Abstract
Water temperature is a major environmental factor affecting fish activity, physiology, behavior, and growth. Alpha lipoic acid (ALA) has garnered much attention lately as an aqua feed supplement because of its antioxidant effects. Thus, this study aimed to assess the effect of dietary ALA on performance, hematological and immunological indices, behavior and oxidative stress, apoptosis, and genetic inflammation in Oreochromis niloticus following 60 days of cold stress. Two hundred forty fish were divided into four groups (15 fish/replicate, N = 60 fish/group). The control (CNT) and ALA groups were reared at 25 °C and fed a basal diet without any supplementation or a basal diet containing 600 mg ALA/kg diet, respectively. The cold water-stressed group (CWS) and ALA + CWS groups were reared at 18 °C and fed basal and basal diets containing ALA, respectively. The results revealed that dietary ALA supplementation significantly improved feed utilization and growth in fish. ALA also mitigated CWS-induced microcytic hypochromic anemia and hyperlipidemia. Moreover, ALA supplementation considerably enhanced the antioxidant status and boosted intestinal α amylase, lipase, protease, and serum acetylcholinesterase activity of CWS-exposed fish. Besides, ALA supplementation significantly reversed CWS-induced upregulation of proinflammatory genes, pro-apoptotic genes, heat shock protein, and proliferating cell nuclear antigen or downregulation of superoxide dismutase and catalase gene expressions in liver and spleen tissues. Furthermore, ALA supplementation reduced CWS-induced histopathological alterations in fish liver, spleen, and intestine. We can conclude that ALA (600 mg/kg diet) could be recommended as an aqua feed supplement to boost growth, antioxidant capacity, and immunity and attenuate inflammatory and apoptotic reactions of fish reared under CWS-induced.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Increases in tilapia production (Oreochromis Spp.), the most commonly consumed freshwater aquaculture species, are needed to cover the increase in the human population (El-Sayed 2020; Ayyat et al. 2022). It is the first species bred in Egypt and the third in the world due to its fast development, excellent food utilization, and favorable edible qualities (Almarri et al. 2023; Ayyat et al. 2024). As a tropical warm-water fish, Nile tilapia (O. niloticus) can withstand a wide variety of temperatures (8–42 °C; Leonard and Skov 2022). It cannot develop well at temperatures beneath 18 °C and cannot endure at temperatures beneath 10 °C for almost a few days (Wu et al. 2019).
The vulnerability of tilapia to cold temperatures is an urgent economic concern, as it restricts growing seasons and causes enormous over-winter death (Liu et al. 2022). Cold water stress (CWS) is a prevalent cause of fish sickness and death, which has reduced the production of tropical aquaculture systems (Almarri et al. 2023). Low temperatures could increase cortisol levels, decrease metabolism, alter blood enzymes, and impair fish immunological responses (Lima de Almeida et al. 2019). During CWS, fish swim deeper into ponds, gather in huge groups, inhibit feeding, become sluggish, and could easily exposed to predation (Crawshaw et al. 2022). Low water temperatures may also reduce the digestibility of nutrients through decreasing digestion, lengthening gut transit time, and decreasing gastrointestinal emptying speed (Zhang et al. 2023). To satisfy the growing energy needs and metabolic changes, CWS-exposed fish require a considerable oxygen amount, resulting in stored electrons at particular sites of the respiratory chain, which drives the reactive oxygen species (ROS) generation (Cheng et al. 2018) and lowers the antioxidant enzymes activity (Yilmaz et al. 2021). Moreover, hypothermia suppresses or delays the production of important innate immune components in adult fish in response to pathogen mimics or pathogens, blocking proinflammatory or antiviral responses (Abram et al. 2017). Studies have found histological abnormalities in the gills (short secondary lamellae, thin gill filament tips, absence of secondary lamellae, and increased cartilage tissue in the gill filament) and livers (severe hepatocytes degeneration and necrosis and severe hemolysis and infiltration) of fish reared under CWS (Refaey et al. 2022).
The significance of dietary approaches in mitigating the adverse effects of CWS on various fish species has recently been explored (Ibrahim et al. 2019a; Peng et al. 2022). Alpha-lipoic acid (ALA) is a lipid and water-soluble antioxidant that can easily cross biological membranes (Li et al. 2021). It exhibits antioxidant effects by directly absorbing metal ions and free radicals and boosting intracellular glutathione (Rezaei Zonooz et al. 2021). ALA showed antioxidant activities in aquatic organisms like grass carp (Shi et al. 2017), Pacific white shrimp (Silva et al. 2018), and Nile tilapia (El-Houseiny et al. 2023). Dietary ALA also boosted grass carp growth, fatty acid β-oxidation, and lipolysis, enhanced protein deposition, augmented immunity and antioxidant effectiveness, mitigated the inflammatory response, and lessened lipid oxidative damage (Ou et al. 2023). ALA can reduce inflammation by inhibiting the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling (Liu et al. 2018). Furthermore, ALA has many metabolic advantages, including anti-obesity, insulin sensitization, and lipid-lowering properties (Xiong et al. 2022). ALA supplementation activates the lipid and carbohydrate metabolisms, increasing non-protein energy availability and decreasing protein breakdown as an energy source (Rifai et al. 2022a).
Therefore, this study aimed to determine the effect of ALA addition to the Nile tilapia diet on their survival, growth performance, feed utilization, intestinal enzyme activities, hematological parameters, serum biochemical and immune indices, behavioral response, and expression of stress, antioxidant, apoptosis, and inflammation-related genes under chronic CWS.
Materials and methods
Diet preparation
ALA (C8H14O2S2Thiotacid) was supplied by the Egyptian International Pharmaceutical Industries Company (10th of Ramadan City, Egypt). Two isoenergetic and isonitrogenous experimental diets, a control diet consisting of a basal diet and an ALA diet in which the basal diet was supplemented with 600 mg ALA/kg diet, were formulated in the Fish Research Unit, Faculty of Veterinary Medicine, Zagazig University to cover the nutrient requirements of Nile tilapia (NRC 2011). The feed components were finely powdered and well mixed before adding water to form a homogenous paste. The meals were minced using an electric meat mincer into small pellets (2.0 mm in diameter), then air-dried at room temperature, and stored in airtight plastic bags at 4 °C. Table 1 shows the proximate chemical analysis of the basal and ALA diets.
The fish were fed the experimental nutritional regimen at a rate of 3% of their total biomass twice daily (8:00 am and 3:00 pm) for 60 days. The introduced food was changed every 2 weeks based on the alteration in fish weight, and the wasted diet was dried and deducted from the supplied feed for an exact measurement of feed intake.
Fish rearing and experimental design
The study was performed at Aquatic Animal Medicine, Faculty of Veterinary Medicine, Zagazig University, Egypt. The Animal Ethics Committee of Zagazig University (ZU-IACUC) approved the experimental methodology with an approval number ZU-IACUC/2/F/276–2023. Nile tilapia (Oreochromis niloticus) (N = 240 with average initial weight 31.03 ± 0.07) were bought from the Fish Research Unit at Zagazig University’s Veterinary Medicine Faculty. The fish were placed in 80 × 40 × 30 cm glass aquaria that contained 60 L of dechlorinated tap water. Fish were subjected to a control diet for 2 weeks just before the start of the study. The water criteria were investigated throughout the experiment and remained within the prescribed ranges (pH = 7.1 ± 0.4, dissolved oxygen = 6.25 ± 0.5 mg/L, ammonia = 0.02 ± 0.001 mg/L, nitrite = 0.016 ± 0.001 mg/L). This study was carried out in December and January during the cold seasons. Throughout the experiment, the water temperature was less than 18 °C and adjusted by the heater to 18 °C and 25 °C for all study groups. Water was exchanged twice weekly with pre-warmed water, and the aquaria were cleaned regularly. The behaviors of fish were observed during the exposure period.
Fish were arbitrarily distributed into four quadruplicate groups (15 fish/replicate, N = 60 fish/group). CNT was fed a basal diet without any supplementation and reared at 25 °C. The ALA group was fed a basal diet containing 600 mg ALA/kg and reared at 25 °C. The selected ALA dietary level was chosen based on earlier studies on different species of fresh water fish, including hybrid tilapia (Xiong et al. 2012), grass carp (Shi et al. 2018b), and Nile tilapia (Lu et al. 2019a). The CWS group was fed on a basal diet and reared at 18 °C (Ibrahim et al. 2019b). The ALA + CWS group was fed a basal diet encompassing ALA and reared at 18 °C.
Growth performance parameters and survivability
Individually weighed fish from each replicate at the onset of the trial for estimating the starting body weight (IBW) and at the termination of the feeding period (60 days) to investigate the final body weight (FBW). The following formulas were used to determine the feed utilization and growth metrics, as well as the survival rate:
Blood and tissue sampling
After a feeding session and before collecting blood, the fish were starving for a single-day at the finish of the experiment. The fish was sedated with 100 mg/L of benzocaine (Neiffer and Stamper 2009) to reduce handling stress. Blood was taken from the caudal blood vessels of the fish without using any anticoagulant and centrifuged at 1750 × g for 15 min to separate the serum (three samples per replicate; 12 samples per group). The collected sera were used to assess some immune and biochemical indices. Another blood sample was drained with a 1-mL heparinized syringe for erythrogram and leukogram measurement. Then, the fish were aseptically necropsied, and the liver, spleen, and intestine specimens were collected for histological analysis. Intestinal enzyme activity (amylase, lipase, and protease) was measured in intestinal samples containing pyloric caeca. The liver, spleen, and intestine parts were taken and stored in 10% neutral buffered formalin for histological investigation. Another fish’s spleen and liver tissues were taken and rinsed in ice-cold phosphate-buffered saline before being stored at − 80 °C for gene expression analysis.
The chemical composition of fish body
Fish (three samples per replication; 12 samples per group) were taken randomly and stored at − 20 °C for proximate examination of the entire body. The estimated moisture, lipids, protein, and ash contents were determined using AOAC (2005) protocol.
Hematological analyses
Red blood cells (RBCs), total and differential white blood cell counts (WBCs), hemoglobin (Hb), packed cell volume (PCV), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), and mean corpuscular hemoglobin concentration (MCHC) were measured using an automatic cell counter (XE-2100 Sysmex, Kobe, Japan) in line with the protocol of Fitzgerald and Haylock (1998).
Biochemical assays
For assessing serum cholesterol and triglycerides, enzymatic colorimetric diagnostic kits (Biodiagnostic Co., Giza, Egypt, Cat. No. CH 12 20 and TR 20 30, receptively) were used. Likewise, total serum proteins, albumin, and glucose concentrations have been established utilizing the commercial colorimetric Biodiagnostic kits (Giza, Egypt) with Cat. No. TP 20 20, AB 10 10, and GL 13 20, respectively. Total globulins were identified by deducting albumin from total protein concentration. Serum levels of cortisol hormone were estimated using the ELISA commercial kit of MyBioSource Inc., San Diego, CA, USA (Cat. No MBS704055).
Serum immune indices
Complement 3 (C3, Catalogue No.; MBS281020) was measured using kits received from MyBioSource Co. (CA, USA) according to the manufacturer’s directives. The serum nitric oxide was estimated in line with the protocol of Bryan and Grisham (2007). The method of Ghareghanipoora et al. (2014) was used to assess serum lysozyme activity through lysing Micrococcus lysodeikticus (Sigma Co., USA). The technique was modified with M. lysodeikticus suspension (pH = 6.2, 0.2 mg/mL in 0.05 M PBS) for 5 min at 25 °C. The optical density was measured at 540 nm every minute for the next 5 min using a 5010 Photometer (BM Co. Germany). To determine the serum’s lysozyme concentration, a calibration curve was developed by serially diluting lyophilized chicken egg-white lysozyme (Sigma Co., USA).
Serum oxidants/antioxidants biomarkers and acetylcholine esterase (AChE) activity
Serum antioxidant enzymes activities, including superoxide dismutase (SOD), glutathione peroxidase (GPx), and catalase (CAT), were detected using commercial kits of Biodiagnostic Co., Cairo, Egypt (SD 25 21, GP 2524, and CA 25 17, respectively). Besides, reduced glutathione (GSH) and malondialdehyde (MDA) levels were quantified by Bio-diagnostic Co. (Giza, Egypt) kits (Cat. No. GR 25 11 and MD 25 29, respectively). Furthermore, using a commercial kit of MyBioSource Inc. (San Diego, CA, USA), serum acetylcholine esterase (AChE) activity was spectrophotometrically (Cat. No MBS280290) estimated at a 450-nm detection wavelength.
Determination of intestinal enzyme activities
The intestinal tissues were collected, placed on ice-cold dishes, rinsed with sterile saline, dried on filter paper, and then frozen at − 20 °C. Following Teflon homogenization, 100 mg of tissue was centrifuged at 6000 × g for 5 min at 4 °C in a container containing 1 mL of a buffer (pH = 7.0 containing 10 mM phosphate/20 mM tris). After centrifugation, the supernatants were collected and stored at − 80 °C until analysis. Commercial MyBioSource Inc. (San Diego, CA, USA) colorimetric kits (Giza, Egypt) were employed concerning the manufacturer’s instructions to assess the activity of lipase (Cat. No MBS2540464) and α amylase (Cat. MBS9719214) enzymes in the intestinal homogenates from different groups. The intestinal protease activity was estimated as per the protocol of Bezerra et al. (2005).
Behavior evaluation
The behavior measures were conducted throughout the study between 9:00 am and 3:00 pm hours using a stopwatch and a video camera. Throughout the experiment, all behavioral patterns were noted at (15-min intervals over 6 h each week. The following behavior patterns were identified: feeding behavior: the definite amount of food consumed during the feeding period (Khalil et al. 2022). Foraging behavior: looking for food (Ramadan et al. 2018). Eliminative behavior: Fish feces that have fallen or been hung from the anus (Ramadan et al. 2018). Surfacing behavior: measured by the incidence of fish air pipping adjacent to the water’s surface in aquariums with low dissolved oxygen (Noga 2010). Swimming behavior: refers to the observation of fish moving quickly or slowly without engaging in any movement at the aquarium surface, middle, or bottom, according to the protocol established by Chen et al. (2001). Aggressive behavior: According to Ramadan et al. (2018) protocol, several behaviors were observed to measure fish aggression, comprising the straight movement of one fish toward another (approach), the rate at which fish swim rapidly after one another (chasing), fishes escaping from the adversary by swimming (fleeing), the rate at which two fish stand with their mouths open against one another (mouth pushing), and all of a fish’s fins extend out or expand (spreading of fins). Resting behavior: Fish have wide eyes and are immobile (Aziz et al. 2022).
Histological evaluation of the liver, spleen, and intestine
For histological analysis, the intestines’ livers, spleens, and mid segments (three samples/replicate) were collected, trimmed, and fixed for 24 h in 10% neutral buffered formalin. Post-fixation, the tissue specimens were washed in distilled water, dehydrated by immersing in ascending grades of ethyl alcohol, cleared in two changes of dimethyl benzene, infiltrated, and embedded in paraffin wax, sectioned by Leica RM2125 RTS manual rotary microtome at 5-μm thick, and stained with Harris hematoxylin and eosin Y stains following the guides of Suvarna et al. (2018). The stained sections were examined microscopically, where any histological changes were recorded. The area fractions occupied by (1) melanomacrophage centers (MMCs) in the hepatic and splenic tissues, (2) cytoplasmic vacuolations in the hepatocytes, and (3) the areas not occupied by any cellular or extracellular elements in the splenic tissue were determined using the Java-based image processing software (Image J v1.53t) (Schneider et al. 2012). In addition, quantitative measurements of the intestinal villus width [(villus apical width + villus basal width)/2], villus height (from the tip to base), and villus surface area [(villus apical width + villus basal width)/2 × villus height] were determined in 10 complete, full-sized villi not showing any bending, mechanical damage, or fixation and/or processing artifacts following the guides of Wilson et al. (2018). All measurements on the intestinal villi were performed using the AmScope ToupView v4.8.15934 software (AmScope, Irvine, CA, USA). Finally, the results were presented as percentages (means ± SE).
Hepatic and splenic stress, apoptosis, antioxidant and inflammatory gene expression
The total RNA was extracted from 50 mg (liver and spleen) for the transcriptome study using 1 ml of Quiazol (Qiagen, Germany) (4 fish/replicate). The extracted RNA efficiency was assessed using the NanoDrop® ND-1000 UV–Vis Spectrophotometer (Thermo Scientific, Waltham, MA, USA), which analyzed the quantity of extracted total RNA at wavelengths 260 and 280. The extracted total RNA acceptable for gene expression was in the range (1.8–2). The cDNA280 was synthesized using a high-capacity reverse transcriptase kit from Applied Biosystem, Foster City, CA, USA (Khamis et al. 2021).
Caspase-3, heat shock protein 70 (hsp70), tumor protein p53 (p53), proliferating cell nuclear antigen (pcna), superoxide dismutase (sod), catalase (cat), interleukin-1 beta (il-1β), and tumor necrosis factor-α (tnf-α) RT-qPCR were assessed by the specified primers (Sangon Biotech, Beijing, China) as listed in Table 2. The sample was first heated to 95 °C for 10 min, then subjected to 40 cycles of melt curve analysis (denaturation for 10 s at 95 °C, annealing for 15 s at 60 °C, and extension for 15 s at 72 °C). Gene expression is often quantified by calculating the fold change in expression in relation to that of the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (gapdh) (Schmittgen and Livak 2008).
Statistical analysis
Kolmogorov–Smirnov (Berger and Zhou 2014) and Levene’s tests (Nordstokke and Zumbo 2010) were used to check the data for normality and variance homogeneity, respectively. When normality assumptions were met, using IBM SPSS Statistics, version 21 (IBM; Armonk, NY, USA; Spss 2011), data were analyzed by one-way analysis of variance (ANOVA) to statistically define the variation between groups, followed by Tukey’s multiple range post hoc test for pairwise comparisons (Benjamini and Braun 2002). The data has been displayed as means ± SE for each group. At P < 0.05, mean differences were considered significant. Moreover, the GraphPad Prism version 8 (GraphPad Software, San Diego, CA, USA) was used for data presentation (Prism 2019).
Results
Clinical observation
Throughout the feeding trial, fish that reared at 25 °C and fed a basal diet or ALA-fortified diet displayed a normal appetite and normal swimming behavior during the experimental period. In contrast, fish groups that reared under 18 °C and fed on the control diet suffered from lethargy and anorexia, and reduced swimming behavior. In contrast, fish reared at 18 °C and fed a supplemented diet showed normal appetite and swimming behavior to a great extent.
Growth performance, feed utilization, and survival of fish
Table 3 shows that the CNT, ALA, and ALA + CWS groups had the best survival rates of any group, reaching 100%. The CWS group had the highest mortality rate (10%). In ALA-supplemented groups, there was a significant increase in final body weight (13.16%), weight gain (20.68%), weight gain % (17.46%), and SGR (11.19%) than the CNT group (P < 0.05) while feed intake value was comparable to the CNT. In contrast, FCR displayed a significant reduction (16.78%) in the ALA-supplemented group compared to CNT (P < 0.05).
A significant reduction in final body weight, weight gain, weight gain, feed intake, and SGR values was recorded in fish exposed to cold stress water at 18 °C for 60 days by 21.54%, 35.64%, 33.93%, 7.94%, and 25.17%, respectively, but a significant increase in FCR by 42.28% than the control fish. Remarkably, the ALA + CWS group elicited a significant (P < 0.05) and similar enhancement in final body weight, weight gain, weight gain %, feed intake, SGR, and FCR values than the CWS group and very relative to the CNT group (P < 0.05).
Whole body proximate composition
As shown in Table 3, dietary supplementation of ALA for fish reared at 25 °C significantly increases the crude protein content (6.60%) compared to the CNT group. The crude protein content decreased (9.72%) significantly in Nile tilapia-fed basal diet and reared at 18 °C compared to those of the CNT group (P < 0.05). Crud protein in the ALA + CWS group showed non-significant change (P > 0.05) compared to the CNT group. The groups had no statistically significant differences (P > 0.05) in the amounts of crude lipid, moisture, or ASH.
Hematological indices
Table 4 summarized the hematological changes in Nile tilapia when they were exposed to cold water stress and/or supplemented with ALA in their diet for 60 days. Compared with the control one, the counts of RBCs (12.99%), HB level (25.33%), PCV % (21.48%), MCV (9.70%), MCH (14.23%), and MCHC (4.97%) notably declined in the fish fed basal diet and reared at 18 °C (P < 0.05). These hematometric values characterize the anemia presented by the CWS-exposed fish as microcytic and hypochromic. On the contrary, the cold-stressed fish supplemented with ALA showed a substantial improvement in RBC count, HB level, PCV %, and MCV value compared with the CWS group and very similar to the CNT group. Moreover, MCH did not improve in the ALA + CWS group, continuing to be comparable to that detected in the CWS group.
The WBCs, heterophils, lymphocytes, eosinophils, and monocytes counts showed a trend toward increase, but non-significant (P > 0.05), in the ALA group by 6.10%, 9.03%, 3.18%, 8.82%, and 10.14%, respectively, compared to the CNT group. Cold stress exposure at 18 °C for fish-fed basal diet in the CWS group elicited a significant decrease in all previous WBC counts by 12.15%, 12.74%, 8.27%, 27.27%, and 17.91%, respectively, compared to the CNT group (P < 0.05). In the ALA + CWS group, there were significant improvements in the total and differential WBC counts compared to the CWS group and very near the CNT group (P < 0.05).
Serum biochemical indicators
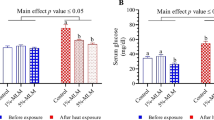
Blood protein profiles (total protein, albumin, and globulin); lipid profiles (cholesterol and triglycerides); and stress markers (cortisol and glucose) levels of Nile tilapia in response to cold water stress exposure and/or dietary supplementation with ALA for 60 days are presented in Table 5. ALA at dose concentrations of 600 mg/kg for fish reared at 25 °C significantly enhanced the total protein (18.49%), albumin (23.81%), and globulin (14.76%) in comparison with the CNT group (P < 0.05). On the other hand, a trend toward a decrease, but non-significant (P > 0.05), was recorded in total protein, albumin, and globulin in the CWS-exposed fish by 12.61%, 10.20%, and 14.76%, respectively, than the control group. On the other hand, a trend toward an increase of the protein profile but non-significant (P > 0.05) was recorded in the ALA + CWS compared to the CWS group.
ALA supplementation (mg/kg) markedly decreased the levels of serum cholesterol (16.15%) and triglycerides (6.90%) of fish reared at 25 °C (P < 0.05). Relative to the control group, rearing at 18 °C with feeding a basal diet markedly increased serum cholesterol (22.69%) and triglycerides (22.03%) compared to the CNT group (P < 0.05). Compared with the CWS group, a significant decrease in cholesterol and triglyceride levels in serum was found in the ALA + CWS group (P < 0.05), similar to the CNT group.
As for serum glucose and cortisol, the ALA group revealed a marked decrease in serum glucose (23.35%) and cortisol (13.22%) concentrations compared with CNT, and all these values were significantly elevated (37.74%) and (52.65%), respectively, in the CWS group compared to CNT (P < 0.05). On the other hand, the ALA + CWS group displayed enhanced values of serum glucose and cortisol compared to the CWS group and near-to-control values (P < 0.05), as shown in Table 5.
Immunological parameters
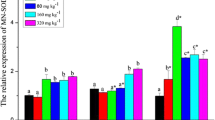
Figure 1 demonstrates the changes in Nile tilapia’s immunological parameters in response to cold water stress exposure and/or dietary supplementation with ALA for 60 days. A marked elevation in the level of serum immune parameters (lysozymes (47.10%), complement 3 (44.58%), and nitric oxide (45.09%)) in the ALA group compared with the CNT one (P < 0.05). However, cold stress exposure in the CWS group revealed a significant decrease in these immune parameters (30.40%), (34.18%), and (32.68%), respectively, in comparison to the CNT group (P < 0.05). In contrast, the ALA + CWS group indicated better serum immune component values than the CWS group, nearly to control values (P < 0.05).
Immune parameters of Nile tilapia fed diets supplemented with 600 mg ALA/kg diet during 60 days and submitted to water temperature stress. CNT: Fish fed a basal diet without any supplementation at 25 °C. Alpha lipoic acid (ALA) group: Fish fed a basal diet supplemented with 600 mg ALA/kg diet at 25 °C. Cold water-stressed group (CWS): Fish fed on a basal diet and reared at 18 °C. ALA + CWS group: Fish fed a basal diet containing ALA and reared at 18 °C. Lysozyme (A), complement 3 (B), and nitric oxide (C). Values are expressed as means ± SE (N = 12/group). The bars with different superscripts differed significantly (P < 0.05, one-way ANOVA)
Serum oxidative stress and AChE biomarkers
The alterations in serum oxidative stress markers of Nile tilapia after 60 days of exposure to cold water stress and/or dietary treatment with ALA are displayed in Fig. 2. Supplementing fish diets with ALA improved their antioxidant defenses. ALA group had the uppermost levels of antioxidants, including SOD (29.46%), CAT (48.58%), GPx (26.89%), and GSH (71.61%) but the lowest MDA levels (12.50%) relative to the CNT group (P < 0.05). Conversely, the only cold stress exposure at 18 °C elicited a significant reduction in antioxidants, including SOD (39.57%), CAT (40.37%), GPx (25.47%), and GSH (27.37%) (P < 0.05). In comparison, serum MDA levels were the highest (119.56%) among all fish groups. The ALA + CWS group significantly improved the changes in serum antioxidant variables and MDA levels compared to the CWS group and near the CNT group (P < 0.05). Compared to the non-cold stressed groups (CNT and ALA), the CWS group showed significantly lower AChE enzyme activity. The fish that consumed an ALA-rich diet during CWS exposure showed a significant (P < 0.05) increase in AChE activity compared to the CWS group, as presented in Table 5.
Serum oxidative/anti-oxidative status of Nile tilapia, fed diets supplemented with 600 mg ALA/kg diet during 60 days and submitted to water temperature stress. CNT: Fish fed a basal diet without any supplementation at 25 °C. Alpha lipoic acid (ALA) group: Fish fed a basal diet supplemented with 600 mg ALA /kg diet at 25 °C. Cold water-stressed group (CWS): Fish fed on a basal diet and reared at 18 °C. ALA + CWS group: Fish fed a basal diet containing ALA and reared at 18 °C. Superoxide dismutase (SOD) (A), catalase (CAT) (B), glutathione peroxidation (GPx) (C), reduced glutathione (GSH) (D), and malondialdehyde (MDA) (E). Values are expressed as means ± SE (N = 12/group). The bars with different superscripts differed significantly (P < 0.05, one-way ANOVA)
Intestinal digestive enzymes’ activities
Changes in intestinal digestive enzyme activity of Nile tilapia exposed to cold water stress and/or supplemented with ALA for 60 days are depicted in Fig. 3. The α amylase (153.04%), lipase (74.83%), and protease (158.83%) activities increased significantly in Nile tilapia fed on basal diet supplemented with ALA. It was reared at 25 °C compared to those fed on the control diet (CNT group) (P < 0.05). Compared with the control one, these intestinal enzymes notably declined by 27.83%, 13.72%, and 26.87% in the fish-fed basal diet and reared at 18 °C (P < 0.05). Exposure to cold stress at 18 °C concurrently with ALA supplementation ameliorated the intestinal enzyme activities compared to the CWS group nearly to CNT values (P < 0.05).
Intestinal digestive enzymes of Nile tilapia fed diets supplemented with 600 mg ALA/kg diet during 60 days and submitted to water temperature stress. CNT: Fish fed a basal diet without any supplementation at 25 °C. Alpha lipoic acid (ALA) group: Fish fed a basal diet supplemented with 600 mg ALA/kg diet at 25 °C. Cold water-stressed group (CWS): Fish fed on a basal diet and reared at 18 °C. ALA + CWS group: Fish fed a basal diet containing ALA and reared at 18 °C. α-amylase (A), lipase (B), and protease (C). Values are expressed as means ± SE (N = 12/group). The bars with different superscripts differed significantly (P < 0.05, one-way ANOVA)
Behavioral observations
The impacts of ALA food supplementation on O. niloticus behavioral performance parameters after 60 days of CWS exposure are shown in Table 6. Compared to the CNT group, there were significant (P < 0.05) decreases in feeding behavior, surfacing, and middle swimming behavior in the CWS groups. Moreover, CWS groups showed considerably (P < 0.05) lower rates of aggression indications (approach, chasing, mouth pushing, fleeing, and spreading of fins). But in contrast, to control fish, CWS groups revealed considerably (P < 0.05) higher rates of resting and bottom swimming behavior. On the other hand, compared to fish-fed non-fortified diets, adding ALA to O. niloticus diets significantly (P < 0.05) enhanced all behavioral indicators except for resting and bottom swimming behavior. Furthermore, all behavioral indicators are significantly affected (P < 0.05) by combining CWS and ALA feed supplementation.
Liver, spleen, and intestinal histopathology
The postmortem examination of the fish of different experimental groups did not reveal obvious PM changes except for the liver enlargement of CWS-exposed fish only. Normal histological pictures were seen in the examined hepatic tissue sections of all tilapias of the CNT group as displayed in Fig. 4A, with a non-significant increase in the area fractions occupied by both hepatocytes’ cytoplasmic vacuolations and melanomacrophage centers (MMCs) of all tilapias of the ALA group as shown in Fig. 4B. The basic effects of the cold stress on the hepatic tissue of the CWS group included a sharp reduction in the hepatocytes’ lipoidal cytoplasmic vacuolations with significant reductions in the sizes of the MMCs (Fig. 4C). Alpha lipoic acid supplementation moderately increased the lipoidal cytoplasmic vacuolations in the hepatocytes of the ALA + CWS group yet did not regain the normal values. Still, it significantly increased the areas occupied by MMCs compared to the CWS group (Fig. 4D).
A–D Representative light micrographs of the Hand E-stained hepatic tissue sections show normal histological pictures in the CNT (A) and ALA tilapias (B), with notable and moderate reductions in both hepatocytes’ cytoplasmic vacuolations and MMCs in the CWS (C), and ALA + CWS (D) tilapias, respectively. The scale bar equals 25 µm. E–H Representative light micrographs of the Hand E-stained splenic tissue sections show normal histological pictures in the CNT (E) and ALA tilapias (F), with notable and moderate reductions in both the cellular and MMCs in the CWS (G) and ALA + CWS (H) tilapias, respectively. The scale bar equals 25 µm. I–L Representative light micrographs of the Hand E-stained intestinal tissue sections show normal histological pictures in the CNT (I), and ALA tilapias (J), with significant reductions in the villus width, height, and surface areas, and the numbers of the mucosal-associated lymphocytes in both the CWS (K), and ALA + CWS (L) tilapias. The scale bar equals 100 µm. Symbols|black arrowhead; hepatocytes cytoplasmic vacuolation, black arrow; MMCs, blue arrowhead; mucosal-associated lymphocytes, green arrowhead; goblet cells. CNT: Fish fed a basal diet without any supplementation at 25 °C. Alpha lipoic acid (ALA) group: Fish fed a basal diet supplemented with 600 mg ALA/kg diet at 25 °C. Cold water-stressed group (CWS): Fish fed on a basal diet and reared at 18 °C. ALA + CWS group: Fish fed a basal diet containing ALA and reared at 18 °C. (N = 12/group)
The spleens of the CNT (Fig. 4E) and the ALA (Fig. 4F) groups showed normal histological pictures without any histological differences. On the contrary, all splenic tissue sections of the CWS tilapias exhibited a substantial rise in the area fractions not occupied by cellular or extracellular elements due to notable depletion in the cellular elements, including the lymphoid, erythroid, and MMCs (Fig. 4G). The spleens of the ALA + CWS tilapias appeared much more cellular, with a hyperactivation to the MMCS compared to the CWS group (Fig. 4H).
The intestine of the CNT (Fig. 4I) and the ALA (Fig. 4J) groups showed normal histological pictures without significant histological differences between them. Still, the ALA group’s intestines showed more mucosal-associated lymphocytes and surface epithelial intermingled goblet cells. The intestines of the CWS tilapias (Fig. 4K) showed significant reductions in the villus width, villus height, and villus surface areas compared to the CNT or ALA groups. In addition, marked reductions in the numbers of mucosal-associated lymphocytes were evident. The effects of ALA supplementation on the intestinal histology were very weak as a non-significant increase in the villus width, villus height, and villus surface areas were observed in the intestines of the ALA + CWS tilapias (Fig. 4L) compared to the CWS group. All the statistical data of the histological parameters in all groups’ hepatic, splenic, and intestinal tissues were summarized in Table 7.
Genes expression levels
Hepatic stress, apoptosis, antioxidant, and inflammatory gene expression
Stress- and apoptosis-related genes such as hsp70, caspase-3, p53, and pcna were significantly elevated in the hepatic tissues of CWS group by 2.51-, 2.82-, 1.84-, and 1.81-fold, respectively, compared to the hepatic tissues of the control ones as shown in Fig. 5. Dietary ALA supplementation significantly reduced this change, with expression levels of the hsp70, caspase-3, and pcna genes significantly lower in the ALA + CWS group compared to the CWS group, though not reaching the levels measured in the CNT, with fold changes of 1.41-, 1.49-, and 0.76-, respectively. Moreover, P53 gene expression did not improve in the ALA + CWS group (1.47-fold), comparable to that detected in the CWS group. In contrast, the cat gene was significantly downregulated (0.50-fold) in the CWS group compared to the CNT group. Also, the CAT mRNA expression levels were significantly increased (1.86-fold) in healthy fishes reared at 25 °C (P < 0.05) when fed an ALA diet. Although the CAT gene expression in the ALA + CWS group did not reach the levels seen in the CNT group (P < 0.05), it did increase by 1.21-fold when compared to the CWS group. SOD mRNA gene expression revealed non-significant change between all groups (P > 0.05).
Quantitative real-time PCR expression of hepatic stress, apoptosis, antioxidant and inflammation-related genes (heat shock protein 70 (HSP70), caspase 3, tumor protein p53 (P53), proliferating cell nuclear antigen (PCNA), superoxide dismutase (SOD), catalase (CAT), interleukin 1 beta (IL 1β) and tumor necrosis factor α (TNF α)) of Nile tilapia fed diets supplemented with 600 mg ALA/kg diet during 60 days and submitted to water temperature stress. CNT: Fish fed a basal diet without any supplementation at 25 °C. Alpha lipoic acid (ALA) group: Fish fed a basal diet supplemented with 600 mg ALA/kg diet at 25 °C. Cold water-stressed group (CWS): Fish fed on a basal diet and reared at 18 °C. ALA + CWS group: Fish fed a basal diet containing ALA and reared at 18 °C. Values are expressed as means ± SE (N = 12/group). The bars with different superscripts differed significantly (P < 0.05, one-way ANOVA)
For inflammation-related genes, a significantly higher expression (P < 0.05) of il-1β (1.78- fold) and tnf-α (1.72- fold) were observed in the CWS group than in the CNT one. However, there was a substantial decrease in the expression of the genes mentioned above in the ALA + CWS group compared to the CWS group, with fold changes (P < 0.05) of 0.81- and 0.55- for il-1β and tnf-α, respectively, as presented in Fig. 5.
Splenic stress, apoptosis, antioxidant, and inflammatory gene expression
Genes involved with stress and apoptosis, including Hsp70, caspase-3, p53, and pcna, were significantly upregulated in the splenic tissues of Nile tilapia in the CWS group by 5.90-, 2.89-, 1.83- and 1.54- fold, respectively, than the control fish as shown in Fig. 6. Such change was significantly mitigated by dietary ALA supplementation, with expression levels of the caspase-3, hsp70, and p53 genes being significantly decreased in ALA + CWS group than the CWS group, though not reaching the levels dignified in CNT with fold changes 2.61-, 1.56- and 0.98-, respectively (P < 0.05). Interestingly, dietary ALA significantly decreased PCNA mRNA gene expression either in control fish reared at 25 °C (0.38-fold) or in cold-stressed fish reared at 18 °C (0.40-fold) compared to the CNT group (P < 0.05).
Quantitative real-time PCR expression of splenic stress, apoptosis, antioxidant and inflammation-related genes (heat shock protein 70 (HSP70), caspase 3, tumor protein p53 (P53), proliferating cell nuclear antigen (PCNA), superoxide dismutase (SOD), catalase (CAT), interleukin 1 beta (IL 1β) and tumor necrosis factor α (TNF α)) of Nile tilapia fed diets supplemented with 600 mg ALA/kg diet during 60 days and submitted to water temperature stress. CNT: Fish fed a basal diet without any supplementation at 25 °C. Alpha lipoic acid (ALA) group: Fish fed a basal diet supplemented with 600 mg ALA/kg diet at 25 °C. Cold water-stressed group (CWS): Fish fed on a basal diet and reared at 18 °C. ALA + CWS group: Fish fed a basal diet containing ALA and reared at 18 °C. Values are expressed as means ± SE (N = 12/group). The bars with different superscripts differed significantly (P < 0.05, one-way ANOVA)
Antioxidant enzyme (sod and cat) mRNA expression values were significantly reduced in the CWS group (0.59-fold and 0.66-fold, respectively) compared to the CNT group (P < 0.05). Whereas, in the fish-fed ALA-containing diet group, these values were significantly elevated in healthy fishes reared at 25 °C (3.41- and 2.91-fold, respectively) (Fig. 6). Yet, the expression of the cat and sod genes significantly increased in ALA + CWS group (1.62- and 1.92-fold, respectively) than the CWS group, though not reaching the values of CNT group (P < 0.05) (Fig. 6).
For inflammation-related genes, a significantly higher expression (P < 0.05) of il-1β (1.71-fold) and tnf-α (1.52-fold) were observed in the CWS group than in the CNT one. Yet, the expression of the genes mentioned above significantly reduced in the ALA + CWS group compared to the CWS group, which were near to the values of the CNT group where (0.89-) and (0.59-) for il 1β and tnf-α, respectively, were fold changes (P < 0.05). Interestingly, dietary ALA significantly decreased TNF α mRNA gene expression in control fish reared at 25 °C (0.37-fold) compared to the CNT group (P < 0.05) (Fig. 6).
Discussion
The study findings demonstrated an elevated stress response after fish exposure to CWS (18 °C) for 60 days on the hemato-biochemical, immunological, behavioral, and molecular markers. The current study discovered a lower survival rate in Nile tilapia rearing at 18 °C for 60 days. The increased mortality rate caused by CWS could be due to fish immune system depression (Hassaan et al. 2019). Cold shock and stress can be fatal if fish cannot acclimatize or survive with temperature shifts, and temperature drops may result in lower survival rates (Reid et al. 2022). ALA dietary supplementation reduced CWS in fish maintained at 18 °C and boosted survival rate, possibly due to its increased antioxidant ability (Xiong et al. 2022). Additionally, Mahmoudi-Nezhad et al. (2021) found that including ALA in a prawn, diet can increase glycolysis, support aerobic oxidation, and result in the aerobic oxidation of carbohydrates, which can lessen damage caused by oxidative stress. This finding may also explain why ALA consumption is associated with a higher survival rate.
In the present study, Nile tilapia subjected to low temperatures (18 °C) demonstrated a substantial decrease in final body weight, weight gain %, feed intake, and SGR with increased FCR. This current finding is congruent with the of Refaey et al. (2023) findings, who attributed the drop to fish fasting during CWS and using stored energy to resist challenging conditions. This pushes the body to consume its conserved energy, which manifests as a loss of body weight (Zhu et al. 2020). Herein, the reduced SGR, the link between feeding rate and feeding efficiency, indicates that low temperatures may diminish the feeding axis (Pang et al. 2016). When ALA (600 mg/kg diet) was added, the fish had enhanced growth performance indices than the control. The enhanced growth rate could be attributable to ALA-induced lipid utilization, which allows for more energy provision for growth (Xu et al. 2019). Increased appetite with feed intake could be generated by ALA’s participation in adenosine monophosphate-activated protein kinase enzyme activation, which provided an alarm to the cells for energy uptake and hunger-full control (Rifai et al. 2022b).
Compared to control fish, fish-reared under CWS had significantly lower intestinal digestive enzymes, indicating the impact of CWS on the entire metabolic processes, resulting in suppression of enzyme activity (Li et al. 2023a). Instead, adding ALA to the diet minimized the reduction of digestive enzymes, showing that ALA played essential roles in metabolism adjustment and energy transformation (Serhiyenko et al. 2018). Besides, the intestinal histological examination exhibited notable decreases in villus width, height, and surface areas compared to the CNT or ALA groups. Similarly, Xing et al. (2019) showed that CWS might alter the integrity of the intestinal mucus layer and disrupt the mucosal mechanical barrier of juvenile Trachinotus ovatus intestinal tissue, indicating CWS-induced deficits in body metabolism (Liu et al. 2022).
The current findings demonstrated a considerable drop in the crude protein of Nile tilapia reared under CWS, which explained alterations in conserved energy mobilization and reduced growth rate (Hossain et al. 2018). Oppositely, the consumption of ALA counteracted the decrease in crude protein induced by CWS in the current study. Similarly, Xu et al. (2019) demonstrated that genetically enhanced farmed tilapia fed various levels of ALA (600, 900, 1200, and 2400 mg/kg diet) significantly increased crude protein contents. In this regard, Shi et al. (2018a) discovered that ALA regulates the phosphorylated activated protein kinase and carnitine palmitoyl transferase 1α pathway to augment protein deposition.
In the present study, CWS induced apparent microcytic and hypochromic anemia. The alterations in hematological parameters were confirmed by splenic histopathological findings, which revealed a significant increase in the area fractions that were not occupied by any cellular or extracellular elements and notable depletion in the cellular elements, including the lymphoid, erythroid, and MMCs. In accordance with existing findings, CWS diminished splenic red blood cells in the spleen in the study of Liu et al. (2022). The same findings were previously reported by Jakiul Islam et al. (2021) in the European seabass, Dicentrarchus labrax. In this regard, CWS impairs erythropoiesis, resulting in decreased oxygen transport capability for organism tissue, and juvenile tilapia cannot keep the minimal number of RBCs required to ensure their health (Barros et al. 2009). Besides, CWS induces metabolism to reset to homeostasis rather than conservation, preventing nutrients from being acquired adequately for optimal HB production (Witeska 2015). Moreover, regarding the detected leukopenia, a possible explanation could be that during CWS, circulation was retarded, and consequently, leukocyte release from the myeloid sinuses into the bloodstream was hampered (Teległów et al. 2022). On the contrary, ALA dietary addition ameliorated CWS-induced hematological alterations in the present study, which is compatible with former studies that demonstrated that ALA significantly elevated Hb concentration and serum iron level (Elewa et al. 2020; Abdel Hamid et al. 2022).
Herein, CWS promoted immunosuppression in Nile tilapia, as evidenced by decreased WBC count, lysozyme, complement 3, and nitric oxide levels. Chronic stress and its key signaling mediator, cortisol, have been thought to decrease immunity (Shepherd et al. 2018; Ayyat et al. 2020). Similarly, decreased lysozyme activities were recorded in O. mossambicus (Ndong et al. 2007) and European seabass (Jakiul Islam et al. 2021) during CWS. The immunostimulant action of ALA may have improved those immunological measures in Nile tilapia grown at 18 °C and fed ALA-supplemented diets. ALA influences the immune system directly and indirectly (Liu et al. 2019). In Lu et al. (2019b) research on Nile tilapia dietary, ALA significantly increased lysozyme activity. Likewise, the ALA group’s total protein, globulin, and albumin contents were significantly higher, which could be attributable to ALA supplementation improving immune function (Ou et al. 2023). Similarly, ALA dietary supplementation in commercial broilers favorably increased total protein, albumin, and globulin (Sakr et al. 2020). The authors of the earlier study correlated the increased serum protein profile with increased body weight gain and enhanced liver function as the organ that synthesizes proteins. Moreover, ALA has been recorded to decrease urinary protein excretion (Kang et al. 2008).
The current study found a considerable rise in total cholesterol and triglycerides in CWS fish, indicating increased energy demands to cope with repair processes (Hu et al. 2022). Under CWS, a rise in total cholesterol has been documented in Lateolabrax maculatus to act as a critical energy source (Wang et al. 2022). Similarly to these findings, cold stress raised triglyceride content in a genetically improved farmed tilapia strain of Nile tilapia (Liu et al. 2011). On the contrary, fish fed an ALA-supplemented diet showed accelerated lipid metabolism and reduced lipid production. According to Xu et al. (2019), dietary ALA enhanced lipolysis dose-dependently by increasing mRNA expression of adipose triglyceride lipase, a rate-limiting enzyme in lipolysis (Li et al. 2018).
In the current investigation, the higher serum glucose and cortisol levels of fish cultivated under CWS and fed a basal diet may be related to thermal adaptation to decreased enzymatic response rate (Dellagostin et al. 2022). It may also reveal a greater demand for energy to combat the effects of stress (Sun et al. 2019). In line with Rotllant et al. (2001), a drop in water temperature alters the pituitary-interrenal axis in gilthead seabream, causing cortisol release. In contrast, dietary ALA also considerably reduced the harmful effects of CWS on Nile tilapia blood glucose and cortisol. The lowering of serum glucose by ALA suggests that ALA increases glycolysis and the conversion of pyruvate to acetyl-CoA while not affecting gluconeogenesis. Besides, dietary ALA has been shown to lower serum cortisol levels (Kim et al. 2015).
According to the present findings, CWS decreased SOD, CAT, GPx, and GSH activity but increased MDA concentration in the serum of CWS-exposed fish. Several recent reports verified that fish’s antioxidant defense system was severely disrupted by CWS, which may have altered SOD, CAT, GSH-Px, and MDA-related enzyme activities and content (Li et al. 2023b; Refaey et al. 2023). In contrast, ALA supplementation at 600 mg/kg improved antioxidant parameters in fish maintained at 25 °C and reversed changes in antioxidant markers caused by cold stress at 18 °C. This suggests that ALA can protect against CWS-induced oxidative stress by increasing antioxidant enzyme activities, replenishing endogenous antioxidants, and scavenging excess ROS (Li et al. 2021). Similarly, the higher antioxidant capacity in genetically modified farmed tilapia-fed ALA diets can be attributed to its ability to suppress ROS production (Xu et al. 2019).
Fish exposed to CWS in the current experiment showed a marked fall in AChE activity, feeding, middle swimming, and aggression, indicating decreased food consumption and activity. CWS-induced decrease in fish activity in the ecosystem may make it easier for predators to catch fish larvae (Crawshaw et al. 2022). Additionally, the decreased activity and altered food-seeking behavior may harm their fitness and growth (Said et al. 2020). A shift in water temperature may influence the aggressive behaviors of fish species, as a decrease in water temperature is followed by a decrease in all kinds of aggressive behavior (Brandão et al. 2018). Moreover, it was noticed that the brain’s AChE-specific activity significantly decreased when tilapia fish were exposed to CWS (Hassan et al. 2013). Due to dietary ALA’s potential to lessen the oxidative stress caused by cold, AChE levels and behavioral responses in fish exposed to CWS were improved. By activating choline acetyltransferase and increasing glucose absorption, ALA promotes the production of acetylcholine (ACh) and provides more acetyl-CoA for ACh synthesis (Maczurek et al. 2008).
The current results revealed that cold stress exposure at 18 °C promoted upregulation of hepatic and splenic HSP70, caspase 3, P53, PCNA, IL 1β, and TNF-α with downregulation of hepatic and splenic SOD and CAT gene expression. Comparably, Mladineo and Block (2009) demonstrated the HSP70 upregulation in Bluefin tuna splenic tissue reared at 14 °C (Iwata et al. 2007). CWS-induced mitochondrial failure significantly induces mitochondria-mediated apoptosis and necrosis (Chapa-Dubocq et al. 2018). CWS-induced apoptotic gene patterns resulted in activating the initiator Caspase 9, followed by the proteolytic maturation of the executioner Caspase 3 (Han et al. 2022). Low temperature promoted apoptosis in puffer fish and grouper livers by upregulating caspase 3, 9, and p53 (Sun et al. 2019). P53 is easily triggered by lots of stress signals, particularly temperature fluctuations in aquatic animals (Qian et al. 2020). Furthermore, p53 can enhance apoptosis by upregulating pro-apoptosis genes (Perfettini et al. 2004). PCNA is a well-known protein that interacts with various enzymes and regulatory proteins to repair DNA in various pathological circumstances (Mailand et al. 2013). The augmented PCNA mRNA in cold-exposed fish muscle tissue could indicate a mechanism to offset muscle catabolism caused by skeletal muscle restructuring (Weber and Bosworth 2005). Similarly, cold shock altered the activity and mRNA expression of antioxidant genes, causing oxidative damage in zebrafish’s liver, gills, and brain (Wu et al. 2015). The present study has shown an increase in tnf-α and il-1β gene expression in the liver and spleen of fish exposed to cold conditions, consistent with Dellagostin et al. (2022). IL-1β has multiple functions within the inflammatory process (Dinarello 2009), while TNF-α is responsible for inducing critical cellular responses ranging from cellular proliferation via the NF-κB pathway to apoptosis induction (Cook et al. 2018).
Stress, apoptosis, and oxidative alterations in Nile tilapia were alleviated by ALA dietary supplementation, as seen by down-regulation of hepatic and splenic HSP70, caspase 3, P53, PCNA, IL-1β, and TNF-α expression, as well as up-regulation of SOD and CAT expression. ALA’s antioxidant, anti-apoptotic, and anti-inflammatory capabilities could explain these benefits. The ability of ALA to alleviate oxidative stress in fish caused by cold stress exposure may be linked to the activation of nuclear factor-erythroid factor 2 (Nrf2) signaling (Giuliani and Regoli 2014). The capacity of ALA to inhibit NF-κB signaling could explain its anti-inflammatory action (Li et al. 2022). ALA inhibited NF-κB signaling and reduced inflammatory responses in immature grass carp’s skin, head, kidney, and spleen against Aeromonas hydrophila (Liu et al. 2018). ALA’s ability to inhibit NF-κB is most likely connected to its ability to reduce inhibitor of nuclear factor kappa B degradation (Gomes and Negrato 2014).
Conclusion
This study concluded that cold exposure at 18 °C causes oxidative stress, cell apoptosis, and inflammation in Nile tilapia. However, dietary supplementation of ALA at 600 mg/kg meal can increase the fish’s development, digestion, and blood parameters, revealing its hemostatic efficacy on RBCs, HB, PCV, and total and differential WBCs. Furthermore, nutritional supplementation with ALA could boost fish innate immunity, antioxidant capability, and welfare, reducing cell apoptosis after cold stress. Moreover, dietary ALA may alleviate cold stress-induced oxidative damage, apoptosis, and inflammation in fish by modulating NF-κB signaling. As a result of ALA’s great antioxidant, anti-inflammatory, and anti-apoptotic activities, it could be recommended as a safe and ecologically acceptable aqua feed supplement (600 mg/kg diet) to alleviate the CSW-induced harmful effects in fish. Future research is required to assess the efficacy of ALA on the health and performance of different fish species, as well as its effect on various water stressors.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- AChE:
-
Acetylcholine esterase
- ALA:
-
Alpha lipoic acid
- C3:
-
Complement 3
- CAT:
-
Catalase
- CWS:
-
Cold water- stress
- FBW:
-
Final body weight
- FCR:
-
Feed conversion ratio
- GPx:
-
Glutathione peroxidase
- Hb:
-
Hemoglobin
- Hsp70:
-
Heat shock protein
- IBW:
-
Initial body weight
- IL-1β:
-
Interleukin-1 beta
- MCH:
-
Mean corpuscular hemoglobin
- MCHC:
-
Mean corpuscular hemoglobin concentration
- MCV:
-
Mean corpuscular volume
- MDA:
-
Malondialdehyde
- Nrf2:
-
Nuclear factor-erythroid factor 2
- PCNA:
-
Proliferating cell nuclear antigen
- PCV:
-
Packed cell volume
- RBCs:
-
Red blood cells
- ROS:
-
Reactive oxygen species
- SGR:
-
Specific growth rate
- SOD:
-
Superoxide dismutase
- TWG:
-
Total weight gain
- WBCs:
-
White blood cell counts
References
Abdel Hamid DZ, Nienaa YA, Mostafa TM (2022) Alpha-lipoic acid improved anemia, erythropoietin resistance, maintained glycemic control, and reduced cardiovascular risk in diabetic patients on hemodialysis: a multi-center prospective randomized controlled study. Eur Rev Med Pharmacol Sci 26:2313–2329
Abram QH, Dixon B, Katzenback BA (2017) Impacts of low temperature on the teleost immune system. Biology 6:39
Almarri SH, Khalil AA, Mansour AT, El-Houseiny W (2023) Antioxidant, immunostimulant, and growth-promoting effects of dietary Annona squamosa leaf extract on Nile tilapia, Oreochromis niloticus, and its tolerance to thermal stress and Aeromonas sobria infection. Animals 13:746
AOAC, 2005. Official Methods of Analysis of the Association of Analytical Chemists International, 18th ed. Association of Official Analytical Chemists, Gaithersburg, Md., USA.
Ayyat MS, Ayyat AMN, Abd El-Latif KM, Hessein AAA, Al-Sagheer AA (2020) Inorganic mercury and dietary safe feed additives enriched diet impacts on growth, immunity, tissue bioaccumulation, and disease resistance in Nile tilapia (Oreochromis niloticus). Aquat Toxicol 224:105494
Ayyat MS, Al-Sagheer AA, Ayyat AMN, Abdelrhman AM, Ahmed NH, Naiel MAE (2022) Effects of a blend of herbal feed supplements on growth, associated blood indices and body chemical analysis in Nile tilapia reared under high stocking density. Aquac Res 53:5475–5485
Ayyat MS, Ayyat AMN, Abdel-Rahman MS, Al-Sagheer AA (2024) Appraisal of leaf protein concentrate derived from sugar beet and carrot as a novel fish meal substitute for juvenile Nile tilapia (Oreochromis niloticus). Anim Feed Sci Technol 307:115833
Aziz E, Ahmed BA, Ramadan S, Mahboub HD (2022) Effects of magnetic water on productive performance, behaviour, and some physiological responses of Nile tilapia fish (Oreochromis niloticus) reared under normoxia and hypoxia conditions. Assiut Vet Med J 68:76–89
Barros M, Ranzani Paiva M, Pezzato L, Falcon D, Guimarães I (2009) Haematological response and growth performance of Nile tilapia (Oreochromis niloticus L.) fed diets containing folic acid. Aquac Res 40:895–903
Benjamini Y, Braun H (2002) John W. Tukey’s contributions to multiple comparisons. Ann Stat: 1576–1594
Berger VW, Zhou Y (2014): Kolmogorov–Smirnov test: overview. Wiley stat
Bezerra RS, Lins EJF, Alencar RB, Paiva PMG, Chaves MEC, Coelho LCBB et al (2005) Alkaline proteinase from intestine of Nile tilapia (Oreochromis niloticus). Process Biochem 40:1829–1834
Brandão ML, Colognesi G, Bolognesi MC, Costa-Ferreira RS, Carvalho TB, Gonçalves-de-Freitas E (2018) Water temperature affects aggressive interactions in a Neotropical cichlid fish. Neotrop Ichthyol 16
Bryan NS, Grisham MB (2007) Methods to detect nitric oxide and its metabolites in biological samples. Free Radic Biol Med 43:645–657
Cao L, Yin G, Du J, Jia R, Gao J, Shao N et al (2023) Salvianolic acid b regulates oxidative stress, autophagy and apoptosis against cyclophosphamide-induced hepatic injury in Nile tilapia (Oreochromis niloticus). Animals 13:341
Chapa-Dubocq X, Makarov V, Javadov S (2018) Simple kinetic model of mitochondrial swelling in cardiac cells. J Cell Physiol 233:5310–5321
Chen TC, Ormond R, Mok HK (2001) Feeding and territorial behaviour in juveniles of three co-existing triggerfishes. J Fish Biol 59:524–532
Chen S-W, Liu C-H, Hu S-Y (2019) Dietary administration of probiotic Paenibacillus ehimensis NPUST1 with bacteriocin-like activity improves growth performance and immunity against Aeromonas hydrophila and Streptococcus iniae in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol 84:695–703
Cheng CH, Guo ZX, Wang AL (2018) The protective effects of taurine on oxidative stress, cytoplasmic free-Ca(2+) and apoptosis of pufferfish (Takifugu obscurus) under low temperature stress. Fish Shellfish Immunol 77:457–464
Cook AD, Lee M-C, Saleh R, Khiew H-W, Christensen AD, Achuthan A et al (2018) TNF and granulocyte macrophage-colony stimulating factor interdependence mediates inflammation via CCL17. JCI Insight 3(6):e99249
Crawshaw LI, Peavy M, Podrabsky JE (2022) Behavioral responses to temperature in fishes, Reference Module in Life Sciences. Elsevier
da Silva MÁ, Artigas Flores J, Porto C, Romano L, Wasielesky Junior W, Caldas S et al (2018) Antioxidant effects of nanoencapsulated lipoic acid in tissues and on the immune condition in haemolymph of Pacific white shrimp Litopenaeus vannamei (Boone, 1931). Aquac Nutr 24:1255–1262
Dellagostin EN, Martins AW, Blödorn EB, Silveira TLR, Komninou ER, Junior ASV et al (2022) Chronic cold exposure modulates genes related to feeding and immune system in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol 128:269–278
Dinarello C (2009) Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol 27:519–550
El-Houseiny W, Arisha AH, Metwally MMM, Abdel-Warith A-WA, Younis EM, Davies SJ et al (2023) Alpha-lipoic acid suppresses gibberellic acid nephrotoxicity in Nile tilapia (Oreochromis niloticus) via modulating oxidative stress, inflammation, cytokine production, and apoptosis. Pestic Biochem Physiol 196:105598
El-Sayed A-FM (2020): Chapter 3 - Tilapia distribution, transfers and introductions. In: El-Sayed A-FM (Editor), Tilapia Culture (Second Edition). Academic Press, pp. 33–45
Elewa HA, Zalat Z, Keshk WA, Werida RH (2020) Role of alpha-lipoic acid in protection from cardiovascular events in patients with hemodialysis. J Biosci Appl Res 6:121–132
Fitzgerald R, Haylock D (1998) Basic techniques in fish haematology. Aqualex Multimedia Consortium, Dublin Ireland
Ghareghanipoora M, Akbary P, Akhlaghi M, Fereidouni M (2014) Non-specific immune responses and immune related genes expression of rainbow trout (Oncorhynchus mykiss, walbaum) fed Zataria multiflora boiss extract. Bull Env Pharmacol Life Sci 3:140–146
Giuliani ME, Regoli F (2014) Identification of the Nrf2-Keap1 pathway in the European eel Anguilla anguilla: role for a transcriptional regulation of antioxidant genes in aquatic organisms. Aquat Toxicol 150:117–123
Goes ESdR, Goes MD, Castro PLd, Lara JAFd, Vital ACP, Ribeiro RP (2019) Imbalance of the redox system and quality of tilapia fillets subjected to pre-slaughter stress. PLoS ONE 14:e0210742
Gomes MB, Negrato CA (2014) Alpha-lipoic acid as a pleiotropic compound with potential therapeutic use in diabetes and other chronic diseases. Diabetol Metab Syndr 6:80
Han S, Wei S, Chen R, Ni M, Chen L (2022) Tissue-specific and differential cold responses in the domesticated cold tolerant fugu. Fishes 7:159
Hassaan MS, El Nagar AG, Salim HS, Fitzsimmons K, El-Haroun ER (2019) Nutritional mitigation of winter thermal stress in Nile tilapia by propolis-extract: associated indicators of nutritional status, physiological responses and transcriptional response of delta-9-desaturase gene. Aquaculture 511:734256
Hassan B, El-Salhia M, Khalifa A, Assem H, Al Basomy A, El-Sayed M (2013) Environmental isotonicity improves cold tolerance of Nile tilapia, Oreochromis niloticus, in Egypt. Egypt J Aquat Res 39:59–65
Hossain S, Koshio S, Ishikawa M, Yokoyama S, Sony NM, Islam J et al (2018) Substitution of dietary fishmeal by soybean meal with inosine administration influences growth, digestibility, immunity, stress resistance and gut morphology of juvenile amberjack Seriola dumerili. Aquaculture 488:174–188
Hu J, Zhao H, Wang G, Sun Y, Wang L (2022) Energy consumption and intestinal microbiome disorders of yellow catfish (Pelteobagrus fulvidraco) under cold stress. Front Physiol 13:985046
Ibrahim RE, El-Houseiny W, Behairy A, Abo-Elmaaty A, Al-Sagheer AA (2019a) The palliative role of Eruca sativa leaves dietary supplementation against oxidative stress, immunosuppression, and growth retardation in temperature-stressed Oreochromis niloticus. J of Therm Biol 84:26–35
Ibrahim RE, El-Houseiny W, Behairy A, Abo-Elmaaty A, Al-Sagheer AA (2019b) The palliative role of Eruca sativa leaves dietary supplementation against oxidative stress, immunosuppression, and growth retardation in temperature-stressed Oreochromis niloticus. J Therm Biol 84:26–35
Iwata E, Sanae Y, Naoko M-S, Kyohei M, Sasaki H (2007) Cloning and expression of HSP70 and 90 mRNA from Bluegill Lepomis macrochirus. J Biol Sci 7:791–795
Jakiul Islam M, James Slater M, Thiele R, Kunzmann A (2021) Influence of extreme ambient cold stress on growth, hematological, antioxidants, and immune responses in European seabass, Dicentrarchus labrax acclimatized at different salinities. Ecol Indic 122:107280
Kang S, Lee S, Kim D-S, Kim T (2008): The effect of α-lipoic acid on proteinuria and renal TGFβ expression in obese type 2 diabetic rat model. Korean Diabetes Journal 32.
Khalil AA, Abd-Elhakim YM, Said EN, Moselhy AA, Abu-Elsaoud AM, El-Houseiny W (2022) Milk thistle and co-enzyme Q10 fortified diets lessen the nickel chloride-induced neurotoxic and neurobehavioral impairments in Oreochromis niloticus via regulating the oxidative stress response, acetylcholinesterase activity, and brain nickel content. Aquaculture 553:738102
Khamis T, Abdelalim AF, Saeed AA, Edress NM, Nafea A, Ebian HF et al (2021) Breast milk MSCs upregulated β-cells PDX1, Ngn3, and PCNA expression via remodeling ER stress/inflammatory/apoptotic signaling pathways in type 1 diabetic rats. Eur J Pharmacol 905:174188
Kim D, Mushtaq M, Parvin R, Kang H, Kim J, Na J et al (2015) Various levels and forms of dietary α-lipoic acid in broiler chickens: Impact on blood biochemistry, stress response, liver enzymes, and antibody titers. Poultry Sci 94:226–231
Kishawy ATY, Roushdy EM, Hassan FAM, Mohammed HA, Abdelhakim TMN (2020) Comparing the effect of diet supplementation with different zinc sources and levels on growth performance, immune response and antioxidant activity of tilapia, Oreochromis niloticus. Aquac Nutr 26:1926–1942
Leonard JN, Skov PV (2022) Capacity for thermal adaptation in Nile tilapia (Oreochromis niloticus): Effects on oxygen uptake and ventilation. J Therm Biol 105:103206
Li M, Kong Y, Wu X, Yin Z, Niu X, Wang G (2021) Dietary α-lipoic acid can alleviate the bioaccumulation, oxidative stress, cell apoptosis, and inflammation induced by lead (Pb) in Channa argus. Fish Shellfish Immunol 119:249–261
Li M, Wu X, Zou J, Lai Y, Niu X, Chen X et al (2022) Dietary α-lipoic acid alleviates deltamethrin-induced immunosuppression and oxidative stress in northern snakehead (Channa argus) via Nrf2/NF-κB signaling pathway. Fish Shellfish Immunol 127:228–237
Li R-x, Amenyogbe E, Lu Y, Jin J-h, Xie R-t, Huang J-s (2023a) Effects of low-temperature stress on intestinal structure, enzyme activities and metabolomic analysis of juvenile golden pompano (Trachinotus ovatus). Front Mar Sci 10:1114120
Li S, Sang C, Zhang J, Li Z, Chen N (2018) Molecular cloning, expression profiling of adipose triglyceride lipase (ATGL) and forkhead box O1 (FoxO1), and effects of dietary carbohydrate level on their expression in hybrid grouper (Epinephelus fuscoguttatus♀× E. lanceolatus♂). Aquaculture 492:103–112
Li S, Wang J, Xiong Y, Zheng J, Zhou D, Zhao J et al (2023b) Protein-sparing effect of <i>α</i>-lipoic acid in diets with different protein/carbohydrate ratios for the oriental river prawn. Macrobrachium Nipponense Aquac Res 2023:5614359
Lima de Almeida CA, de Lima Almeida CK, de Fátima Ferreira Martins E, Gomes AM, da Anunciação Pimentel L, Pereira RT et al (2019) Effect of the dietary linoleic/α-linolenic ratio (n6/n3) on histopathological alterations caused by suboptimal temperature in tilapia (Oreochromis niloticus). J Therm Biol 85:102386
Liu B, Wang M, Xie J, Xu P, Ge X, He Y et al (2011) Effects of acute cold stress on serum biochemical and immune parameters and liver HSP70 gene expression in GIFT strain of Nile tilapia (Oreochromis niloticus). Acta Ecol Sin 31:4866–4873
Liu HX, Zhou XQ, Jiang WD, Wu P, Liu Y, Zeng YY et al (2018) Optimal α-lipoic acid strengthen immunity of young grass carp (Ctenopharyngodon idella) by enhancing immune function of head kidney, spleen and skin. Fish Shellfish Immunol 80:600–617
Liu R, Liu R, Song G, Li Q, Cui Z, Long Y (2022) Mitochondria dysfunction and cell apoptosis limit resistance of Nile tilapia (Oreochromis niloticus) to lethal cold stress. Animals 12:2382
Liu W, Shi LJ, Li SG (2019) The immunomodulatory effect of alpha-lipoic acid in autoimmune diseases. BioMed Res Int 2019:8086257
Lu D-L, Limbu SM, Lv H-B, Ma Q, Chen L-Q, Zhang M-L et al (2019a) The comparisons in protective mechanisms and efficiencies among dietary α-lipoic acid, β-glucan and l-carnitine on Nile tilapia infected by Aeromonas hydrophila. Fish Shellfish Immunol 86:785–793
Maczurek A, Hager K, Kenklies M, Sharman M, Martins R, Engel J et al (2008) Lipoic acid as an anti-inflammatory and neuroprotective treatment for Alzheimer’s disease. Adv Drug Deliv Rev 60:1463–1470
Mahmoudi-Nezhad M, Vajdi M, Farhangi MA (2021) An updated systematic review and dose-response meta-analysis of the effects of α-lipoic acid supplementation on glycemic markers in adults. Nutrition 82:111041
Mailand N, Gibbs-Seymour I, Bekker-Jensen S (2013) Regulation of PCNA-protein interactions for genome stability. Nat Rev Mol Cell Biol 14:269–282
Mladineo I, Block BA (2009) Expression of Hsp70, Na+/K+ ATP-ase, HIF-1α, IL-1β and TNF-α in captive Pacific bluefin tuna (Thunnus orientalis) after chronic warm and cold exposure. J Exp Mar Biol Ecol 374:51–57
Ndong D, Chen YY, Lin YH, Vaseeharan B, Chen JC (2007) The immune response of tilapia Oreochromis mossambicus and its susceptibility to Streptococcus iniae under stress in low and high temperatures. Fish Shellfish Immunol 22:686–694
Neiffer DL, Stamper MA (2009) Fish sedation, anesthesia, analgesia, and euthanasia: considerations, methods, and types of drugs. ILAR J 50:343–360
Noga EJ (2010) Fish disease: diagnosis and treatment. John Wiley and Sons
Nordstokke DW, Zumbo BD (2010) A new nonparametric Levene test for equal variances. Psicológica 31:401–430
NRC (2011) Nutrient requirements of fish and shrimp. National Academies Press.
Ou G, Xie R, Huang J, Huang J, Wen Z, Li Y et al (2023) Effects of dietary alpha-lipoic acid on growth performance, serum biochemical indexes, liver antioxidant capacity and transcriptome of juvenile hybrid grouper (Epinephelus fuscoguttatus♀× Epinephelus polyphekadion♂). Animals 13:887
Pang X, Fu S-J, Zhang Y-G (2016) Acclimation temperature alters the relationship between growth and swimming performance among juvenile common carp (Cyprinus carpio). Comp Biochem Physiol Part A Mol Integr Physiol 199:111–119
Peng L-B, Wang D, Han T, Wen Z, Cheng X, Zhu Q-L et al (2022) Histological, antioxidant, apoptotic and transcriptomic responses under cold stress and the mitigation of blue wavelength light of zebrafish eyes. Aquac Rep 26:101291
Perfettini JL, Roumier T, Castedo M, Larochette N, Boya P, Raynal B et al (2004) NF-kappaB and p53 are the dominant apoptosis-inducing transcription factors elicited by the HIV-1 envelope. J Exp Med 199:629–640
Prism G (2019): Prism 8 for windows. GraphPad Software Inc
Qian B, Qi X, Bai Y, Wu Y (2020) The p53 signaling pathway of the large yellow croaker (Larimichthys crocea) responds to acute cold stress: evidence via spatiotemporal expression analysis of p53, p21, MDM2, IGF-1, Gadd45, Fas, and Akt. PeerJ 8:e10532
Ramadan SG, Mahboub HD, Helal MA, Ahmed WS (2018): Behavioral responses of Nile tilapia (Oreochromis niloticus) to feed restriction regime. Alexandria J Vet Sci 59
Refaey M, Mehrim A, Zenhom O, Mansour A (2022) Effect of fatty acids manipulation on survival and physiological response of hybrid red tilapia under chronic cold stress. Aquaculture 561:738663
Refaey MM, Mehrim AI, El-Komy MM, Zenhom OA, Mansour AT (2023) Chronic cold-stress induced histopathological changes, oxidative stress, and alterations in liver functions and nutrient composition of hybrid red tilapia and the potential protection of unsaturated fatty acids. Front Mar Sci 10:1148978
Reid CH, Patrick PH, Rytwinski T, Taylor JJ, Willmore WG, Reesor B et al (2022) An updated review of cold shock and cold stress in fish. J Fish Biol 100:1102–1137
Rezaei Zonooz S, Hasani M, Morvaridzadeh M, Beatriz Pizarro A, Heydari H, Yosaee S et al (2021) Effect of alpha-lipoic acid on oxidative stress parameters: a systematic review and meta-analysis. J Funct Foods 87:104774
Rifai R, Jusadi D, Suprayudi M, Alimuddin A, Nuryati S (2022a) Evaluation of dietary α-lipoic acid supplementation on the growth performance and physiological status of striped catfish Pangasianodon hypophthalmus. Jurnal Akuakultur Indonesia 21:198–206
Rifai R, Jusadi D, Suprayudi MA, Nuryati S (2022b) Evaluation of dietary α-lipoic acid supplementation on the growth performance and physiological status of striped catfish Pangasianodon hypophthalmus. Jurnal Akuakultur Indonesia 21:198–206
Rotllant J, Balm PH, Pérez-Sánchez J, Wendelaar-Bonga SE, Tort L (2001) Pituitary and interrenal function in gilthead sea bream (Sparus aurata L., Teleostei) after handling and confinement stress. Gen Comp Endocrinol 121:333–342
Said EN, Ahmed FAA, Saleem A-SY, Mohammed HH, Youssef MY, Fattah AFA (2020) Behavioural response, welfare, and performance of Nile tilapia (Oreochromis niloticus) under different water temperatures. Int J Fish Aquat Stud 8:1–11
Sakr OA, Nassef E, Fadl ES, Omar H, Waded E, El-Kassas S (2020) The impact of alpha-lipoic acid dietary supplementation on growth performance, liver and bone efficiency, and expression levels of growth-regulating genes in commercial broilers. J World’s Poultry Res 10:172–179
Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative CT method. Nat Protoc 3:1101–1108
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675
Serhiyenko V, Serhiyenko L, Suslik G, Serhiyenko A (2018) Alpha-lipoic acid: mechanisms of action and beneficial effects in the prevention and treatment of diabetic complications. MOJ Public Health 7:174–178
Shepherd BS, Spear AR, Philip AM, Leaman DW, Stepien CA, Sepulveda-Villet OJ et al (2018) Effects of cortisol and lipopolysaccharide on expression of select growth-, stress- and immune-related genes in rainbow trout liver. Fish Shellfish Immunol 74:410–418
Shi X-c, Jin A, Sun J, Tian J-j, Ji H, Chen L-q et al (2018a) The protein-sparing effect of α-lipoic acid in juvenile grass carp, Ctenopharyngodon idellus: effects on lipolysis, fatty acid β-oxidation and protein synthesis. Br J Nutr 120:977–987
Shi XC, Jin A, Sun J, Yang Z, Tian JJ, Ji H et al (2017) α-lipoic acid ameliorates n-3 highly-unsaturated fatty acids induced lipid peroxidation via regulating antioxidant defenses in grass carp (Ctenopharyngodon idellus). Fish Shellfish Immunol 67:359–367
SPSS (2011): IBM SPSS statistics for Windows, version 20.0. New York: IBM Corp 440, 394
Standen BT, Peggs DL, Rawling MD, Foey A, Davies SJ, Santos GA et al (2016) Dietary administration of a commercial mixed-species probiotic improves growth performance and modulates the intestinal immunity of tilapia, Oreochromis niloticus. Fish Shellfish Immunol 49:427–435
Sun Z, Tan X, Liu Q, Ye H, Zou C, Xu M et al (2019) Physiological, immune responses and liver lipid metabolism of orange-spotted grouper (Epinephelus coioides) under cold stress. Aquaculture 498:545–555
Suvarna KS, Layton C, Bancroft JD (2018): Bancroft’s theory and practice of histological techniques. Elsevier Health Sciences
Teległów A, Romanovski V, Skowron B, Mucha D, Tota Ł, Rosińczuk J et al (2022) The effect of extreme cold on complete blood count and biochemical indicators: a case study. Int J Environ Res Public Health 19:424–438
Wang Z, Dong Z, Yang Y, Wang J, Yang T, Chen X et al (2022) Histology, physiology, and glucose and lipid metabolism of Lateolabrax maculatus under low temperature stress. J Therm Biol 104:103161
Weber TE, Bosworth BG (2005) Effects of 28 day exposure to cold temperature or feed restriction on growth, body composition, and expression of genes related to muscle growth and metabolism in channel catfish. Aquaculture 246:483–492
Wilson F, Cummings T, Barbosa T, Williams C, Gerard P, Peebles E (2018) Comparison of two methods for determination of intestinal villus to crypt ratios and documentation of early age-associated ratio changes in broiler chickens. Poul Sci 97:1757–1761
Witeska M (2015) Anemia in teleost fishes. Bull Eur Assoc Fish Pathol 35:148–160
Wu F, Yang C, Wen H, Zhang C, Jiang M, Liu W et al (2019) Improving low-temperature stress tolerance of tilapia, Oreochromis niloticus: a functional analysis of Astragalus membranaceus. J World Aquac Soc 50:749–762
Wu J-J, Liu W, Jiang M, Zhou Y, Wang W-M, Wen H et al (2020) Beneficial effects of dietary exogenous protease on the growth, intestinal health and immunity of GIFT (Oreochromis niloticus) fed plant-based diets. Aquac Nutr 26:1822–1834
Wu SM, Liu JH, Shu LH, Chen CH (2015) Anti-oxidative responses of zebrafish (Danio rerio) gill, liver and brain tissues upon acute cold shock. Comp Biochem Physiol A Mol Integr Physiol 187:202–213
Xing X, Song R-X, Wang L, Niu C-J, Zhang Z-B (2019) The different response of the intestinal mucosal histological features in different sections of juvenile Chinese soft-shelled turtle under acute cold stress. Acta Hydrobiol Sin 43:102–108
Xiong D, Zhang X, Liu X (2012) Effects of anethole trithione and lipoic acid on growth performance and antioxidant ability of Oreochromis niloticus× O. aureus. China Feed 23:28–31
Xiong Y, Li Q, Ding Z, Zheng J, Zhou D, Wei S et al (2022) Dietary α-lipoic acid requirement and its effects on antioxidant status, carbohydrate metabolism, and intestinal microflora in oriental river prawn Macrobrachium nipponense (De Haan). Aquaculture 547:737531
Xu F, Xu C, Xiao S, Lu M, Limbu S, Limbu M et al (2019) Effects of α-lipoic acid on growth performance, body composition, antioxidant profile and lipid metabolism of the GIFT tilapia (Oreochromis niloticus) fed high-fat diets. Aquac Nutr 25:1–12
Yilmaz S, Ergün S, Celik ES, Banni M, Ahmadifar E, Dawood M (2021) The impact of acute cold water stress on blood parameters, mortality rate and stress-related genes in Oreochromis niloticus, Oreochromis mossambicus and their hybrids. J Therm Biol 100:103049
Zhang T, Zhang L, Yin T, You J, Liu R, Huang Q et al (2023) Recent understanding of stress response on muscle quality of fish: from the perspective of industrial chain. Trends Food Sci Technol 140:104145
Zhu Q, Song H, Zhang Y, Chen R, Tian L, Xu D (2020) Effects of cold stress and starvation on the liver of yellow drum Nibea albiflora: histological alterations and transcriptomic analysis. Aquac Environ Interact 12:359–369
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
AB: conceptualization, methodology, software, data curation, and writing—original draft. HAG: conceptualization, methodology, and writing—review and editing. NAA-A: conceptualization, methodology, software, data curation, and writing—review and editing. WE-H: conceptualization, methodology, investigation, software, data curation, and writing—original draft. AHA: conceptualization, methodology, software, data curation, visualization, and writing—review and editing. MMMM: conceptualization, methodology, software, data curation, and writing—review and editing. BAE: conceptualization, software, data curation, visualization, resources, and writing—review and editing. AAA-S: conceptualization, software, and writing—review and editing. EMMM: conceptualization, software, and writing—review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
All experimental steps and protocols involving fish were sanctioned by the Animal Ethics Committee of Zagazig Universitys (ZU-IACUC), with an approval number ZU-IACUC/2/F/276–2023.
Competing interest
The authors declare no competing interests.
Additional information
Handling Editor: Amany Abbass
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Behairy, A., Ghetas, H.A., Abd-Allah, N.A. et al. Dietary alpha-lipoic acid boosts growth, immune-antioxidant traits, behavior, and transcriptomes of antioxidant, apoptosis, and immune-related genes to combat cold stress in Nile tilapia (Oreochromis niloticus). Aquacult Int (2023). https://doi.org/10.1007/s10499-023-01365-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10499-023-01365-4