Abstract
Eight-week feeding trial was conducted to estimate the effects of dietary probiotic (P), garlic (G), and onion (O) powders on growth, hematology, and serum parameters in the juvenile rainbow trout (Oncorhynchus mykiss). Seven practical diets were formulated to be isonitrogenous (49% protein) and isolipidic (20% lipid) and the diets included the P, O, and G at the concentrations of 0.1, 1, and 1%, respectively, at the replace of wheat flour. Rainbow trout fed the G supplemented diet displayed a higher specific growth rate (SGR) when compared with fish fed the P and the mix of probiotics and onion (PO) (P < 0.05). The feed conversion ratio (FCR) of the fish fed the G diet was lower than the P diet (P < 0.05). Net protein utilization (NPU) of the juveniles fed the O and PO diets was higher than the other experimental groups, except the G (P < 0.05). The group fed the G diet exhibited significant differences in the packed cell volume (PCV) and hemoglobin (Hb) levels of groups fed the P and PO (P < 0.05). There were no significant differences in terms of white blood cell (WBC) and red blood cell (RBC) counts (P > 0.05). Significant differences were recorded among the serum alkaline phosphatase (ALP), while juveniles fed the G and PG diets were higher than the P and O groups (P < 0.05). The results of this study demonstrated that supplementing diets with garlic can improve some growth parameters such as SGR and PER for rainbow trout.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Through the years, aquaculture has emerged as one of the most promising agricultural sectors, mainly due to the depletion of fisheries and the growing demand for fish due to the increasing world population (Tacon and Metian 2008; Welch et al. 2010). As aquaculture production intensifies, the risk of infectious diseases in water organisms becomes a threat due to environment, host, pathogen, and pollution (Jeyavani et al. 2022). Vaccination, antibiotics, or chemotherapeutics are mainly applied in intensive fish farming to deal with fish diseases (Zhang et al. 2022). This has led to significant environmental and consumer concerns, including some drug residues, water pollution, and the development of antibiotic-resistant microorganisms (Van Hai 2015). Thus, herbal plants and/or probiotics applications in fish feed as immunostimulants have been considered, and research on these materials’ effects on fish health and growth has been performed. (Dimitroglou et al. 2011; Olusola et al. 2013).
Garlic (Allium sativum) for human nutrition is a natural antibiotic due to its allicin content and has been proven to have blood pressure, cholesterol, and blood sugar lowering effects (Olusola et al. 2013). In aquaculture, diets supplemented with garlic can promote growth, strengthen the immune system, stimulate appetite, and improve flesh quality (Lee and Gao 2012). These improvements were achieved mainly due to the presence of allicin in garlic (Valenzuela-Gutiérrez et al. 2021). Allicin, an organosulfur-containing compound, was suggested to promote fish growth by improving digestion and stimulating the intestinal flora, thus enhancing energy utilization (Khalil et al. 2001). There have been several studies reporting the beneficial effects of garlic on fish growth performance and health (Khattab et al. 2004; Shalaby et al. 2006; Mohebbi et al. 2012; Zaefarian et al. 2017; Zare et al. 2021). On the other hand, some researchers have discovered controversial results, such as no significant (Aly and Mohamed 2010) or adverse effects of garlic on growth and/or health (Ndong and Fall 2011). From these reports, it might be suggested that the inclusion of garlic into fish feed might be dependent on dose concentration, fish species, fish size, development stage, and the duration of the feeding with garlic-supplemented feed.
Onion (Allium cepa) is a well-known medicinal plant that is widely used in folk medicine for protection against infections, spasm relaxation, blood pressure reduction, and as a diuretic (Younes et al. 2021). The presence of flavonoids and thiosulfinates in the onion bulb, which act as growth and immuno-stimulants, can improve digestion by enhancing the synthesis of vitamins and enzymes, thus leading to increased growth rates (Olusola et al. 2013). Onion has also been proved to have good antioxidant and anti-cancer effects due to its high quercetin content and its derivatives (Jeong et al. 2009). Thus, in recent years, there has been an interest in the inclusion of onion into aquaculture feeds and determining its efficacy on fish growth and health (Kaya et al. 2021; Younes et al. 2021). Cho and Lee (2012) reported that onion has improved the growth performance of olive flounder (Paralichthys olivaceus). Furthermore, Cho and Lee (2012) fed olive flounder an onion powder–supplemented feed and found out that, although the inclusion of onion did not enhance fish survival and growth performance, onion powder at 0.5% concentration had a positive immune-stimulant effect on fish.
Aquaculture has a wide range of probiotic sources, including Gram-positive, Gram-negative bacteria, bacteriophages, microalgae, and yeasts (Govindaraj et al. 2021). Lactic acid bacteria (LAB) as probiotics have been the subject of studies in aquaculture with mechanisms that increase feed conversion rate, feed digestion, inhibition of pathogenic microorganisms, and fish growth by improving the immune system (Dimitroglou et al. 2011; Merrifield et al. 2010). In recent years, the synergistic effects of various dietary supplements have gained a specific concern (Lee et al. 2018; Jasim et al. 2022). Positive effects of dietary inclusions of probiotic and herbal mixtures on healthy growth have been reported in South Asian carp (Catla catla) (Bhatnagar and Saluja 2021), olive flounder (Paralichthys olivaceus) (Harikrishnan et al. 2011), and Nile tilapia (Oreochromis niloticus) (Abarike et al. 2020). The present study was therefore conducted to assess the effect of garlic powder, onion powder, probiotic separate, or in combination on the growth performance and hematological and biochemical parameters of juvenile trout (Oncorhynchus mykiss) was investigated.
Material and methods
Ethics statement
The Local Ethics Committee approved this experiment of Animal Experiments of the Tokat Gaziosmanpaşa University, Tokat, Türkiye, under protocol number 51879863–04.
Experimental diets
Seven practical diets were formulated to be isonitrogenous (49% protein) and isolipidic (20% lipid). Fish meal, soybean meal, wheat gluten, and corn gluten were used as the main protein sources, and wheat meal and fish oil were used as the carbohydrate and lipid sources, respectively. A commercially available probiotic (P) (Bacillus subtilis: 2 × 109 cfu/g, Sim Pro, Simbiyotek), onion (O), and garlic (G) powders were used as a dietary additive. All experimental diets were prepared to contain P, O, and G at the concentrations of 0.1, 1, and 1% for diets, respectively, at the expense of wheat flour (Table 1). A nutritionally balanced basal diet that meets the requirements of rainbow trout was prepared (NRC 2011). Dietary ingredients were combined with warm water in a food mixer (model no: IBT-22; Dirmak Food Equipment, İzmir, Türkiye) until a soft slightly moist consistency was achieved. This was then cold press extruded (PTM P6; Yalova, Türkiye) to produce fingerlings with a diameter of 3 mm. After being fan dried, the moist pellets were frozen at − 18 °C until use.
Rearing systems and fish
The feeding trial was carried out at the Fisheries Unit of Tokat Gaziosmanpaşa University, Tokat, Türkiye. Fish were obtained from a commercial rainbow trout facility in Tokat province. The fish were acclimatized to experimental conditions for 2 weeks prior to the start of the experiment. During the adaptation period, the fish were fed a sinking extruded commercial rainbow trout diet containing 50% protein and 18% lipid (Çamlı Feed Company, Türkiye). Fish weighing 8.51 g were randomly distributed into 21 300-L square tanks with 20 fish per tank at the start of the trial. Temperature, dissolved oxygen, and pH of the water were measured daily with the oxygen meter and the pH meter. In an indoor system that allowed natural light, fish were subjected to the natural photoperiod. To ensure suitable water conditions, each tank received an 8-L/min flow of sand-filtered fresh water throughout the study. The pH of the water was 8.30 ± 0.02, the temperature was 17.45 ± 0.05 °C, and the dissolved oxygen level was 9.70 ± 0.09 ppm. Fish were fed ad libitum three times a day (08:00, 12:00, and 16:00) for 8 weeks.
Sampling protocols
All fish were starved for 48 h after the feeding trial to ensure the digestive tract was free of feed. Six fish were chosen randomly from the start of the experiment, and two fish from each tank (six fish per treatment) were sampled at the end of the trial to determine whole body proximate analysis. The samples were kept frozen at − 80 °C until they were analyzed. Three fish were chosen randomly, individually weighed, and dissected to obtain liver to determine biological parameters; the same livers were then used for histology observations. Two fish were randomly captured in each tank, anesthetized with clove oil, and blood samples were drawn from the caudal arch using a 32-gauge needle and 2-mL heparinized syringes to determine white blood cell (WBC) and red blood cell (RBC), packed cell volume (PCV), and hemoglobin levels (Hb).
Proximate analyses
The whole fish and diets were analyzed for crude protein, moisture, fiber, and ash using standard methods (AOAC 2000). The dry matter was determined by drying at 105 °C until constant weight. Ash was measured by burning in a muffle furnace at 525 °C for 12 h. The Gerhardt system acid digested crude protein (N*6.25) for Kjeldahl analysis. Acid/alkali hydrolysis of filtered residue and 3 h of drying sample ignition determined crude fiber. Folch et al. (1957) extracted dietary and whole-body lipids with chloroform/methanol (2:1 v/v). Nitrogen-free extract (NFE) was calculated by subtracting 100 from the sum of moisture, crude protein, lipid, ash, and crude fiber (Olvera-Novoa et al. 1994). Protein, lipid, and carbohydrate conversion factors of 23.7, 39.5, and 17.2 kJ/g were used to calculate dietary gross energy (Brett and Groves 1979).
Hematology and serum analyses
Fresh blood was drawn into microhematocrit tubes and centrifuged at 3600 g for 6 min in a microhematocrit centrifuge to determine hematocrit levels. Hematocrit values were calculated and expressed as a percentage of packed cell volume (% PCV). Sahli’s method was used to calculate hemoglobin levels (Güroy et al. 2016). Using Dacie’s diluting fluid, the RBC count was determined using a Thoma hemocytometer. A semi-automatic chemistry analyzer was used to determine blood parameters such as calcium (Ca, mg/dL), glucose (Glu, mg/dL), total protein (TP, g/dL), and alkaline phosphate (ALP, U/L) (BA-88A, Mindray, China).
Calculations and somatic indices
Growth performance, in terms of feed intake (FI), specific growth rate (SGR), feed conversion ratio (FCR), protein efficiency ratio (PER), and net protein utilization (NPU) were determined using the following formulas:
Six fish were randomly selected from the initial pool of fish at the start of the experiment, and three fish were randomly selected from each tank (nine fish per treatment) at the end of the trial to determine somatic indices.
Statistical analysis
Before proceeding with further analysis, the Shapiro–Wilk W and Levene tests were used to ensure that the variance was normal and homogeneous. All data were subjected to a one-way analysis of variance (ANOVA), and if a significant difference between treatments was discovered, Duncan’s multiple range test was used to rank the groups using Statgraphics 4.0 (Manugistics, Rockville, MD, USA) statistical software (Zar 1999). All data are presented in the form of mean standard error of the mean calculated from all replicates. Before being analyzed, all percentage data were arcsine transformed. Differences were considered significant at the 95% confidence interval.
Results
The effect of the dietary inclusion of garlic, onion, and probiotics on the growth performance of juvenile trout is presented in Table 2. As shown in Table 2, the values of SGR were significantly (P < 0.05) improved in fish fed with garlic-supplemented diet when compared to fish fed with probiotic (P) and probiotic and onion (PO). Similarly, the diet containing garlic led to lower FCR when compared with the diet containing probiotics (P < 0.05), but was not significantly different from the other diets. The protein efficiency ratio (PER) was higher for fish fed with onion diet (O) compared with fish fed control diet and diet containing probiotics (P < 0.05), but it was not significantly different from the PER of fish fed diets G and PO. The net protein utilization (NPU) of fish fed diets O and PO was higher than fish fed control, P and PG diets (P < 0.05). Furthermore, fish fed diets G and PG also exhibited higher NPU than fish fed diets C and P (P < 0.05). On the other hand, there was no statistically significant difference in terms of NPU among diets G, O, and PO.
The whole-body composition of the fish fed the trial diets is given in Table 3. No statistically significant differences were observed among the moisture, protein, lipid, and ash composition of the fish fed the diets.
The hematological parameters of fish fed the trial diets (n = 6) for 8 weeks are presented in Table 4. No statistical differences were observed among the fish fed the trial diets in terms of WBC and RBC. The PCV of the fish fed diet P and PO were statistically lower than G diet. The HGB of fish fed garlic supplemented diet (G) was higher than the fish fed diets P, O, and PO (P < 0.05) and not statistically different from the HGB of the fish fed diets C and PG.
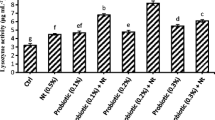
The serum parameters (Ca, Glu, TP, and ALP) of fish fed at the end of the feeding trial are given in Table 5. The serum Ca levels ranged from 5.3 to 6.4 mg/dL, while the serum TP values were in the range of 2.9–4.1 g/dL. There was no statistical difference in the serum Ca and TP values of fish fed the experimental diets. On the other hand, there were statistical differences among the serum Glu parameters of fish fed C, P, and O diets (P < 0.05). Inclusion of probiotic and onion into the diets of trout proved to decrease significantly the Glu levels in the fish blood serum, with the Glu level of the fish fed onion supplemented feed being the lowest. Interestingly, the diet containing a mixture of probiotics and onion (PO) led to higher serum Glu level than the diet containing only onion supplementation (O) (P < 0.05) but did not led statistically different Glu levels when compared with the diet containing only probiotics (P). The serum ALP levels were lowest for the fish fed diet P and highest for fish fed garlic containing diets (G and PG) (P < 0.05). Similarly, The ALP values were significantly lower in fish fed with probiotic (P) than those received the onion (O and PO) diets (P < 0.05). Inclusion of probiotics into trout diets proved to decrease the serum ALP levels, while inclusion of garlic increased the serum ALP levels of fish.
Discussion
In the present study, inclusion of garlic powder proved to have a beneficial effect on the growth performance of juvenile trout. In literature, there is a number of studies reporting the positive effects of garlic on the growth performance of Nile tilapia (Oreochromis niloticus) (Khalil et al. 2001; Shalaby et al. 2006). Garlic contains allicin that has a strong flavor that might stimulate the appetite and further improve digestion. Besides this, allicin can improve digestion by promoting the intestinal flora and inhibiting the pathogenic bacteria, thus improving the general health status of cultured fish (Khalil et al. 2001). Inclusion of onion did not have any adverse effect on the growth when compared with the control group, while the PER and NPU of fish fed onion powder supplemented were significantly higher than that of fish fed control diet. Cho and Lee (2012) obtained similar results for juvenile olive flounder (Paralychthys oilvaceus) when onion powder was supplemented at 0, 0.5, 1, 2, 3, and 5% into the trial diets. In literature, the positive effects of probiotics supplementation on the growth of cultured fish are well documented (Dimitroglou et al. 2011; Merrifield et al. 2010; Elgendy et al. 2023). On the other hand, Akrami et al. (2015) reported significantly higher weight gain and SGR for beluga (Huso huso) fed diet containing 1% onion powder. Giri et al. (2014) prepared four different mixtures of probiotics and included them into the Labeo rohita fingerlings. Only the diet containing the mixture of B. subtilis, P. aeruginos, and L. plantarum had a positive effect on the growth performance of the fish. Similarly, to our results, the other probiotic-containing experimental diets did not exhibit significantly different results from the control group in terms of growth parameters. Furthermore, in our study, supplementation of a mixture with onion powder and probiotics and garlic powder and probiotics did not result in any significant difference in terms of weight gain, SGR, and FCR when compared with the control group. On the other hand, the PER of fish fed G, O, and PO diet and NPU of fish fed G, PG, O, and PO diet were significantly higher when compared with the fish-fed control diet. Harikrishnan et al. (2011) found out that supplementation of a mixture of probiotics and herbal extracts significantly increased the final weight of infected olive flounder (P. olivaceus) after 6–12 weeks of the feeding trial.
In the present study, no significant effect of herbs and probiotics on the whole-body composition of juvenile trout was detected. Similar to our results, Cho and Lee (2012) reported no impact of onion powder inclusion on the chemical composition of olive flounder (P. olivaceus). Similarly, Lee et al. (2012) found that garlic extract supplementation had no effect on the whole-body amino acid composition of juvenile sterlet sturgeon (Acipenser ruthenus).
The hematological parameters of cultured fish are usually known as indicators of disease and stress of fish (Campbell 2004). The changes in the WBC, RBC, Hb, and PCV values indicate the stress and health of living creatures. In the present study, the highest hemoglobin level was detected for fish fed the G diet. The inclusion of garlic proved to increase the Hb level of the juvenile diet, while diets G and PG did not affect the PCV when compared with the control diet. Lee et al. (2012) reported that garlic extracts significantly increased juvenile steroid sturgeon’s PCV and Hb levels (A. ruthenus). Similar to our results, Akrami et al. (2015) found no effect of onion powder supplementation on the Hb values of juvenile beluga (H. huso).
The inclusion of herbal extracts and probiotics is generally known to have a hypoglycemic effect in fish. Lee et al. (2012) reported a significant decrease in blood glucose level of juvenile sterlet sturgeon (A. ruthenus) fed garlic extracts. Akrami et al. (2015) found out that glucose levels decrease in juvenile beluga (H. huso) fed onion powder. Our results were similar. On the contrary, Cho and Lee (2012) observed no significant effect of onion powder on the GLU level of olive flounder (P. olivaceus). Harikrishnan et al. (2011) also reported no effect of probiotics and herbal mixtures on the GLU level of olive flounder (P. olivaceus).
Total protein values in the blood serum of fish are related to the protein synthesized in the liver. An increase in the serum protein is suggested to be related to a stronger innate immune response (Wiegertjes et al. 1996). In contrast to our results, Akrami et al. (2015) reported that onion powder significantly increased the blood serum TP levels of juvenile beluga (H. huso). Similarly to our results, for olive flounder (P. olivaceus), Cho and Lee (2012) and Harikrishnan et al. (2011) showed no effect on the TP level of supplementation of onion powder and probiotics and herbal mixtures, respectively. Alkaline phosphatase (ALP) activity can be used as an indicator of liver damage. Increased levels of this type of enzyme may suggest the degeneration and/or destruction of the liver. Furthermore, enzymes such as AST, ALT, and ALP might be used to assess the toxicity of diets and/or supplements (Bhardwaj et al. 2010). Mohebbi et al. (2012) have found out that garlic might improve the antioxidative status of rainbow trout (Oncorhynchus mykiss), but the dose of garlic in the feed should be well balanced. Interestingly, the garlic containing diets G and PG led to increased levels of ALP, while the probiotics and onion containing diets P and O resulted in lowered ALP levels with the P diet leading to the lowest ALP concentration. Thus, it can be concluded that probiotics- and onion powder–containing diets did positively affect the liver health of juvenile trout. Akrami et al. (2015) reported no change in the ALP levels of juvenile beluga (H. huso) when compared with the control group. Yet, since the normal levels of fish are still to be determined, these results might be used with precaution.
In conclusion, this study revealed probiotics, onion powder, and a mixture of these supplements did not positively affect the growth performance, blood parameters, and health status of juvenile trout. But, according to the findings of the present study, supplementing diets with garlic could improve some growth parameters such as the SGR and PER. For future studies, the authors recommend approaches that may improve the dietary efficacy of garlic in rainbow trout diets.
Data availability
All data analyzed during this study are included in this published article.
References
Abarike ED, Jian J, Tang J, Cai J, Sakyi EM, Kuebutornye FK (2020) A mixture of Chinese herbs and a commercial probiotic Bacillus species improves hemato-immunological, stress, and antioxidant parameters, and expression of HSP70 and HIF-1α mRNA to hypoxia, cold, and heat stress in Nile tilapia. Oreochromis Niloticus Aquac Rep 18:100438
Akrami R, Gharaei A, Razeghi M, Galeshi A (2015) Effects of dietary onion (Allium cepa) powder on growth, innate immune response and hemato e biochemical parameters of beluga (Huso huso Linnaeus, 1754) juvenile. Fish Shellfish Immunol 45:828–834
Aly SM, Mohamed MF (2010) Echinacea purpurea and Allium sativum as immunostimulants in fish culture using Nile tilapia (Oreochromis niloticus). J Anim Physiol Anim Nutr 94:e31–e39
AOAC (2000) Official methods of analysis, 17th edn. Association of Official Analytical Chemists, Gaithersburg, MD, USA
Bhardwaj S, Srivastava MK, Kapoor U, Srivastava LP (2010) A 90 days oral toxicity of imidacloprid in female rats: morphological, biochemical and histopathological evaluations. Food Chem Toxicol J 48:1185–1190
Bhatnagar A, Saluja S (2021) Role of Zingiber officinale and autochthonous probiotic Bacillus coagulans in feeds of Catla catla (Hamilton, 1822) for growth promotion, immunostimulation, histoprotection, and control of DNA damage. Fish Physiol Biochem 47:2081–2100
Brett J, Groves T (1979) Physiological energetics. In Hoar S, Randal Dj, Brett J (eds). Fish physiology: bioenergetics and growth. New York, NY: Academic Press, pp. 279–352
Campbell T (2004) Clinical chemistry of fish and amphibians. In: Thrall M, Baker D, Campbell T, DeNicola D, Fettman M, Lassen E, Rebar A, Weiser G (eds) Veterinary hematology and clinical chemistry. Lippincott Williams & Wilkins, Pennsylvania, pp 499–517
Cho SH, Lee SM (2012) Onion powder in the diet of the olive flounder, Paralichthys olivaceus: effects on the growth, body composition, and lysozyme activity. J World Aquac Soc 43:30–38
Dimitroglou A, Merrifield DL, Carnevali O, Picchietti S, Avella M, Daniels C, Güroy D, Davies SJ (2011) Microbial manipulations to improve fish health and production: a Mediterranean perspective. Fish Shellfish Immunol 30:1–16
Elgendy MY, Ali SE, Abdelsalam M, Abd El-Aziz TH, Abo-Aziza F, Osman HA, Authman MMN, Abbas WT (2023) Onion (Allium cepa) improves Nile tilapia (Oreochromis niloticus) resistance to saprolegniasis (Saprolegnia parasitica) and reduces immunosuppressive effects of cadmium. Aquacult Int 31(3):1457–1481
Folch J, Lees M, Sloane-Stanley GH (1957) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226:497–509
Giri SS, Sukumaran V, Sen SS, Jena PK (2014) Effects of dietary supplementation of potential probiotic Bacillus subtilis VSG1 singularly or in combination with Lactobacillus plantarum VSG3 or/and Pseudomonas aeruginosa VSG2 on the growth, immunity and disease resistance of Labeo rohita. Aquac Nutr 20:163–171
Govindaraj K, Samayanpaulraj V, Narayanadoss V, Uthandakalaipandian R (2021) Isolation of lactic acid bacteria from intestine of freshwater fishes and elucidation of probiotic potential for aquaculture application. Probiotics Antimicrob Proteins 13(6):1598–1610
Güroy B, Mantoğlu S, Merrifield DL, Güroy D (2016) Effects of dietary Nutrafito Plus on growth, haemotological parameters and total ammonia-nitrogen excretion of juvenile striped catfish Pangasianodon hypophthalmus. Aquac Res 47:1770–1777
Harikrishnan R, Kim M, Kim J, Balasundaram C, Heo M (2011) Probiotics and herbal mixtures enhance the growth, blood constituents, and nonspecific immune response in Paralichthys olivaceus against Streptococcus parauberis. Fish Shellfish Immunol 31:310–317
Jasim SA, Hafsan H, Saleem HD, Kandeel M, Khudhair F, Yasin G, Iswanto AH, Mohammed HT, Izzat SE, Dadras M (2022) The synergistic effects of the probiotic (Lactobacillus fermentum) and cinnamon, Cinnamomum sp. powder on growth performance, intestinal microbiota, immunity, antioxidant defence and resistance to Yersinia ruckeri infection in the rainbow trout (Oncorhynchus mykiss) under high rearing density. Aquac Res 53(17):5957–5970
Jeong C, Heo HJ, Choi S, Shim K (2009) Antioxidant and anticancer properties of methanolic extracts from different parts of white, yellow and red onion. Food Sci Biotechnol 18:108–112
Jeyavani J, Sibiya A, Sivakamavalli J, Divya M, Preetham E, Vaseeharan B, Faggio C (2022) Phytotherapy and combined nanoformulations as a promising disease management in aquaculture: a review. Aquacult Int 30(2):1071–1086
Kaya D, Sardogan A, Genç E (2021) Onion powder as feed additive in Procambarus clarkii culture. J Surv Fish Sci 7(2):41–47
Khalil RH, Nadia BM, Soliman MK (2001) Effects of biogen and levamisol HCl on the immune response of cultured Oreochromis niloticus to Aeromonas hydrophila vaccine. Beni-Suef Vet Med J Egypt 11:381–392
Khattab Y, Shalaby A, Sharaf S, El-Markby H, Rizkallaeh E (2004) The physiological changes and growth performance of the Nile tilapia Oreochromis niloticus after feeding with Biogen as growth promoter. Egypt J Aquat Biol Fish 8:145–158
Lee J, Gao Y (2012) Review of the application of garlic, Allium sativum, in aquaculture. J World Aquac Soc 43:447–458
Lee D, Lim S, Ra C, Kim J (2012) Effects of dietary garlic extracts on whole body amino acid and fatty acid composition, muscle free amino acid profiles and blood plasma changes in juvenile sterlet sturgeon, Acipenser ruthenus. Asian Australas J Anim Sci 25:1419–1429
Lee S, Katya K, Hamidoghli A, Hong J, Kim DJ, Bai SC (2018) Synergistic effects of dietary supplementation of Bacillus subtilis WB60 and mannanoligosaccharide (MOS) on growth performance, immunity and disease resistance in Japanese eel, Anguilla japonica. Fish Shellfish Immunol 83:283–291
Merrifield DL, Dimitroglou A, Foey A, Davies SJ, Baker RTM, Bøgwald J, Castex M, Ringø E (2010) The current status and future focus of probiotic and prebiotic applications for salmonids. Aquaculture 302:1–18
Mohebbi A, Nematollahi A, Dorcheh EE (2012) Influence of dietary garlic (Allium sativum) on the antioxidative status of rainbow trout (Oncorhynchus mykiss). Aquac Res 43:1184–1193
Ndong D, Fall J (2011) The effect of garlic (Allium sativum) on growth and immune responses of hybrid tilapia (Oreochromis niloticus x Oreochromis aureus). J Clin Immunol Immunopathol 3:1–9
National Research Council (NRC) (2011) Nutrient requirements of fish and shrimp. National Academy Press, Washington, DC, p 376
Olusola SE, Emikpe BO, Olaifa FE (2013) The potentials of medicinal plant extracts as bio-antimicrobials in aquaculture. Int J Med Aromat Plants 3:404–412
Olvera-Novoa MA, Martínez-Palacios CA, de Real LE (1994) Nutrition of fish and crustaceans – a laboratory manual, Mexico City: FAO. GCP/RLA/102/ITA, Field Document No. 19, p 58
Shalaby A, Khattab Y, Abdel-Rahman A (2006) Effects of garlic, Allium sativum and chloramphenicol on growth performance, physiological parameters and survival of Nile Tilapia, Oreochromis niloticus. J Venom Anim Toxins Incl Trop Dis 12:172–201
Tacon AGJ, Metian M (2008) Global overview on the use of fish meal and fish oil in industrially compounded aquafeeds : trends and future prospects. Aquaculture 285:146–158
Valenzuela-Gutiérrez R, Lago-Lestón A, Vargas-Albores F, Cicala F, Martínez-Porchas M (2021) Exploring the garlic (Allium sativum) properties for fish aquaculture. Fish Physiol Biochem 47(4):1179–1198
Van Hai N (2015) The use of medicinal plants as immunostimulants in aquaculture: a review. Aquaculture 446:88–96
Welch A, Hoenig R, Stieglitz J, Benetti D, Tacon A, Sims N, Hanlon BO, Hoenig R, Stieglitz J, Benetti D, Tacon A, Sims N (2010) From fishing to the sustainable farming of carnivorous marine finfish from fishing to the sustainable. Rev Fish Sci 18:235–247
Wiegertjes G, Stet R, Parmentier H, van Muiswinkel W (1996) Immunogenetics of disease resistance in fish: a comparative approach. Dev Comp Immunol 20:365–381
Younes AM, Gaafar AY, Abu-Bryka AEDZ, Awad ES, Abo-Aziza F, Abd El-Aziz TH, Darwish DA, Authman MMN, Abbas WT (2021) Effects of onion (Allium cepa) in diets of Oreochromis niloticus: growth improvement, antioxidant, anti-inflammatory and disease resistance perspectives. Aquac Res 52(5):2324–2334
Zaefarian A, Yeganeh S, Adhami B (2017) Dietary effects of garlic powder (Allium sativum) on growth, blood indices, carcass composition, and lysozyme activity in brown trout (Salmo caspius) and resistance against Yersinia ruckeri infection. Aquacult Int 25:1987–1996
Zar J (1999) Biostatistical analysis, 4th edn. Prentice-Hall Inc, Upper Saddle River
Zare M, Tran HQ, Prokešová M, Stejskal V (2021) Effects of garlic Allium sativum powder on nutrient digestibility, haematology, and immune and stress responses in Eurasian perch Perca fluviatilis juveniles. Animals 11(9):2735
Zhang W, Zhao J, Ma Y, Li J, Chen X (2022) The effective components of herbal medicines used for prevention and control of fish diseases. Fish Shellfish Immunol 126:73–83
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK).
Author information
Authors and Affiliations
Contributions
Derya Güroy: project leader; responsible for every step of the experiment, calculations, statistical analysis, and writing MS draft. Nesrin Emre: hematological and blood analysis. Fatma Banu Yalım: hematological and blood analysis. Onur Karadal: making experimental feed, hematological analysis, calculations, statistical analysis, and writing MS draft. Doğukan Kaya: design of the experiment, consulting during the study, and writing MS draft. Nazan Arifoğlu: whole-body analysis and writing MS draft.
Corresponding author
Ethics declarations
Ethical approval
The Local Ethics Committee approved this experiment of Animal Experiments of the Tokat Gaziosmanpaşa University, Tokat, Türkiye, under protocol number 51879863–04.
Competing interests
The authors declared no competing interests.
Additional information
Handling Editor: Brian Austin
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
We dedicate this article to the memory of Prof. Dr. Yılmaz Emre, who passed away untimely.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Güroy, D., Emre, N., Yalım, F.B. et al. Interaction of dietary garlic (Allium sativum), onion (Allium cepa), and probiotic on the growth performance and health status of juvenile rainbow trout (Oncorhynchus mykiss). Aquacult Int (2024). https://doi.org/10.1007/s10499-024-01388-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10499-024-01388-5