D, Kuehn A, Planchon S et al (2020) Protein changes as robust signatures of fish chronic stress: a proteomics approach to fish welfare research. BMC Genomics 21(1):309
Rasal KD, Iquebal MA, Dixit S, Vasam M, Raza M, Sahoo L et al (2020) Revealing alteration in the hepatic glucose metabolism of genetically improved carp, Jayanti rohu Labeo rohita fed a high carbohydrate diet using transcriptome sequencing. Int J Mol Sci 21(21):8180
Reda RM, El-Murr A, Abd Elhakim Y, El-Shahat W (2022) Aeromonas veronii detection in Egyptian fish farms with summer tilapia mortality outbreaks and the role of formic acid in limiting its spread. Aquac Res 53(3):940–956
Romano N, Simon W, Ebrahimi M, Fadel AH, Chong CM, Kamarudin MS (2016) Dietary sodium citrate improved oxidative stability in red hybrid tilapia (Oreochromis sp.) but reduced growth, health status, intestinal short chainatty acids and induced liver damage. Aquaculture 458:170–176
Samaras A, Tsoukali P, Katsika L, Pavlidis M, Papadakis IE (2023) Chronic impact of exposure to low dissolved oxygen on the physiology of Dicentrarchus labrax and Sparus aurata and its effects on the acute stress response. Aquaculture 562:738830
Seibel H, Baßmann B, Rebl A (2021) Blood will tell: what hematological analyses can reveal about fish welfare. Front Vet Sci 8:616955
Shafique L, Afzal M, Shah SZH, Fatima M, Naz H, Saddique Q (2018) Acidified diet increases the trace mineral content in whole body fish Labeo rohita fingerlings. Punjab Univ J Zool 33:103–106
Shahid S, Sultana T, Sultana S, Hussain B, Al-Ghanim KA, Al-Bashir F et al (2022) Detecting aquatic pollution using histological investigations of the gills, liver, kidney, and muscles of Oreochromis niloticus. Toxics 10(10):564
Sobhy HM, El Moghazy GM, Abdel A’al MH, Ibrahim HE (2018) Impact of protected and non-protected lactic acid used as an acidifier in the diet on Oreochromis niloticus. Egypt J Aquat Biol Fish 22(5):87–97
Soltan MA, Hassaan MS, Meshrf RN (2017) Response of Nile tilapia (Oreochromis niloticus) to diet acidification: effect on growth performance and feed utilization. J Appl Aquac 29(3–4):207–219
Suvarna KS, Layton C, Bancroft JD (2018) Bancroft’s theory and practice of histological techniques. E-Book.Elsevier Health Sciences, Churchill Livingstone. Elsevier, England, Eighth edition edn
Takase K (2023) Research that contributes to improving the physiological functions of fish and promoting sustainable aquaculture using biological resources. Salt Seawater Sci Technol 3:45–57
Torrecillas S, Terova G, Makol A, Serradell A, Valdenegro-Vega V, Izquierdo M et al (2021) Dietary phytogenics and galactomannan oligosaccharides in low fish meal and fish oil-based diets for european sea bass (Dicentrarchus labrax) juveniles: effects on gill structure and health and implications on oxidative stress status. Front Immunol 12:663106
Tran-Ngoc KT, Huynh ST, Sendão J, Nguyen TH, Roem AJ, Verreth JAJ et al (2019) Environmental conditions alter the effect of organic acid salts on digestibility and intestinal morphology in Nile tilapia (Oreochromis niloticus). Aquac Nutr 25(1):134–144
Verma DK, Satyaveer MN, Kumar P, Jayaswa R (2022) Important water quality parameters in aquaculture: an overview. Aquac Environ 3:24–29
Vijayaram S, Ringø E, Zuorro A, van Doan H, Sun Y (2023) Beneficial roles of nutrients as immunostimulants in aquaculture: a review. Aquac Fish https://doi.org/10.1016/j.aaf.2023.02.001
Zhao Q, Feng K, Zhang L, Bai Y, Yao W (2021) Effects of acute ammonia stress on antioxidant responses, histopathology and ammonia detoxification metabolism in triangle sail mussels (Hyriopsis cumingii). Water 13(4):425
Acknowledgements
The authors would like to thank all staff members of the Aquaculture and Biotechnology Dept., Faculty of Aquaculture and Marine Fisheries, Arish University, Egypt, and all staff members of Aquaculture Division, National Institute of Oceanography and Fisheries (NIOF), Egypt for supporting this research.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Mohamed F. A. Abdel-Aziz, Mohammed F. El Basuini, Mohamed F. Sadek, Mohamed A. Elokaby, Ashraf Y. El-Dakar, Mohamed M. M. Metwally, Ahmed Shehab, Mahmoud Mabrok, Afaf N. Abdel Rahman: conceptualization, data curation, formal analysis, investigation, methodology, resources, validation, visualization. Mohamed F. A. Abdel-Aziz & Afaf N. Abdel Rahman: Writing – original draft. Mohamed F. A. Abdel-Aziz, Mahmoud Mabrok, & Afaf N. Abdel Rahman: Writing – review & editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Handling editor: Brian Austin
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Nile tilapia reared under unchanged water shows growth retardation and physiological disturbances.
• The unchanged water induces histopathological changes and suppresses antioxidant-immune responses.
• Dietary formic acid and commercial organic acids do not protect from these detrimental effects.
• Dietary lactic acid (1%) alleviates these hazards and improves health status.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abdel-Aziz, M.F.A., El Basuini, M.F., Sadek, M.F. et al. Unchanged water stress induces growth retardation, histopathological alterations, and antioxidant-immune disruptions in Oreochromis niloticus: the promising role of dietary organic acids. Aquacult Int (2024). https://doi.org/10.1007/s10499-024-01454-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10499-024-01454-y