Abstract
The use of phytobiotic as functional feed additives is a promising inducer for growth performance and immune response in the Pacific whiteleg shrimp (Litopenaeus vannamei). It can be also commercially applied in combination with chemotherapeutic agents and vaccines. This study was carried out to evaluate the use of a commercial phytobiotic feed additive, Sanacore® GM, containing vegetable fatty acids and inactivated Saccharomyces cerevisiae with herbal extracts, on growth, body composition, non-specific immune response, antioxidant index, intestinal microflora count, and fungal resistance to Fusarium solani challenge in Pacific white shrimp, Litopenaeus vannamei. Shrimp juveniles (5.00 ± 0.1 g) were randomly distributed into 12 hapas (1 m3) in triplicates at a stocking density of 20 shrimp per hapa and fed increasing levels of Sanacore® (0, 0.1, 0.2, and 0.3%) for 63 days. At the end of the feeding trial, the experimental shrimp were infected with F. solani, and mortality was recorded for 15 days. Results revealed that growth performance, feed utilization, and whole-body protein deposition were improved with increasing dietary sanacore levels. A significant improvement was reported in cellular (hemocyte counts, phagocytosis, phagocytic index, respiratory burst activity) and humoral (lysozyme and phenoloxidase) immune responses with higher dietary sanacore levels. Superoxide dismutase, catalase, and glutathione peroxidase were notably improved in shrimp-fed sanacore-supplemented diets. Meanwhile, malondialdehyde level was significantly decreased in all sanacore-supplemented shrimp. Furthermore, the dietary sanacore consistently increased probiotic bacterial counts and decreased enteric and Clostridium sp. counts in the intestine. Juveniles fed with Sanacore exhibited significantly lower mortality rates when challenged with F. solani. In conclusion, dietary sanacore could exhibit a profitable effect on the growth performance, antioxidant capacity, immunity, intestinal microbial, and the health condition of L. vannamei. Thus, sanacore can be used as an attractive feed supplement in the L. vannamei diet (0.2–0.3%), providing more insights into its application in aquaculture as a functional immunostimulant feed additive.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Shrimp farming is one of the fast-growing aquaculture sectors with an 86% increase in global production accounting for more than 6.5 mt and about 40 billion U.S. dollars in 2019 (Karthikeyan et al. 2015; Abbas et al. 2020; Eissa et al. 2022). In 2022, world shrimp production has increased and reached 9.4 mt, showcasing a remarkable rebound from the challenges experienced during the COVID-19 pandemic in 2020. Notably, Pacific white shrimp (Litopenaeus vannamei) constituted more than half of the total global shrimp production (FAO 2023).
Pacific white shrimp (Litopenaeus vannamei) accounts for the major share of penaeid production with 83.1% of global yield compared with other penaeid shrimp (FAO 2020; Islam et al. 2020; Mansour et al. 2022b). Besides, L. vannamei has favorable biological features for selective breeding that involve its lower requirement of dietary protein, grazing microbial particles, higher stocking density culture, disease tolerance, and relative ease of domestication (Kim et al. 2015; Emerenciano et al. 2022; Mansour et al. 2022a). Thus, the highly intensive farming of L. vannamei advancement has cut-edge technologies fitted to high-density production (Samocha 2019; Ciji and Akhtar 2021).
As a result of high-density farming and environmental changes, diseases have been the major obstacle to global shrimp farming (Zou et al. 2020; Abbas et al. 2023). Of these, the outbreaks of black spot disease (BSD), which is caused by Fusarium solani, a serious fungal pathogen of L. vannamei (Sharawy et al. 2022). Fusarium belongs to Sordariomycetes class, Hypocreales order is a genus of fungi in the family Nectriaceae. Fungal Fusarium spp. is a saprotrophic fungi with wide distribution in plants, soil, freshwater, and brackish water (Palmero et al. 2009; Eissa et al. 2023). The fungal pathogen was first isolated from Kuruma prawn, Penaeus japonicus (Ishikawa 1968), as well as from L. vannamei shrimp (Lin et al. 2020; Yao et al. 2022). Mass mortalities have been observed in pond-cultured shrimp populations that occurred due to BSD, showing black gill symptoms related to water quality variables (Yao et al. 2022).
A crucial strategy is needed to boost the shrimp’s immunity during farming. Feed additives are substances derived from microbes, plants, and animals including yeast extracts, phytobiotics, probiotics, prebiotics, and organic acids. These additives have health-promoting and disease-preventive effects with faster growth and higher production, consequently, they are remarkable components for farm management to decrease the risk of microbial pathogens (Patra et al. 2019; Hendam et al. 2023).
Prebiotics are popular alternatives to antibiotics for sustaining growth and health status, as well as diminishing the risk of infectious disease in shrimp aquaculture (Hill et al. 2014; Ringø 2020; Eissa et al. 2021). Most of the research has indicated the physiological and immunological responses of host-fed probiotics-supplemented diets, in addition to improving host resistance efficacy, and growth performance (Hoseinifar et al. 2018; Ringø 2020). A natural product, Saccharomyces cerevisiae cell wall or extract has been authenticated to augment the immune responses and growth indices in a variety of aquatic species, comprising β-glucan, nucleic acids, mannan oligosaccharides, and chitin (Sheikhzadeh et al. 2012).
Moreover, the augment in intestinal balance could be implemented by the functional feed additives strategy, including the production of chemicals that decelerate the growth of possible pathogens, promoting the immune response of the host, and improve digestion (Sanders 2008; Vargas-Albores et al. 2017; Abo-Taleb et al. 2020). Understanding the effects of feed additive administration is needed to recognize the protective functions of intestinal microbiota in penaeid species (Tzuc et al. 2014; Abdelrhman et al. 2022; Mabrouk et al. 2022).
For shrimp culture, many studies have proved that botanical feed additives have growth-promoting effects, as well as higher survivability and immune response against early mortality syndrome, which is provoked by Vibrio parahaemolyticus in shrimp (Al-Musalam et al. 2014; Tran et al. 2015). Besides natural compounds of probiotics, organic acids, yeast extracts, and phytobiotics have been capable of favorably modulating the gut microflora in shrimp (Lem et al. 2014). Previously, phytogenic feed additives have improved the growth performance and well-being of aquatic organisms (Chakraborty et al. 2014; Kesselring et al. 2021). Also, the enhanced effects of phytogenic supplementation (e.g. feed palatability, gut functions, and antioxidant responses) have been addressed in several aquatic species (Peterson et al. 2014; Rodrigues et al. 2018).
There is a well-studied point of view on the role of phytobiotic in the immune system of aquatic animals. While studies on the potential of phytobiotics to improve immune response have been reported on several fish species, their specific effects on white shrimp (L. vannamei) have not been extensively studied. However, previous research has documented the use of biosynthetic plant-derived compound derivatives in other contexts (Ly et al. 2019; Rahardjo et al. 2022). Rahardjo et al. (2022) concluded that supplementation of S. ferox and Z. zerumbet could enhance the production performance and hemato-immunological parameters of whiteleg shrimp. In the same line, the inclusion of phytogenic products derived from yeast cells and grape pomace showed an enhancement in the phagocytic activity of L. vannamei (Chuchird et al. 2017). The presence of S. cerevisiae, which contains β-glucans (βGs) and oligosaccharides, has the potential to potentiate the immune response in L. vannamei. This is achieved by activating the shrimp β-glucan binding protein (βGBP), which interacts with βGs to form a complex called βGBP-βGs. This compound then activates blood cells and stimulates the proPO cascade system (Chuchird et al. 2017; Ayiku et al. 2020).
The current work aims to examine whether the growth performance, immune response, antioxidant activity, and resistance toward F. solani could be provoked when the L. vannamei shrimp were fed with functional feed additive, Sanacore® GM (Adisseo), as dietary supplements. As well, the shrimp’s intestinal general health will be assessed concerning beneficial and harmful intestinal bacterial counts after feed additive supplementation.
Materials and methods
Diet assembling
Four experimental diets were prepared by the addition of Sanacore® GM (Adisseo, Dendermonde, Belgium), a commercial natural health-promoting feed additive at a rate of 0, 0.1, 0.2, and 0.3%, denoted as T0, T1, T2, and T3, respectively. Sanacore® GM is derived from a mixture of vegetable fatty acids and inactivated S. cerevisiae with herbal extracts on a mineral component. Briefly, the experimental diet was prepared as follows: all ingredients were milled into powder using a hammer mill machine and then sieved through 80-mm mesh before mixing thoroughly with minerals and vitamins in a V-mixer machine for 30 min. Thereafter, 200 ml of warm water (45 °C) per kg was added to make a dough. The feed dough was then pushed through a meat miner to produce a spaghetti-like shape. The resultant diets were dried overnight, crushed to ~2-mm diameter pellets, and then kept in a refrigerator at −4 °C until use. The diet formula and proximate composition are presented in Table 1.
Shrimp rearing and culture conditions
A total of 240 healthy juveniles of L. vannamei (5.00 ± 0.1 g) are categorized into 4 experimental groups into 12 hapas (in triplicates) which were set up in an earthen pond located on a private farm at Damietta governorate, Egypt. Each hapa was sized 1 m3 (1 m × 1 m × 1 m), and the shrimp’s initial stocking density was 20 shrimp/hapa. Shrimp were acclimatized in these hapas for 14 days before the beginning of the feeding experiment. The shrimp were fed the control diet during the acclimatization period at a rate of 5%.
Shrimp were hand-fed 4 times daily at 8:00, 12:30, 16:00, and 20:00 h for 63 days at 3–5% of their average body weight to apparent satiation. During the experimental period, the water exchange rate was increased by increasing shrimp size by 8%, 10%, and 15%, every 20 days. Feed intake and mortality were monitored. Also, water quality parameters have been tested during the feeding trial in the earthen pond according to standard methods (APHA 2012). The average of measured water quality parameters was 26–29.5 °C for temperature range, 22–25‰ for salinity values, 6.24–7.20 mg L−1 for dissolved oxygen values, 7.60–8.2 for pH values, 0.02–0.04 mg L−1 for nitrite, 0.02–0.03 mg L−1 for nitrates, and 0.3–0.5 mg L−1 for NH4.
Sample collection
Shrimp fasted for 24 h before sampling and then transported to the Laboratory of Fish Diseases and Management, Veterinary Medicine, Mansoura University. The numbers and the body weight of shrimp from each hapa were noted. After that, three shrimps were picked and kept at −20 °C for the carcass examination. Before hemolymph collection, shrimp were drugged with clove oil at 0.2 ml L−1. Hemolymph samples were drawn from the ventral sinus of the third pleopods of five shrimps per group with a sterile 1-ml syringe, which was loaded with a precooled (4 °C) anticoagulant solution (0.114 M trisodium citrate, 450 mM NaCl, 10 mM KCl, and 10 mM HEPES at pH 7.4) (Nonwachai et al. 2010). The previous mixture estimated the total hemocyte count (THCs), phagocytosis, and respiratory burst activities. Other hemolymph samples were collected in Eppendorf tubes, then they were immediately centrifuged at 800 × g for 10 min at 4 °C, and finally, plasma was separated and stored at −20 °C for further analysis of lysozyme and phenoloxidase (PO) activity.
For evaluation of antioxidant enzymes and oxidative stress markers, hemolymph samples were centrifuged at 3000 rpm and 4 °C for 10 min, and the formed pellet was washed with 3 ml of 0.9% NaCl and centrifuged again. Then, the pellet was again resuspended with 2 ml of distilled water; the final mixture was reserved at 4 °C. Following the sampling of hemolymph, shrimp were dissected, and intestinal tissues were used for the analysis of bacterial counts.
Growth performance analysis
The weights of five L. vannamei shrimp were recorded from each group before and 9 weeks after the feeding to record the initial and final body weight (IBW and FBW, respectively). Besides, the overall amount of feed supplemented to prepared groups was evaluated. The initial and final numbers were reported to investigate the survival rate (SR%). The weight gain rate (WGR, %), specific growth rate (SGR), and feed conversion ratio (FCR) were calculated using the following formulas:
where ln is the natural logarithmic of FBW and IBW; t = time in days;
Diets and shrimp carcass composition analysis
Formulated feed and shrimp body samples (n= 3) were examined for proximate composition using a standard method (Lee 1995). Crude protein was determined by the Kjeldahl method using boric acid to trap NH3 (Kjeldahl procedure: N × 6.25). Dry matter was analyzed by oven-drying samples at 105 °C. The ash content was detected in a muffle furnace at 550 °C for 4 h by burning 2 g of samples. Total lipids were extracted by ether using the Soxtect System HT extraction unit.
Total hemocyte counts
The anticoagulant–hemolymph mixture of 50 μl was added in 150 μl of formaldehyde (4%) and then 20 μl of the mixture was placed on a hemocytometer (Neubauer). The counts of total hemocytes using a light microscope were assessed (Blaxhall and Daisley 1973). THC counts were calculated with the following formula: THC (cells ml−1) = count ×104 × dilution factor.
Immune parameter analysis
Phagocytosis assay
Phagocytosis assay was carried out with slight modifications according to a previous protocol (Itami et al. 1992). Hemocytes were washed with shrimp saline (a solution of NaCl 28.4 g, MgCl2-6H2O 1.0 g, MgSO4-7H2O 2.0 g, CaCl2-2H2O 2.25 g, KCl 0.7 g, glucose 1.0 g, and HEPES 2.38 g/L) to set the viable cell numbers to 1 × 106 cells/ml. A 200 μl of cellular suspension was transferred on a cover slip. After that, removal of the suspension was performed 20 min later and washed with the same saline three times. Two milliliters of a prepared fungal suspension of Candida albicans (C. albicans) were inoculated with the previous suspension for 2 h. Following the removal of C. albicans preparation, the cell suspension was again rinsed with shrimp saline five times to adjust 5 × 108 cells/ml and fixed with 100% methanol. Finally, the coverslip was stained with Giemsa stain and covered with paramount slide mounting fluid. The phagocytic activity as well as the phagocytic index were determined using the following equations:
Respiratory burst assay
The respiratory burst (RB) activity was evaluated according to a previous protocol of Song and Hsieh (1994), which was based on spectrophotometric detection of formazan from the nitroblue tetrazolium, NBT-O2− redox reaction. Fifty microliters of a hemolymph-anticoagulant were incubated with 50 μl of Hank’s balanced salt solution (MCHBSS, Sigma, USA) in a 96-well microtiter plate and incubated for 30 min at room temperature. After that, the supernatants were discarded and replaced with 50 μl of MCHBSS medium. Then, 50 μl of 0.3% NBT was distributed to each well and incubated for 2 h. The hemocytes were fixed by the addition of 200 μl absolute 70% methanol and dried. The formazan deposits were made soluble in 120 μl, 2 M KOH, and 140 μl dimethyl sulfoxide (DMSO; Sigma, USA). The contents were read at 620 nm in a spectrophotometer.
Lysozyme activity
The efficacy of shrimp plasma lysozyme was evaluated using the turbidimetric method previously described (Engstad et al. 1992). Lyophilized Micrococcus lysodekticus (0.2 mg ml−1) as a substrate was adjusted in phosphate buffer saline to pH 5.75. Plasma (50 μl) was added to 3 ml of fresh bacterial suspension. Following the incubation of the mixture for 30 min at 37 °C, the 540-nm absorbance was used for measuring the mixture by spectrophotometer.
Phenoloxidase activity
The efficacy of the phenoloxidase enzyme was detected spectrophotometrically by noting the dopachrome formation produced from l-dihydroxyphenylalanine according to a published modified protocol of Supamattaya et al. (2000). Washing three times for the hemolymph mixture was done with shrimp saline with centrifugation at 1000 rpm at 4 °C for 10 min. Preparation of hemocyte lysate from hemocytes was performed in cacodylate buffer (pH 7.4; 0.01 M sodium cacodylate, 0.45 M sodium chloride, 0.01 M calcium chloride, and 0.26 M magnesium chloride; pH 7.0) via a sonicator at 30 amplitudes for 5 s, and followed by centrifugation of the suspension at 10,000 rpm at 4 °C for 20 min. Then, with the addition of 0.25% trypsin in cacodylate buffer, along with hemocyte lysate and l-dihydroxyphenylalanine substrate, the phenoloxidase enzyme activity was finally measured as the absorbance of dopachrome at 490-nm wavelength.
Antioxidant and oxidant index determination
Superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) activities were estimated following previously described protocols (Paglia and Valentine 1967; McCord and Fridovich 1969; Aebi 1984), respectively using commercial kits (Biodignostic, Cairo, Egypt). The SOD enzyme assay has the ability to prohibit the phenazine methosulphate-mediated reduction of nitroblue tetrazolium dye, whereas colorimetric measurement of CAT activity depends on the inhibition of oxidant H2O2 in the presence of peroxidase enzyme. GPx enzymes catalyze the reduction of H2O2 to water and organic peroxides to stable alcohols using reducing agents such as glutathione (GSH). Malondialdehyde (MDA) content (a lipid peroxidation biomarker) was determined via a thiobarbituric acid reaction following previously described methods (Ohkawa et al. 1979).
Intestinal bacterial counts
Sampled shrimp intestine was ground till complete homogeneity in 1-ml sterilized saline. Serial dilution was made by inoculating 500 μl from the prepared shrimp intestine solution in 5-ml sterilized saline to form the 1st dilution. The previous step was performed till the 5th dilution factor. Then, 1 ml from the dilutions from each group was inoculated in sterilized Petri dishes and then poured with pre-prepared, warm sterilized media as follows: for total aerobic bacterial count, nutrient agar medium was used, and the plates were incubated aerobically at 30 °C for 24–48 h. For total fecal bacterial counts, MacConkey agar was applied, and plates were grown at 37 °C for 48 h under aerobic conditions. Following the procedure previously described by APHA (2005), the probiotic bacterial counts were determined by inoculation on De Man, Rogosa, and Sharpe agar plates (MRS), then put in an incubator at 37 °C for 48–72 h through ambient light conditions. Finally, tryptic sulfite cycloserine agar plates (TSC) were used for Clostridium perfringens count and incubated in anaerobic jars for 48 h at 37 °C.
Challenge study with Fusarium solani
Pathogenic F. solani was obtained from the Microbiology Department of Fish Diseases, Animal Health Institute, Egypt. Fungal spores were prepared by culturing F. solani on potato dextrose agar (PDA) at 25 °C for 7 days. Subsequently, the conidial mass was produced via the addition of 20 ml of sterile distilled water into the culture plate. The suspension (30 ml) was collected in sterile tubes that were filtered via sterile medical gauze to accumulate the fungal conidia. Concentrations of the fungal conidia were adjusted to 5 × 104 conidia ml−1 using an erythrocyte counting chamber. Healthy L. vannamei shrimp (13.5–18.5 g) fed with supplemented groups were pooled in a 50-l aquarium (10 shrimp per tank × 3 tanks per treatment = 30 shrimp per treatment). Shrimp were challenged with F. solani by intramuscular injection in the third abdominal segment with 0.1 ml of 5 × 104 of the conidial suspension (Khoa et al. 2004; Le et al. 2005). All shrimp were fed twice daily with the basal diet for 15 days at the rate of 3% of their body weight. After that, two shrimp were randomly taken from each tank after 1, 3-, 5-, 7-, and 15-days post-inoculation to record any abnormal behavior, clinical signs, and daily mortality. Gills and muscles from freshly dead shrimps were microscopically checked and inoculated into PDA plates for re-isolation and identification of the fungus. The cumulative mortality percentage (%) was calculated as previously described (Adel et al. 2017).
Statistical analysis
One-way analysis of variance (ANOVA) was used to determine the significant differences among means followed by Tukey’s multiple comparison test. Significance levels of p < 0.05 were used in overall tests. Data were presented as means ± standard deviations (SD). All statistical analyses and their relevant graphics were preceded using GraphPad Prism (GraphPad Software, Inc., USA, version 8.0).
Results
Growth parameters and body composition
The final body weight, WG, SGR, and SR were significantly improved in fish fed with sanacore-supplemented diets compared to the control (p > 0.05). Furthermore, Table. 2 observed that the FCR in all groups supplemented with sanacore exhibited significantly lower values when compared to the control group (p < 0.05). Crude protein and ash were significantly increased with increasing sanacore supplementation up to 2 g kg−1 (Table 3). Meanwhile, lipids content and moisture were not affected.
Total hemocyte counts and immune response
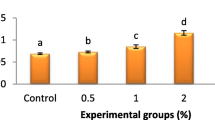
Marked increment in THCs (p < 0.05) was shown in L. vannamei fed sanacore-supplemented feeds (1, 2, 3 g kg−1) (Fig. 1). A significant augment in the phagocytic and respiratory burst activities (p < 0.05) was observed in shrimp fed on sanacore-supplemented diets (Figs. 1 and 2). Shrimp fed with sanacore-supplemented diets (T2 and T3) exhibited the highest phagocytic index followed by the T1 groups. In addition, the lysozyme and PO activities (p < 0.05) were notably higher in sanacore-supplemented groups. No remarkable difference was observed in the immune parameters among sanacore-supplemented groups.
Effects of Sanacore®GM on the total haemocyte counts (THCs) (A), phagocytosis (B), and phagocytic index (C) of L. vannamei shrimp. Control, T1, T2, and T3 represent Sanacore® GM supplementation at levels of 0, 0.1, 0.2, and 0.3%, respectively. Each bar represents mean ± SD (n = 3). Data with different letters are significantly different among treatments
Effects of Sanacore®GM on the lysozyme (A), respiratory burst (B), and phenoloxidase (C) activities of L. vannamei shrimp. Control, T1, T2, and T3 represent Sanacore® GM supplementation at levels of 0, 0.1, 0.2, and 0.3%, respectively. Each bar represents mean ± SD (n = 3). Data with different letters are significantly different among treatments
Antioxidants activity
Feeding of shrimp with a sanacore diet at levels of 1, 2, and 3 g kg−1 exhibited a notable increase in the activities of SOD (p < 0.05), CAT (p < 0.05), and GSH (p < 0.05) enzymes compared to the control group (Fig. 3). Moreover, a significant decrease in MDA levels (p < 0.05) was reported in L. vannamei fed sanacore diets compared to the control (Fig. 3).
Hemolymph SOD (A), CAT (B), GPx (C), and MDA (D) of L. vannamei shrimp in different feeding patterns of Sanacore®GM. Control, T1, T2, and T3 represent Sanacore® GM supplementation at levels of 0, 0.1, 0.2, and 0.3%, respectively are mean values of 3 replicates expressed as mean ± SD (n = 3). Data with different superscripts show significant differences among treatments
Intestinal bacterial counts
The bacterial counts in the intestinal tracts of L. vannamei fed the test diets were markedly affected by sanacore supplementation (Table 4). The counts of intestinal microflora composition pronounced in total aerobic and fecal bacteria together with Clostridium perfringens counts were significantly decreased with increasing sanacore concentrations compared with the control group (p < 0.05). Total probiotic bacteria counts were significantly increased (p < 0.05) by increasing the dose of sanacore supplementation. The best administration level of sanacore which showed the highest effectiveness was a 3 g kg−1 diet (p < 0.05) compared to the control shrimp (basal diet).
Challenge study with Fusarium solani
The morphological criteria of F. solani were characterized by dense aerial white to cream mycelium, floccose, and reverse brown coloration with ellipsoidal, fusiform, or kidney-shaped microconidia in fresh isolates. In addition, black-colored gills were revealed in severely infected shrimps. According to Kabaln-Miere survival curve, shrimp fed with diets containing sanacore have higher survival percentage and were more resistant to F. solani infection (Fig. 4).
Discussion
Our study elucidates that the inclusion of phytobiotic feed additive, sanacore, had a highly significant influence on L. vannamei growth performance and feed utilization. Whereas, significant improvement in the final body weight, WG, and SGR, and a noticeable decrease in FCR were observed among L. vannamei groups fed sanacore. Several reports showed the promoting abilities of sanacore on nutritional value, feed utilization, growth performance, and health of L. vannamei, Nile tilapia, and sea bream (Coutteau 2010; Piñeiro et al. 2013; Emerenciano et al. 2022). Herbal extracts have a tonic to improve the growth ability and stimulate the appetite in aquatic animals (Van Hai 2015). Besides, the improved feed digestibility and growth performance have been proven in shrimp via dietary S. cerevisiae supplementation because of the valuable nutrients in yeast cultures, such as protein, carbohydrates, vitamins, and nucleic acids (Ayiku et al. 2020). Similarly, dietary yeast products boosted the growth rate and FCR in L. vannamei (Xiong et al. 2018; Ayiku et al. 2020).
Evaluation of whole-body composition is a good indicator of penaeid physiology as modulations in feed constituents can unfavorably affect nutrient content in shrimp whole body (Xiong et al. 2018; Ayiku et al. 2020). A noteworthy change in the shrimp body composition after sanacore supplementation except for lipids. These results suggest that dietary inclusion of sanacore plays a role in reinforcing feed intake and, subsequently, fish body composition, which may explain the remarkable role of S. cerevisiae in promoting nutrient utilization and digestibility via increasing the activity of digestive enzymes, and so the deposited nutrients increased (Xiong et al. 2018).
The sustainability of shrimp aquaculture depends mainly on disease-preventive strategies and the immune response of shrimp, which are vital parameters for assessing the health status of shrimp (Al-Musalam et al. 2014). Shrimp have only an inherent immune system, consisting of cellular and hormonal reactions. Hemocytes are the main components of phagocytosis, nodule formation, encapsulation, cell adhesion, and cytotoxicity (Roy et al. 2020). Whereas, the prophenoloxidase-activating cascade (proPO) and immune-related proteins (e.g., lysozymes, lectins, and antimicrobial peptides) are mediated via humoral reactions (Cerenius et al. 2008; Söderhäll 2016). Our study assessed the physiological and immunological benefits of sanacore on the health and immune status of L. vannamei through hemolymph examination. Mainly hemocytes, PO, and lysozyme in shrimp represent a protective immune response against a variety of pathogens, subsequently, they have been widely employed as markers of the adaptive immune response in shrimp species (Söderhäll 2016; Kuo et al. 2021). Excess numbers of hemocytes refer to the potential ability of crustaceans immune during environmental stress and infections, and so disease resistance (Söderhäll 2016). Also, the PO mechanism oxidizes the phenols to quinones, resulting in the production of melanin, which blockades pathogens combined with promoted phagocytosis and cytotoxic reactions (Amparyup et al. 2012). Lysozyme enzyme defends against several types of bacteria by damaging N-acetylmuramic acid and N-acetylglucosamine bonds in the cell walls of microorganisms (Kaizu et al. 2011; Kuo et al. 2021). The supplemental sanacore at the inclusion levels of 1, 2, and 3 g kg−1 in the diets notably induced THCs, PO, and lysozyme activities. In accordance, phytogenic products from a spent hop, yeast cell, and grape pomace improved the immune response of L. vannamei through the proliferation of hemocytes, as well as PO and lysozyme activities (Chuchird et al. 2017), as well as hairy eggplant, and bitter ginger phytogenic extracts enhanced the hematological parameters in L. vannamei (Rahardjo et al. 2022). The phagocytic activity and respiratory burst of L. vannamei received sanacore diets showed a significant effect. In line with these studies, the phagocytic activity of L. vannamei increases in diets containing seaweed Gracilaria verrucosa extract at concentrations of 3 g kg−1 (Jasmanindar et al. 2018). Respiratory burst activity of L. vannamei has been stimulated in diets containing Bacillus NP5 RfR and mannan oligosaccharide combination at 1% and 2% (w/w) (Febrianti and Yuhana 2016). In general, both phagocytic activity and respiratory burst increased in line with the increment of sanacore doses, which could be a valid modulator for the non-specific immunity of L. vannamei shrimp.
A notable augment in the survival rate of supplemented shrimp infected with F. solani suggests that sanacore could participate in improving the resistance and providing the immune response of shrimp against fungal infections. These results are consistent with a previous study showing significant improvements in L. vannamei survival trials fed Sanacore for 3 weeks and challenged with Vibrio parahaemolyticus (Tran et al. 2015). Further reports on yeast at a level of 3% fed to shrimp species have enhanced resistance against vibriosis (Sarlin and Philip 2011; Ayiku et al. 2020).
The activities of antioxidant enzymes, including glutathione peroxidase (GPx), superoxide dismutase (SOD), and catalase (CAT), are considered the main defense line against reactive oxygen species (ROS). SOD converts superoxide radical (O2.−) to peroxide (H2O2). CAT reduces H2O2 to water and glutathione peroxidase. While, GPx detoxifies H2O2 and organic hydroperoxide (Halliwell and Gutteridge 2015; Di Giulio et al. 2020). In the present experiment, higher SOD, CAT, and GPX activities with lower MDA concentrations of L. vannamei were displayed in the group fed with sanacore diets. Similar reports have shown that dietary supplementation of yeast cells has higher SOD, CAT, and GPX activities capacity in the hemolymph of white leg shrimp (Yang et al. 2010; Ayiku et al. 2020) and Chinese mitten crab (Eriocheir sinensis) (Zhang et al. 2019). Additionally, a significant diminish of MDA levels was reported in freshwater prawns, Macrobrachium rosenbergii fed probiotic, Clostridium butyricum (Wangari et al. 2021). Our data pointed to the robust antioxidant activities of L. vannamei mediated by S. cerevisiae extract which possesses bioactive substances, such as protein, amino acids, polysaccharides, β-vitamins, fatty acids, and carotenoids (Zhang et al. 2019; Ayiku et al. 2020). Rahardjo et al. (2022) investigated the potential application of the extracts of bitter ginger (Zingiber zerumbet) and hairy eggplant (Solanum ferox) as phytobiotic agent supplementation on whiteleg shrimp (Litopenaeus vannamei). They observed a significant improvement in growth performance, survival, SGR, and FCR values. Notably, using a dose of 100 ml/l, the total number of hemocytes and the number of hyaline hemocytes were considerably increased compared to the control treatment. They concluded that supplementing 100 ml/l of S. ferox and Z. zerumbet could enhance the production performance and hemato-immunological parameters of whiteleg shrimp. These findings suggest the potential for developing phytobiotic-based commercial diets for shrimp.
The intestine is a vital organ for growth, nutrient breakdown, and absorption, with protection against pathogenic microorganisms (Zhang et al. 2019). The present study revealed that the beneficial probiotic bacteria were significantly increased along with a decrease of total enteric bacteria and Clostridium sp. in the intestine of the shrimp-fed diets containing sanacore (0.3%). This agreed well with the finding of Adel et al. (2017) who reported that L. vannamei fed probiotic, Pediococcus pentosaceus as Bacillus sp. counts were increased, meanwhile pathogenic Vibrio sp. counts were diminished. Moreover, yeast culture remarkably increased the beneficial probiotic bacterial sp. and reduced the colonization of Vibrio sp. in the L. vannamei intestine (Ayiku et al. 2020). This could be due to sanacore having bactericidal and quorum-quenching factors, which is a principle strategy in the recent biosecurity measures to prevent the risk of transmission of diseases. Moreover, a healthy gut microbiota indicates overall health status and immune defenses, which may illustrate the positive effect of gut modulators on prohibiting the impact of different pathogens.
Conclusion
Dietary sanacore could exhibit a profitable effect on the growth performance, antioxidant capacity, and immunity of L. vannamei through a notable enhancement in THC, phagocytosis, lysozyme, and phenoloxidase activities. In addition, intestinal microbial counts influence the intestinal bacterial composition, and the health condition of L. vannamei shrimp was positively influenced in response to sanacore supplementation. There was also an improvement in the resistance of the shrimp to the F. solani challenge. Thus, sanacore can be used as a feed supplement in the L. vannamei diet, providing more insights into its application in aquaculture as a functional immunostimulant feed additive.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Abbas EM, Al-Souti AS, Sharawy ZZ, El-Haroun E, Ashour M (2023) Impact of dietary administration of seaweed polysaccharide on growth, microbial abundance, and growth and immune-related genes expression of the Pacific whiteleg shrimp Litopenaeus vannamei. Life 13:344
Abbas EM, Ali FS, Desouky MG, Ashour M, El-Shafei A et al (2020) Novel comprehensive molecular and ecological study introducing coastal mud shrimp (Solenocera crassicornis) recorded at the Gulf of Suez Eqypt. J Mar Sci Eng 9:9. https://doi.org/10.3390/jmse9010009
Abdelrhman AM, Ashour M, Al-Zahaby MA, Sharawy ZZ, Nazmi H et al (2022) Effect of polysaccharides derived from brown macroalgae Sargassum dentifolium on growth performance, serum biochemical, digestive histology and enzyme activity of hybrid red tilapia. Aquacult Rep 25:101212
Abo-Taleb HA, Zeina AF, Ashour M, Mabrouk MM, Sallam AE et al (2020) Isolation and cultivation of the freshwater amphipod Gammarus pulex (Linnaeus, 1758), with an evaluation of its chemical and nutritional content. Egyp J Aquatic Biol Fisheries 24:69–82. https://doi.org/10.21608/EJABF.2020.78232
Adel M, Yeganeh S, Dawood M, Safari R, Radhakrishnan S (2017) Effects of Pediococcus pentosaceus supplementation on growth performance, intestinal microflora and disease resistance of white shrimp. Litopenaeus vannamei Aquacult Nutr 23:1401–1409
Aebi H (1984) Catalase in vitro, Methods in enzymology. Elsevier
Al-Musalam L, Al-Ameeri A, Saheb A, Al-Yaqout A (2014) Effect of herbal feed additive on the growth, survival and immune response of green tiger prawn Penaeus semisulcatus. Pak J Nutr 13:366
Amparyup P, Sutthangkul J, Charoensapsri W, Tassanakajon A (2012) Pattern recognition protein binds to lipopolysaccharide and β-1, 3-glucan and activates shrimp prophenoloxidase system. J Biol Chem 287:10060–10069
APHA (2005) Standard methods for the examination of water and wastewater. American Public Health Association (APHA), Washington, DC, USA
APHA (2012) Standard methods for the examination of water and wastewater, 22nd edn. APHA-AWWA-WEF, Washington DC
Ayiku S, Shen J-F, Tan B-P, Dong X-H, Liu H-Y (2020) Effects of dietary yeast culture on shrimp growth, immune response, intestinal health and disease resistance against Vibrio harveyi. Fish Shellfish Immunol 102:286–295
Blaxhall P, Daisley K (1973) Routine haematological methods for use with fish blood. J Fish Biol 5:771–781
Cerenius L, Lee BL, Söderhäll K (2008) The proPO-system: pros and cons for its role in invertebrate immunity. Trends Immunol 29:263–271
Chakraborty SB, Horn P, Hancz C (2014) Application of phytochemicals as growth-promoters and endocrine modulators in fish culture. Rev Aquac 6:1–19
Chuchird N, Niyamosatha H, Rairat T, Keetanon A (2017) Effect of dietary phytobiotics products on growth, immune responses and vibriosis resistance in Litopenaeus vannamei. J Fish Aquat Sci 12:184–190
Ciji A, Akhtar MS (2021) Stress management in aquaculture: a review of dietary interventions. Rev Aquac 13:2190–2247
Coutteau P (2010) Application of novel feed additives based on botanical extracts to improve productivity and economics in aquaculture. Avances en Nutrición Acuicola
Di Giulio RT, Benson WH, Sanders BM, Van Veld PA (2020) Biochemical mechanisms: metabolism, adaptation, and toxicity. In: Fundamentals of aquatic toxicology. CRC Press, pp 523–561
Eissa E-SH, Ahmed RA, Abd Elghany NA, Elfeky A, Saadony S et al (2023) Potential symbiotic effects of β-1, 3 glucan, and fructooligosaccharides on the growth performance, immune response, redox status, and resistance of Pacific white shrimp, Litopenaeus vannamei to Fusarium solani infection. Fishes 8:105
Eissa EH, Che-Zulkifli C, El-Badawi AA, Ali MAM, Baghdady ES et al (2021) Growth-promoting and immunomodulatory impacts of commercial stimulants on kuruma shrimp, Penaeus japonicus (Bate, 1888) juveniles. Egypt J Aquat Biol Fisheries 25:607–617
Eissa EH, Ahmed NH, El-Badawi AA, Munir MB, Abd Al-Kareem OM et al (2022) Assessing the influence of the inclusion of Bacillus subtilis AQUA-GROW® as feed additive on the growth performance, feed utilization, immunological responses and body composition of the Pacific white shrimp. Litopenaeus vannamei Aquacult Res 53:6606–6615
Emerenciano MG, Rombenso AN, Vieira FDN, Martins MA, Coman GJ et al (2022) Intensification of penaeid shrimp culture: an applied review of advances in production systems, nutrition and breeding. Animals 12:236
Engstad RE, Robertson B, Frivold E (1992) Yeast glucan induces increase in lysozyme and complement-mediated haemolytic activity in atlantic salmon blood. Fish Shellfish Immunol 2(4):287–297. https://doi.org/10.1016/S1050-4648(06)80033-1
FAO (2020) The state of world fisheries and aquaculture 2020: sustainability in action; The State of World Fisheries and Aquaculture (SOFIA). Rome, Italy, FAO ISBN 978-92-5-132692-3
FAO (2023) Global farmed shrimp production increased in 2022 despite low demand. GLOBEFISH Food and Agriculture Organization of the United Nations
Febrianti D, Yuhana M (2016) Dietary synbiotic microcapsule influence the immune responses, growth performance and microbial populations to white spot syndrome virus in pacific white shrimp (Litopenaeus vannamei). J Fish Aquat Sci 11:28
Halliwell B, Gutteridge JM (2015) Free radicals in biology and medicine. Oxford university pr ess, USA
Hendam BM, Munir MB, Eissa MEH, El-Haroun E, van Doan H, Chung TH, Eissa ESH (2023) Effects of water additive probiotic, Pediococcus acidilactici on growth performance, feed utilization, hematology, gene expression and disease resistance against Aspergillus flavus of Nile tilapia (Oreochromis niloticus). Anim Feed Sci Technol 303:115696. https://doi.org/10.1016/j.anifeedsci.2023.115696
Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, Calder PC, Sanders ME (2014) Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol 11(8):506–514. https://doi.org/10.1038/nrgastro.2014.66
Hoseinifar SH, Sun YZ, Wang A, Zhou Z (2018) Probiotics as means of diseases control in aquaculture, a review of current knowledge and future perspectives. Front Microbiol 9:2429. https://doi.org/10.3389/fmicb.2018.02429
Ishikawa Y (1968) A fungus caused black gill condition in cultured kuruma prawn. Fish Pathol 3:34–49
Islam T, Hossain MI, Alam MM, Khalil SMI, Rahman MM et al (2020) Status of shrimp diseases and their management practices at Satkhira in Bangladesh. J Entomol Zool Stud 8:1017–1026
Itami T, Takahashi Y, Tsuchihira E, Igusa H (1992) Enhancement of disease resistance of Kumura prawn Penaeus japonicus and increase in phagocytic activity of prawn hemocytes by oral administration of peptidoglycan and b-1, 3-glucan. Abstract only, Third Annual Asia Fisheries Symposium, Singapore
Jasmanindar Y, Sukenda S, Zairin M Jr, Alimuddin A, Utomo NB (2018) Dietary administration of Gracilaria verrucosa extract on Litopenaeus vannamei immune response, growth, and resistance to Vibrio harveyi. Aquacult Aquar , Conserv Legislat 11:1069–1080
Kaizu A, Fagutao FF, Kondo H, Aoki T, Hirono I (2011) Functional analysis of C-type lysozyme in penaeid shrimp. J Biol Chem 286:44344–44349. https://doi.org/10.1074/jbc.M111.292672
Karthikeyan V, Selvakumar P, Gopalakrishnan A (2015) A novel report of fungal pathogen Aspergillus awamori causing black gill infection on Litopenaeus vannamei (Pacific white shrimp). Aquaculture 444:36–40
Kesselring J, Gruber C, Standen B, Wein S (2021) Effect of a phytogenic feed additive on the growth performance and immunity of Pacific white leg shrimp, Litopenaeus vannamei, fed a low fishmeal diet. J World Aquacult Soc 52:303–315
Khoa L, Hatai K, Aoki T (2004) Fusarium incarnatum isolated from black tiger shrimp, Penaeus monodon Fabricius, with black gill disease cultured in Vietnam. J Fish Dis 27:507–515
Kim S-K, Guo Q, Jang I-K (2015) Effect of biofloc on the survival and growth of the postlarvae of three penaeids (Litopenaeus vannamei, Fenneropenaeus chinensis, and Marsupenaeus japonicus) and their biofloc feeding efficiencies, as related to the morphological structure of the third maxilliped. J Crustac Biol 35:41–50
Kuo H-W, Chang C-C, Cheng W (2021) Synbiotic combination of prebiotic, cacao pod husk pectin and probiotic, Lactobacillus plantarum, improve the immunocompetence and growth of Litopenaeus vannamei. Fish Shellfish Immunol 118:333–342
Le VK, Hatai K, Yuasa A, Sawada K (2005) Morphology and molecular phylogeny of Fusarium solani isolated from kuruma prawn Penaeus japonicus with black gills. Fish Pathology 40:103–109
Lee, M., 1995. Official methods of analysis of AOAC International (16th): Patricia A. Cunniff, AOAC International, 1995. $359.00 (North America)/$399.00 (elsewhere)(xxvi+ 1899 pages) ISBN 0 935 584 54 4. Elsevier.
Lem A, Bjørndal T, Lappo A (2014) Economic analysis of supply and demand for food up to 2030 Special focus on fish and fishery products. FAO, FAO
Lin J-M, Ge H, Lin K-B, Yang Z-W, Zhou C et al (2020) Isolation, identification and antibiotic sensitivity analysis of bacterial pathogen from Litopenaeus vannamei with black gill disease. Biotechnol Bull 36:120
Ly MA, Liou C-H, Cheng A-C (2019) Dietary potassium diformate (Formi) supplementation on juvenile white shrimp (Litopenaeus vannamei) diets for growth and survival support. J Appl Biosci 134:13722–13729
Mabrouk MM, Ashour M, Labena A, Zaki MAA, Abdelhamid AF et al (2022) Nanoparticles of Arthrospira platensis improves growth, antioxidative and immunological responses of Nile tilapia ( Oreochromis niloticus) and its resistance to Aeromonas hydrophila. Aquac Res 53:125–135. https://doi.org/10.1111/are.15558
Mansour AT, Ashry OA, Ashour M, Alsaqufi AS, Ramadan KM et al (2022a) The optimization of dietary protein level and carbon sources on biofloc nutritive values, bacterial abundance, and growth performances of whiteleg shrimp (Litopenaeus vannamei) juveniles. Life 12:888
Mansour AT, Ashour M, Abbas EM, Alsaqufi AS, Kelany MS et al (2022b) Growth performance, immune-related and antioxidant genes expression, and gut bacterial abundance of Pacific white leg shrimp, Litopenaeus vannamei, dietary supplemented with natural astaxanthin. Front Physiol 13. https://doi.org/10.3389/fphys.2022.874172
McCord JM, Fridovich I (1969) Superoxide dismutase: an enzymic function for erythrocuprein (Hemocuprein). J Biol Chem 244:6049–6055
Nonwachai T, Purivirojkul W, Limsuwan C, Chuchird N, Velasco M et al (2010) Growth, nonspecific immune characteristics, and survival upon challenge with Vibrio harveyi in Pacific white shrimp (Litopenaeus vannamei) raised on diets containing algal meal. Fish Shellfish Immunol 29:298–304
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70:158–169
Palmero D, Iglesias C, De Cara M, Lomas T, Santos M et al (2009) Species of Fusarium isolated from river and sea water of southeastern Spain and pathogenicity on four plant species. Plant Dis 93:377–385
Patra AK, Amasheh S, Aschenbach JR (2019) Modulation of gastrointestinal barrier and nutrient transport function in farm animals by natural plant bioactive compounds–a comprehensive review. Crit Rev Food Sci Nutr 59:3237–3266
Peterson BC, Bosworth BG, Li MH, Beltran R, Santos GA (2014) Assessment of a phytogenic feed additive (Digestarom PEP MGE) on growth performance, processing yield, fillet composition, and survival of channel catfish. J World Aquacult Soc 45:206–212
Piñeiro P, Ferreiro UV, Calvar NE, Leal JM (2013) New additives and ingredients in the formulation of aquafeeds. Centro Tecnológico del Mar Fundación CETMAR Special Report
Rahardjo S, Vauza MAT, Rukmono D, Wiradana PA (2022) Supplementation of hairy eggplant (Solanum ferox) and bitter ginger (Zingiber zerumbet) extracts as phytobiotic agents on whiteleg shrimp (Litopenaeus vannamei). J Adv Vet Anim Res 9:78
Ringø E (2020) Probiotics in shellfish aquaculture. Aquacult Fisheries 5:1–27
Rodrigues V, Colen R, Ribeiro L, Santos G, Gonçalves RA et al (2018) Effect of dietary essential oils supplementation on growth performance, nutrient utilization, and protein digestibility of juvenile gilthead seabream Fed a Low-Fishmeal Diet. J World Aquacult Soc 49:676–685
Roy S, Bossier P, Norouzitallab P, Vanrompay D (2020) Trained immunity and perspectives for shrimp aquaculture. Rev Aquac 12:2351–2370
Samocha TM (2019) Sustainable biofloc systems for marine shrimp. Academic Press, Academic Press
Sanders ME (2008) Probiotics: definition, sources, selection, and uses. Clin Infect Dis 46:S58–S61
Sarlin PA, Philip R (2011) Efficacy of marine yeasts and baker's yeast as immunostimulants in Fenneropenaeus indicus: a comparative study. Aquaculture 321:173–178
Sharawy ZZ, Ashour M, Labena A, Mansour AT, Abbas EM (2022) Effects of dietary Arthrospira platensis nanoparticles on growth performance, feed utilization, and growth-related gene expression of Pacific white shrimp. Litopenaeus vannamei. Aquaculture 551:737905. https://doi.org/10.1016/j.aquaculture.2022.737905
Sheikhzadeh N, Heidarieh M, Pashaki AK, Nofouzi K, Farshbafi MA et al (2012) Hilyses®, fermented Saccharomyces cerevisiae, enhances the growth performance and skin non-specific immune parameters in rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol 32:1083–1087
Söderhäll I (2016) Crustacean hematopoiesis. Dev Comp Immunol 58:129–141
Song Y-L, Hsieh Y-T (1994) Immunostimulation of tiger shrimp (Penaeus monodon) hemocytes for generation of microbicidal substances: analysis of reactive oxygen species. Dev Comp Immunol 18:201–209
Supamattaya K, Pongmaneerat J, Klowklieng T (2000) The effect of β–glucan (MacroGard®) on growth performance, immune response and disease resistance in black tiger shrimp, Penaeus monodon Fabricius. Songklanakarin J Sci Technol 22:677–688
Tran L, Phuc NH, Oanh HB, Trang DN, Wu M et al (2015) Experimental challenge show the potential of functional feed additives for EMS/AHPND prevention. Aqua Culture Asia Pacific 11:38–40
Tzuc JT, Escalante DR, Rojas Herrera R, Gaxiola Cortés G, Ortiz MLA (2014) Microbiota from Litopenaeus vannamei: digestive tract microbial community of Pacific white shrimp (Litopenaeus vannamei). SpringerPlus 3:1–10
Van Hai N (2015) The use of medicinal plants as immunostimulants in aquaculture: a review. Aquaculture 446:88–96
Vargas-Albores F, Porchas-Cornejo MA, Martínez-Porchas M, Villalpando-Canchola E, Gollas-Galván T et al (2017) Bacterial biota of shrimp intestine is significantly modified by the use of a probiotic mixture: a high throughput sequencing approach. Helgol Mar Res 71:1–10
Wangari MR, Gao Q, Sun C, Liu B, Song C et al (2021) Effect of dietary Clostridium butyricum and different feeding patterns on growth performance, antioxidant and immune capacity in freshwater prawn (Macrobrachium rosenbergii). Aquac Res 52:12–22
Xiong J, Jin M, Yuan Y, Luo JX, Lu Y et al (2018) Dietary nucleotide-rich yeast supplementation improves growth, innate immunity and intestinal morphology of Pacific white shrimp (Litopenaeus vannamei). Aquac Nutr 24:1425–1435
Yang S-P, Wu Z-H, Jian J-C, Zhang X-Z (2010) Effect of marine red yeast Rhodosporidium paludigenum on growth and antioxidant competence of Litopenaeus vannamei. Aquaculture 309:62–65
Yao L, Wang C, Li G, Xie G, Jia Y et al (2022) Identification of Fusarium solani as a causal agent of black spot disease (BSD) of Pacific white shrimp. Penaeus vannamei Aquacult 548:737602
Zhang R, Jiang Y, Zhou L, Chen Y, Wen C et al (2019) Effects of dietary yeast extract supplementation on growth, body composition, non-specific immunity, and antioxidant status of Chinese mitten crab (Eriocheir sinensis). Fish Shellfish Immunol 86:1019–1025
Zou Y, Xie G, Jia T, Xu T, Wang C et al (2020) Determination of the infectious agent of translucent post-larva disease (TPD) in Penaeus vannamei. Pathogens 9:741
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
El-Sayed Hemdan Eissa and Moaheda E. Hemdan: Conceptualization, Methodology, Investigation and Follow-up publication. Samia Elbahnaswy: Methodology, Writing-original draft, Formal analysis, Writing-review & editing, and Follow-up publication. Amira Hussein El-Baz and Hoda Kabary: Microbiology and Writing-original draft. Ehab El-Haroun, Mohamed Ashour, and Abdallah Tageldein Mansour: Writing, Review, Editing, Response to reviewer comments, and Follow-up publication. Saadea Saadony, Adel F. El-Saeed, Samyah D. Jastaniah, Bothaina A. Alaidaroo, Manal E. Shafi, Mohamed E. Abd El-Hack, and Elsayed A.A. Eldessouki: Conceptualization, Methodology, and Investigation.
Corresponding authors
Ethics declarations
Ethical approval
All protocols were approved and conducted following the Scientific Research Ethics Committee of the Faculty of Agriculture, Suez Canal University following the general guidelines of the Canadian Council on Animal Care (reference no. 70/2022).
Conflict of interest
The authors declare no competing interests.
Additional information
Handling editor: Amany Abbass
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Eissa, ES.H., Elbahnaswy, S., El-Baz, A.H. et al. Effects of dietary commercial phytobiotic “Sanacore® GM” on Pacific white shrimp (Litopenaeus vannamei) growth, immune response, redux status, intestinal health, and disease resistance against Fusarium solani. Aquacult Int 32, 3041–3060 (2024). https://doi.org/10.1007/s10499-023-01310-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-023-01310-5