Abstract
The primary goal of this trial was to examine how Paulownia leaf extract (PLE) dietary supplementation affected performance, feed utilization and efficiency, redox status, immunological responses, intestinal microbial content, and disease resistance of Oreochromis niloticus. Nile tilapia fry (0.52 ± 0.19 g) were fed four formulated diets enriched with graded amounts of PLE at 0.0, 0.25, 0.5, and 1 g kg−1 diet, respectively, for 60 days. At the end of the feeding trial, fish were intraperitoneal injected with Aeromonas hydrophila then the survival percentage was recorded daily and calculated mortality rate in all treated groups for 14 days. The performance findings showed that enriched tilapia diets with modest levels of PLE (0.25 or 0.5 g kg−1) substantially enhanced performance, consumed feed, feed efficiency, and feed utilization parameters compared to the control and other treatment groups, whereas the total serum protein and globulin concentrations were significantly (P < 0.001) higher in the fish provided a small quantity of PLE (2.5 g kg-1 diet) than in the control group. Compared to other treatment groups, all estimated liver enzymes and kidney function indicators improved significantly (P < 0.001) at PLE0.5, followed by PLE2.5, whereas the digestive enzymes’ activity increased significantly (P < 0.001) with increasing PLE level up to 0.5 g/kg diet compared to the un-supplemented group. In the same context, enriched tilapia diets with 0.5 g PLE/kg significantly (P < 0.001) boosted redox status and innate immune parameters as compared to other groups. Furthermore, increased PL inclusion levels in tilapia diets significantly (P < 0.05) increased crude protein and ash contents while decreasing fat levels in fish flesh. Moreover, fortified tilapia diets with high PLE levels significantly enhanced the survival percentage and diminished the development of all gram-negative intestinal microbial content. Finally, the results strongly recommend enriched tilapia diets with 0.25 or 0.5 g /Kg for promoting performance and health status.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tilapia is a commonly cultivated cichlid African species that is an economically valuable and avid breeder fish species that afford higher-protein quality (Naiel et al. 2023c). Until now, tilapia has been categorized as the world’s second-largest cultivated freshwater species after carp (Ayyat et al. 2020). In Egypt, the production from farmed tilapia (Oreochromis niloticus) recorded a 71.2% of the total freshwater fish production with a total output of 4.2 million tonnes (FAO 2018). As the aquaculture of tilapia has developed worldwide, so have the worries about fish health status and infectious disease treatment and control (Naiel et al. 2023a). Moreover, high stocking rates and low water quality in intensive fish cultivation systems may increase fish vulnerability to various pathogens such as bacteria, viruses, and parasites (Naiel et al. 2020a). Thus, experts are investigating novel techniques to increase fish growth and safety owing to economic benefits and the ongoing risk of pathogens in the farmed fish (Yilmaz 2019; Abdelnour et al. 2023; Naiel et al. 2023b). The aforementioned goals might be met by applying safe feed additives to boost fish performance and enhance redox status and non-specific immunological activities (Paray et al. 2020; Negm et al. 2021; Naiel et al. 2022).
Recently, the incorporation of herbs or their extracts in fish feed has become essential in the aquaculture industry (Tan et al. 2018; Abdelghany et al. 2020; Abd-Elaziz et al. 2023). Remarkably, a higher correlation was found between the herb dietary supplementation in farmed fish and boosting blood biochemical parameters, antioxidant activity, and innate immune response (Acar et al. 2018; Parrino et al. 2019; Zargar et al. 2019). In addition, several recent findings have demonstrated that different herb ethanolic extracts may be used in the treatment or prevention of fish pathogens (Pratheepa and Sukumaran 2014; Sutthi et al. 2020) as well as promote growth and developed feed efficiency in several fish species (Lee et al. 2012; Tan et al. 2017a).
Fodder is an agricultural expression for all forage trees, shrubs, and some plant parts (woody plants with leaves, flowers, fruit, or pods) which could be used as feed stuffs, additives, and supplements in animal diets (Jamala et al. 2013). A variety of benefits include the fact that the fodder trees can be used as a feed additive: (a) sustainability of fodder production throughout the year because of its ability to resist the sudden climatic changes during dry seasons; (b) fodder trees, herbs, and its constituents could boost fish health status owing to a higher level of protein, vitamins, minerals, and bioactive molecules (Stewart et al. 2018). There are numerous fast-growing wood trees such as Paulownia tomentosa, Paulownia fortune, and Paulownia elongata (Bodnár et al. 2014). In several countries, like Korea, America, China, Japan, and Bulgaria, cultivating Paulownia tress became widespread and common (Zhu et al. 1986; Stewart et al. 2018). Many attempts to use Paulownia in animal feed have been recorded due to its relevant nutritional value (Al-Sagheer et al. 2019). In addition, the Paulownia tree could grow faster allowing for the production of large quantities of leafy biomass for feed (Alagawany et al. 2020). Besides the higher nutritional qualities of their leaves, Paulownia trees are well-recognized for their therapeutic properties (Yadav et al. 2013; He et al. 2016).
Schneiderová and Šmejkal (2015) investigated that several active compounds (Flavonoids, lignans, phenolic glycosides, quinones, terpenoids, glycerides, phenolic acids) and other compounds could be isolated from several parts of Paulownia tomentosa (Sakr et al. 2022b). Thus, Paulownia tomentosa was found to be usually used in Chinese herbal medicine (Sakr et al. 2022a). The primary biological benefits (including antioxidant, antibacterial, and antiphlogistic activities) of PLE inclusion into animal feed might be attributed to higher thymol levels in their extract, which accounted for 82.37% of total bioactive compounds identified in PLE (He et al. 2016). Thus, determining the optimal desired amount of PLE inclusion within the fish feed is critical since phytogenic chemicals such as limonene and thymol have been proven to have growth-promoting effects (Abd El-Naby et al. 2020). In addition, there has not been any experimental work done on the potential role of Paulownia tomentosa leaf extract in promoting the performance and health of Oreochromis niloticus. Thus, the aim of this study was to evaluate the potential influences of Paulownia tomentosa leaf extract on performance, feed efficiency, serum biochemical, antioxidant status, intestinal microbial content, the innate immune response, and disease resistance against Aeromonas hydrophila infection of O. niloticus.
Materials and methods
Collecting Paulownia leaves and extract procedure
Fresh leaves from the Paulownia tomentosa plant were collected from one hundred trees belonging to the Egyptian Association for Wood Trees and Environmental Protection farm located at Bani Salama Village, Wadi El Natrun, Beheira Governorate, Egypt (N 30° 14′ E ′26°). According to the Panase et al. (2018) method, the collected leaves were washed twice with water to remove any dirt and then steeped for 20 min in a 10% Clorox solution. The leaves were washed again, cut into tiny parts, and then left for air-dried under sterile conditions. The extraction method followed Sutthi et al. (2020) procedure with a small modification. Briefly, the small pieces of leaves were weighed and then blended with a 70% ethanol solution in the ratio of 1:2 (weight per volume) in 1000-mL dark flasks. At room temperature, the prepared mixture was shaken for 24 h using an automatic shaker. The obtained mixture was filtered using a sterile muslin cloth. The obtained solvent was then evaporated using a rotary evaporator at 65 °C and then determined the dry weight was using a freeze-drying method described by Harikrishnan et al. (2009). The remains powder was stored at − 20 °C until use.
Preparing diet and experimental design
Apparently, three hundred healthy Nile tilapia (Oreochromis niloticus) fry (weighed, 0.52 ± 0.19 g) were purchased from a governmental fish hatchery pertinence to the Central Laboratory for Aquaculture Research, Abassa, Abu Hammad, Sharkia, Egypt. Prior to transporting process, all fry were clinically examined and observed no abnormality in the external appearance. The polyethylene bags were used for transporting the live fish fry after being filled with one-third dechlorinated water and fortified with two-thirds oxygen pure. The fish were acclimated to laboratory conditions for 2 weeks. All experimental fish were randomly allocated to equal four groups assigned in five aquariums (15 fish per aquarium; 40 × 65 × 35 cm) and enriched with dechlorinated tap water. The photoperiod duration was 12-h light:12-h dark. The Paulownia leaf extract experimental groups were specified as 0, 0.25, 0.5, and 1 g PLE kg−1 diets. During the feeding trial, the fish were fed ad libitum. Daily, the aquariums were siphoned to remove the solid wastes and replenish them with fresh water. All water quality measurements were determined to be within the suitable ranges in terms of the water temperature range at 26 ± 1 °C, pH 7.8–8.3, dissolved oxygen 7.5–8.2 mg L−1, salinity 2 ppt., and total NH4-N 0.22 mg L−1. The examined diets were prepared to contain 0.0, 0.25, 0.5, and 1 g PLE kg−1 diet. The components and the analysis of the tested diet are presented in Table 1. All ingredients were blended with oil, and then water was added until a solid dough was obtained. Using a mincer, each separate diet was extruded. The performed layers were air-dried, broken, pelletized, and stored at 4 °C in plastic bags till use.
Gas chromatograph-mass analysis (GC–MS) for Paulownia leaf extract
GC–MS analysis of the ethanol extract of Paulownia was accomplished using a Perkin–Elmer GC Clarus 500 system comprising an AOC-20i auto-sampler and a gas chromatograph interfaced to a mass spectrometer (GC–MS) linked with an Elite-5MS (5% diphenyl/95% dimethyl polysiloxane) fused a capillary column (30 × 0.25 μm ID × 0.25 μm df). Helium gas (99.999%) was applied as a transporter gas at a stable flow rate of 1 ml/min, and an injection capacity of 2 μl was directed (10:1 ratio). The MS detection followed the Ezhilan and Neelamegam (2012) protocol. The relative percentage amount of each constituent was estimated by comparing its average peak area to the total areas. The Turbo-Mass Gold-Perkin-Elmer software was used as a mass-detector in this analysis. The spectrum of the unknown molecules was associated with the spectrum of known compounds listed in the National Institute Standard and Technology (NIST) library. The name, molecular weight, and structure of the main components of the tested solvent are identified and presented in Table 2.
Performance and feed efficiency
Throughout the feeding experiment, the consecutive live body weight was recorded biweekly, and the fish were fed twice a day (9.00 and 13.00 h) until apparent satiation. Also, growth performance, feed efficiency, and survival rate were estimated using the following formula:
Aeromonas hydrophila challenge test
The A. hydrophila strain was obtained from the Department of Plant Microbiology, Faculty of Agriculture, Zagazig University, Zagazig, Egypt. The identification of A. hydrophila strain was previously demonstrated based on colonial appearance (creamy color, glist convex), microscopic inspection of stained smears (Gram negative, non-sporulated, and short rod-shaped), and biochemical investigation, according to the technique described by Assane et al. (2021). The pathogenic bacteria were cultivated under 25 °C for 24 h using Tryptic Soy Broth (TSB, Difco, USA). Then, the prepared broth was centrifuged at 3000 g for 10 min. The obtained pellets were washed using phosphate-buffered saline (pH 7.4, each liter consisting of 80 g NaCl, 2 g KCl, 14.4 g Na2HPO4 2H2O, 2.4 g KH2PO4, and 800 mL distilled water) twice and the remaining supernatant was thrown. Following the Bailone et al. (2010) procedure, the solution optical density (OD) was caliper to 0.5 at 456 nm, which parallels 1 × 107 cells ml−1. The fish (10 fish per aquarium) were injected intraperitoneally with 0.1 ml of A. hydrophila prepared solution at the end of the feeding trial (Zahran et al. 2018). Fish were continued on the same feeding regime during this post-challenge period (14 days), and mortality was estimated and recorded in all fish groups daily. The relative percentage survival (RPS) had been estimated using recorded mortality following Amend (1981) equation: RPS = 1 − [(Mortality percentage in the treated group) / (Mortality percentage in the control group)] × 100.
Analysis of diets and fish samples
Fish and diet samples have been analyzed following the A.O.A.C (2005) standard procedures to estimate moisture, crude protein, fat, dry matter, and ash levels. The nitrogen-free extract (NFE) was computed as follows:
NFE (g kg−1) = 1000 − (crude protein + crude lipids + ash + crude fiber).
Also, the gross energy (GE, Kcal/g) was assessed by NFE, fat, and protein factors, which were 4.11, 9.44, and 5.64 kcal/g, respectively, as shown in Table 1. All analyses are measured in five replicates.
Sample collection (blood, mucus, and tissues)
All sampled fish (five fish from each treated group) were anesthetized using 120 mg L−1 aminobenzoic acid (Sigma–Aldrich). Then, the blood samples were collected from the caudal vein using sterile synergy. The obtained serum was centrifuged at 3000 rpm for 15 min at 4 °C and then kept under − 20 °C until the biochemical, digestive enzyme, and immune activity analyses were applied. The fish was carefully placed in position, and the swab was softly slid along the body from head to tail, just above the lateral line, to collect mucus samples and avoid skin damage. After that, the mucus sample is collected within a sterile Eppendorf tube and stored at − 20 °C till analysis. Decapitation was employed to kill the same fish used for blood sampling. Eviscerated liver and intestine tissues were washed with ice-cold physiological saline solution (0.59% NaCl) and kept at − 85 °C until antioxidant activity and intestinal microbial content were assessed.
Blood biochemical analysis
Colorimetric techniques were applied for all serum biochemical measurements. According to Sundeman (1964) procedure, serum total protein and albumin levels were determined. In addition, globulin contents were calculated by subtracting albumin values from total protein levels in serum. As ascribed by Reitman and Frankel (1957) and Henry (1964) protocols, the aspartic aminotransferase (AST), alanine aminotransferase (ALT), uric acid, and creatinine were estimated using commercial kits (Diamond Diagnostic Co. for Modern Laboratory Chemicals, Egypt).
The liver glutathione peroxidase (GPX) level (µg/tissue) was estimated spectrophotometrically following Photometer 5010, BM Co., Germany, commercial kit instruments, as stated by Ismail (2019). while the lipid peroxidase contents (U/L) were assessed by measurement malondialdehyde (MDA) activities according to the spectrophotometric method ascribed by Uchiyama and Mihara (1978). The catalase (CAT) level was measured by the absorption of hydrogen peroxide reduction at 240 nm, as illustrated by Aebi (1984).
Mucus innate immune assay
The serum complement C3 activity contents were determined using the procedure reported by Sunyer and Tort (1995). Also, the polarographic analysis was used to measure serum hydrogen peroxide levels. The enzymatic process was inhibited by the injection of 1.0 ml of 8.5 mol/l 3-amino-1,2,4-triazole. Polarographic analysis was performed to determine the hydrogen peroxide content of the reaction mixture, which was then calculated as ascribed by Clark and Leland (1979).
Serum lysozyme activity was performed as described by Zahran et al. (2018) based on the lysis method of Micrococcus luteus (Sigma Chemical Co). A 0.25 ml of blood sera was mingled with 0.75 ml Micrococcus luteus suspension (0.2 mg/mL in 0.05 M PBS, pH 6.2). The mixture interaction was measured using a spectrophotometer (BM Co. Germany) to test optical density (OD) at 540 nm after 5 min of incubation below 25 °C. Each enzyme activity unit was defined as the enzyme quantity that impedes a decrease of 0.001 in absorbance. The obtained serum lysozyme unit was presented in (U/L), a stander curve made with lyophilized hen egg-white lysozyme (Sigma-Aldrich).
The intestinal microbial content analysis
The same fish used for collecting blood samples were dissected to obtain intestine samples. The distal intestine was carefully aseptically excised from each fish specimen (using sterile tools) and homogenized in 10 mL of 3% sterile sodium chloride solution. Briefly, ten-time dilutions of the stock samples were carried out to obtain serially diluted samples from 10−1 to 10−5. The bacteria population was determined by assays of growth on plated selective agar media, by taking 1 ml from the last dilution. The total count of viable bacteria (TBC) was determined by using a nutrient agar medium (Zhang et al. 2010), whereas Bacillus spp. was determined by using MEYP (Mannitol Egg Yolk Polymyxin Agar) (Fritze and Claus 2003). For Escherichia coli, modified fecal coliform (mFC) agar was used (ISO-International Organization for Standardization) No. 9308/1, 1990). While, MacConkey Agar medium (M081B-100G, Hi-Media Lab., India) was used for the enumeration Coliform bacteria (Feng et al. 2002). In addition, Tryptic Soy Broth (TSB, Difco, USA) was applied for counting A. hydrophila counts (Kaper et al. 1981). Lastly, Pseudomonas CN Selective Supplement (SR0102, Oxoid Limited, Thermo Fisher Scientific Inc.) medium was used for counting Pseudomonas spp. (Aranega Bou 2017). Incubation of the plates was carried out at 30 °C for 24–48 h for enumeration, except for the mFC medium, which was incubated at 44 °C for 24 h. The tools were cleaned and sterilized between individual fish specimens. All samples were in five replicates for analysis.
Data analysis
All collected data were tested for normality and homogeneity using Shapiro–Wilk test. All data following the normal distribution curve were statistically analyzed using the one-way analysis of variance (ANOVA). Differences between means were defined statistically at P < 0.05. Tukey’s range test was used to compare means. Likewise, collected data were examined if the significance trends were linear or quadratic using the SPSS software (v.22.0). Data are exhibited as mean ± SE.
Results
Performance
Final live body weight (FW), weight gain (WG), and weight gain percent (WG%) of tilapia is shown in Table 3 as influenced by various level of paulownia leaf extract. FW, WG, and WG% were significantly higher (quadratic, P < 0.01) in the fish group fed 0.25 or 0.5 g PLE-supplemented diets compared to the control. The highest values were recorded in the fish group fed 0.5 g PLE-supplemented diets. The lower feed intake and FCR levels were exhibited in fish fed the 0.5 g PLE diet. The protein efficiency ratio (PER) was quadratically affected by the level of dietary Paulownia leaf extract (quadratic; P = 0.002) (Table 3). Furthermore, the highest PER level was observed in PLE0.5 group followed by PLE0.25 group in comparison with other treatments. Enriched tilapia diets with Paulownia leaf extract had a significantly improving effect on survival rate (SR) (linear response, P = 0.008) (Table 3). The polynomial regression statistical analysis revealed that the highest weight gain was observed at 0.5 g kg−1 PLE and it proved that the optimum range of PLE supplementation in tilapia diets was between 0.25 and 0.5 g kg−1 (Fig. 1).
Serum biochemical indices
Table 4 illustrates the influence of PLE on Nile tilapia biochemical indices including total protein, albumin, globulin, alanine amine transferase (ALT), aspartate amine transferase (AST), creatinine, and ammonia. The red blood cell (RBCs) and white blood cell (WBCs). There was a linear and quadratic response in total protein, globulin, and AST of fish as the level of PLE increased in the diet (linear and quadratic, P˂0.001). The highest values of both total protein and globulin and the lowest AST value were recorded in the tilapia fish group fed a diet supplemented with 0.5 g kg−1 Paulownia leaf extract. The levels of albumin, ALT, creatinine, and ammonia in the blood exhibit a quadratic response (quadratic, P˂0.001), when the PLE level in the diet increases, whereas the lowest ALT, creatinine, and ammonia values were exhibited in the fish group fed enriched diets with 0.5 g kg−1 Paulownia leaf extract in comparison with other treated groups.
Digestive enzymes, antioxidant, and immune status
Significant quadratic responses in digestive enzymes (quadratic, P˂0.001) were observed with the increased levels of Paulownia leaf extract in tilapia diets (Table 5). The highest amylase and lipase concentrations were found in the fish group fed supplemented diets with 0.5 g kg−1 followed by 0.25 g kg−1 Paulownia leaf extract in comparison with other treated groups. Furthermore, there was a linear and quadratic significant reaction in catalase and alkaline phosphatase levels of fish with the increased levels of PLE in fish diets (linear, P = 0.002, P = 0.011; quadratic, P = 0.017, P˂0.001, respectively), while glutathione peroxidase shows a significant quadratic response (quadratic, P˂0.001) (Table 5). The modest dose of Paulownia leaf extract (0.5 g kg−1) verified a significantly improving effect on antioxidant enzyme activity through increasing catalase and glutathione peroxidase levels and decreasing alkaline phosphatase concertation compared with other experimental groups.
Regarding the immunological results, a significant quadratic improvement in lysozyme and complement C3 activity was illustrated in the fish group fed high levels of PLE-supplemented diets (Table 5). The high level of lysozyme and complement C3 were achieved in the fish group that received diets supplemented with 0.5 g kg−1 Paulownia leaf extract. However, the H2O2 activity revealed no significant differences between all experimental groups.
Whole-body chemical analysis
Table 6 shows the whole-body chemical analysis of tilapia fish as altered by various doses of paulownia leaf extract. There was a significant quadratic response in crude protein and crude fat levels with increasing the PLE in fish diets. The high retention level of crude protein and the lowest level of crude fat in tilapia fish flesh were found in both fish group fed diets supplemented with 0.5 or 1 g kg−1 Paulownia leaf extract. The ash values show linear and quadratic significant effects under different levels of Paulownia leaf extract (Table 6). The high ash content was recorded in the fish group fed a diet supplemented with 1 g kg−1 Paulownia leaf extract. On the contrary, moisture and dry matter percentages revealed no significant differences between all experimental groups.
Bacterial challenge test
Data in Table 7 presented the relative protection level of the fish fed diets supplemented with various levels of PLE. The RLP % data demonstrated that high doses of PLE incorporation into fish feed exhibited a higher survival rate following A. hydrophila injection. Specifically, fish offered diets supplemented with 0.5 or 1 g PLE kg−1 had the greatest RLP (100%) and the lowest death rate (0.0%), when compared to other experimental groups.
Intestinal bacterial count
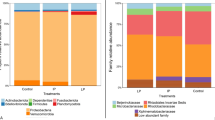
The total bacterial count (TBC) was lower in fish fed diets supplemented with 1 g kg−1 PLE than in fish fed a PLE-free diet (P < 0.05) (Fig. 2). Furthermore, fish treated with any level of PLE exhibited lower Bacillus sp., Pseudomonas sp., and A. hydrophila counts than fish fed basal diets. The lowest counts of Bacillus sp., Pseudomonas sp., and A. hydrophila has been illustrated in fish fed diets supplemented with 1 g kg−1 PLE followed by 0.5 then 0.25 g kg−1 PLE, respectively. In the same context, coliforms sp. and E. coli counts decreased significantly in fish treated with 1 and 0.5 g kg−1 PLE than other fish groups (P < 0.05).
Discussion
Infectious diseases are one of the most dangerous threats to aquaculture sustainability (Naiel et al. 2021b). Thus, discovering novel compounds to promote fish growth and health is of primary importance to fish farmers due to the higher economic losses caused by disease outbreaks in farmed fish (Naiel et al. 2020b). Recently, using herbs or their derivatives has been shown to be more effective in improving fish performance and stimulating antioxidant and non-specific immune responses against infectious diseases (Abd El-Gawad et al. 2020; Khafaga et al. 2020). Several leaf extract for instance oak (Quercus castaneifolia) (Paray et al. 2020) and marjoram (Origanum majorana) (Yousefi et al. 2021a) in common carp, Psidium guajava in tilapia (Gobi et al. 2016), and mistletoe (Viscum album) in rainbow trout (Yousefi et al. 2021b) demonstrated higher fish production and stimulated immunity when used as a feed additive. Despite the pharmacological aspects associated with PL-derived compounds (He et al. 2016), there is at present a lack of knowledge about the beneficial effects and safe application of PL as feed additives in farmed O. niloticus.
The existing trial investigated that enriched tilapia diets with PLE significantly promoted the performance, utilization of consumed feed, and body chemical composition (high crude protein level and lowest fat percentage). The polynomial regression results confirmed our findings that the optimal PLE content in tilapia diets is 0.5 g kg−1 diet. Despite evidence of anti-inflammatory, antioxidant, and antimicrobial properties of Paulownia leaf extract on other fish species (Khademi Hamedi et al. 2019) and animals (WANG et al. 2013; Al-Sagheer et al. 2019; Alagawany et al. 2020), no research has been conducted on the effects of paulownia leaf extract characteristics on tilapia. According to the PLE gas chromatography analysis, the majority of the prenylated flavonoids retrieved from P. tomentosa belong to the C-geranylated group of flavonoids. Thymol was discovered to be the primary bioactive component produced from Paulownia leaves (Schneiderová and Šmejkal 2015). Several recent studies have shown that small doses of thymol in fish diets promote growth and nutrient digestibility (Alagawany et al. 2021). For instance, Amer et al. (2018) investigated that enriched tilapia diets with a low level of thymol essential oil (1 ml kg−1) could promote growth and some blood parameters significantly in comparison with the control and other treated groups. Also, Abd El-Naby et al. (2020) indicated that enriched tilapia diets with 0.5 g thymol kg−1 and nano-chitosan could significantly improve growth and some health indicators of tilapia. The authors proposed that thyme may enhance nutrition metabolism and absorption in the gut, and therefore has the potential to reduce the incidence of high mortality rates and disease outbreaks in fish through antimicrobial properties in the gastrointestinal tract (Sönmez et al. 2015; Zargar et al. 2019).
Moreover, digestive enzymes such as protease, amylase, and lipase are essential for growth and correlated with promoting nutrient metabolism of Nile tilapia (Naiel et al. 2021a). Several studies have previously verified that feeding behaviors and feed supplements influence the distribution and activity of digestive enzymes within the gut (Guerreiro et al. 2021; Naiel et al. 2021b). According to the findings of our experiment, the addition of 0.5 g PLE kg−1 to the tilapia diet significantly increased lipase and amylase activity. In line with our findings, Yuan et al. (2020) exhibited that the total pathogenic bacterial load within the gut may disrupt normal intestinal mucosa structure, resulting in decreased digestive enzyme production. Recent phytochemical investigations proved that Paulownia plants contained several bioactive molecules such as quinones, lignans, triterpenes, phenylpropanoid glycosides, and flavonoids that may be played a vital antimicrobial role (Schneiderová and Šmejkal 2015). In addition, Yuan et al. (2021) investigated the antimicrobial role of Paulownia leaf extract, which could correlate with a high level of flavonoid compounds, especially the C-geranylated group. Other studies have coincided with our results; Khalil et al. (2020) stated that thymol activated the digestive enzyme secretions, enhanced the metabolism of the nutrient, modified the biodiversity of the intestinal microbial content, and reduced the pathogen bacterial counts in tilapia. Thus, the better growth performance in the supplemented fish might be attributed to the stimulating effects of thymol, a major bioactive molecule in PLE, on digestive enzymes, feed utilization, modulating intestinal microbial content, and fish welfare.
Currently, blood biochemical measures may be applied as biological markers to indicate the performance and health of farmed fish (Ismael et al. 2021). In this case, many blood indicators have been assembled that have effectively assisted fish health in a number of previous research, including total serum protein, albumin, globulin, AST, ALT, ammonia, and creatinine (Fazio et al. 2013; El-Rahman et al. 2019). The total serum protein content could be diffracted into two main types of protein, globulins and albumins (Xu et al. 2014). Globulins are a type of synthetic macrophage that play a critical role in immune responses against infections (Kumar et al. 2013), whereas serum AST and ALT values are regarded as significant indicators of cellular damage and hepatic membrane leakage (El-Moghazy et al. 2014). In our experiment, supplemented tilapia diets with 0.5 g PLE kg−1 significantly increased the serum total protein and globulin levels, while the same level of PLE significantly decreased AST, ALT, ammonia, and creatinine concentrations. Decreased serum kidney and liver enzymes of fish fed PLE-enriched diets could clarify the ameliorative protective role of PLE against hepatocytes and kidney disorders induced by contaminated diets, diets containing high fat levels, and ecological stressors (Roncarati et al. 2006). These findings were in consist with Hoseini and Yousefi (2019) findings that the highest thyme extract concentration in the rainbow trout feed diet significantly reduced serum liver enzyme activities, which proved the relation between promoted growth and the health benefits of thymol-derived PLE on fish. Similarly, many research investigated the findings given here (Yılmaz et al. 2012; Khademi Hamedi et al. 2019; Mirghaed et al. 2020).
Recently, using immunostimulants to promote the innate (or nonspecific) immune activities is growing rapidly to avoid pathogen outbreaks in cultivated fish species (Abd El-Naby et al. 2019; El-Hakim et al. 2020). Catalase and glutathione peroxidase are antioxidant enzymes that play a critical role in the antioxidant protection capability of biological systems against cellular damage caused by free radicals (Monteiro et al. 2006). Besides, alkaline phosphatase has been found to play a vital role in the non-specific immune system by detoxifying well-known toxins from internal and external sources (Chandrupatla et al. 2018). Recent in vivo studies have shown that antioxidant supplementation may substantially enhance some immune responses against bacterial and viral infection (Djordjevic et al. 2009; Tan et al. 2017b, 2018). Also, the main components of the non-specific immune system are complement C3 and lysozyme (Magnadóttir 2006). Lysozyme has been defined in invertebrates as an antibacterial protein and a component of the non-specific immune activity that could be isolated from mucus, lymphoid tissue serum, various body fluids, and fish ova (Watts et al. 2001). Furthermore, it is commonly recognized that fish mucosal secretion includes a diverse range of innate immune molecules such as complement proteins, lysozyme, proteases, esterase, and other anti-microbial proteins (Chiu et al. 2008; Biller-Takahashi et al. 2012). The current study indicated that supplemented tilapia diets with 0.5 g PLE kg−1 significantly promoted redox status through stimulating catalase and glutathione peroxidase activities and decreased alkaline phosphatase levels. Moreover, the same supplementation level exhibited higher immune responses by increasing mucus lysozyme and complement activity. The observed findings are consistent with data from earlier research, which show that herbal treatment and its extracts/effective components have a significant impact on activating the immune response in stressed fish and/or infectious diseases. Bıtıs et al. (2010) informed that ethanolic extract of Rosa agrestis leaves contains high levels of flavonoids which promoted the antioxidant activity. Furthermore, dietary supplement with oak (Quercus castaneifolia) leaf ethanolic extract revealed significant increments in plasma catalase, glutathione peroxidase, lysozyme, complement, and reduced malondialdehyde levels in common carp (Cyprinus carpio) (Paray et al. 2020), while Khademi Hamedi et al. (2019) presented that fortified O. mykiss diets with 1% Paulownia extracts significantly improved the non-specific immunity system via increasing lysozyme activity. The leaves and branches of Paulownia tomentosa have a high concentration of flavonoids, making it an ideal choice for stimulating antioxidant and immunological activity (Pontaza-Licona et al. 2019). Thus, the significant increase in antioxidant and non-specific immunological activities in Nile tilapia fed PLE-supplemented diets might be attributed to the immunomodulatory capabilities of bioactive flavonoid molecules.
Intestinal microbial content and the surrounding water environment form an interconnected and highly complicated ecosystem. Thus, the accurate balance between these systems is critical to maintaining the health of fish (Egerton et al. 2018). A variety of variables, including host genetics, feed additives, age, and antibiotics, may alter the gastrointestinal microbial population (Hasan and Yang 2019). It was remarkable to prove that several herbs’ ethanolic extracts for instance Origanum vulgare, Ocimum tenuiflorum, Cymbopogon, Rosmarinus officinalis, thyme, and Aloe vera exhibited strong antibacterial properties against bacterial infections (Aqil et al. 2005; Oskay et al. 2009; Mehrotra et al. 2010). According to our intestinal microbial results, the fish fed a high level of PLE showed strong antibacterial features against gram-negative bacteria, Pseudomonas sp., Bacillus sp., and A. hydrophila. In line with these findings, the fish group fed highest dietary supplementation level of PLE achieved the maximum RPS percentage and low mortality rate. On the same line, Popova and Baykov (2013) investigated that prepared aqueous solution from fresh P. elongata leaves showed higher antibacterial action against gram-negative bacterial strains. A high quantity of isoatriplicolide tiglate (PCAC) molecules may explain the stronger antibacterial activities of P. tomentosa leaves’ extract against gram-positive bacteria (Jung et al. 2012).
In conclusion, fortified tilapia diets with PLE promoted redox status, immune responses, and fish health. Similarly, dietary treatment of PLE may reduce the risk of A. hydrophila infection in tilapia production by boosting redox status and diminishing mortality percentage. Therefore, incorporating PLE into tilapia diets at a level of 0.5 g kg−1 diet may be an effective immunostimulatory agent and might be validated as an efficient method in aquaculture farms for disease outbreak prevention, improved fish welfare, and a sustainable aquaculture sector.
Data availability
Available under reasonable request from the corresponding author.
Code availability
Not applicable.
References
Abd El-Gawad EA, El Asely AM, Soror EI, Abbass AA, Austin B (2020) Effect of dietary Moringa oleifera leaf on the immune response and control of Aeromonas hydrophila infection in Nile tilapia (Oreochromis niloticus) fry. Aquacult Int 28:389–402
Abd El-Naby FSA, Naiel MA, Al-Sagheer AA, Negm SS (2019) Dietary chitosan nanoparticles enhance the growth, production performance, and immunity in Oreochromis niloticus. Aquaculture 501:82–89
Abd El-Naby AS, Al-Sagheer AA, Negm SS, Naiel MA (2020) Dietary combination of chitosan nanoparticle and thymol affects feed utilization, digestive enzymes, antioxidant status, and intestinal morphology of Oreochromis niloticus. Aquaculture 515:734577
Abd-Elaziz RA, Shukry M, Abdel-Latif HM, Saleh RM (2023) Growth-promoting and immunostimulatory effects of phytobiotics as dietary supplements for Pangasianodon hypophthalmus fingerlings. Fish Shellfish Immunol 133:108531
Abdelghany MF, El-Sawy HB, Abd El-hameed SA, Khames MK, Abdel-Latif HM, Naiel MA (2020) Effects of dietary Nannochloropsis oculata on growth performance, serum biochemical parameters, immune responses, and resistance against Aeromonas veronii challenge in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol 107:277–288
Abdelnour SA, Ghazanfar S, Abdel-Hamid M, Abdel-Latif HM, Zhang Z, Naiel MA (2023) Therapeutic uses and applications of bovine lactoferrin in aquatic animal medicine: an overview. Vet Res Commun 1–15. https://doi.org/10.1007/s11259-022-10060-3
Acar Ü, Parrino V, Kesbiç OS, Lo Paro G, Saoca C, Abbate F, Yılmaz S, Fazio F (2018) Effects of different levels of pomegranate seed oil on some blood parameters and disease resistance against Yersinia ruckeri in rainbow trout. Front Physiol 9:596
Aebi H (1984) Catalase in vitro, methods in enzymology. Elsevier, pp 121–126
Alagawany M, Farag MR, Abdelnour SA, Elnesr SS (2021) A review on the beneficial effect of thymol on health and production of fish. Rev Aquac 13:632–641
Alagawany M, Farag MR, Sahfi ME, Elnesr SS, Alqaisi O, El-Kassas S, Al-Wajeeh AS, Taha AE, Abd E-Hack ME (2020) Phytochemical characteristics of Paulownia trees wastes and its use as unconventional feedstuff in animal feed. Anim Biotechnol 33:1–8
Al-Sagheer AA, El-Hack A, Mohamed E, Alagawany M, Naiel MA, Mahgoub SA, Badr MM, Hussein EO, Alowaimer AN, Swelum AA (2019) Paulownia leaves as a new feed resource: chemical composition and effects on growth, carcasses, digestibility, blood biochemistry, and intestinal bacterial populations of growing rabbits. Animals 9:95
Amend DF (1981) Potency testing of fish vaccines. Fish Biologics: Serodiagnostics and Vaccines 49:447–454
Amer SA, Metwally AE, Ahmed SA (2018) The influence of dietary supplementation of cinnamaldehyde and thymol on the growth performance, immunity and antioxidant status of monosex Nile tilapia fingerlings (Oreochromis niloticus). Egypt J Aquat Res 44:251–256
Aqil F, Khan MSA, Owais M, Ahmad I (2005) Effect of certain bioactive plant extracts on clinical isolates of β-lactamase producing methicillin resistant Staphylococcus aureus. J Basic Microbiol: an Int J Biochem, Physiol, Genet, Morphol Ecol Microorganisms 45:106–114
Aranega Bou P (2017) Characterising the microbial communities associated with the water distribution system of a broiler farm and their role in Campylobacter infection. PhD thesis. School of Environment and Life Science, University of Salford, pp 1–314
Assane IM, de Sousa EL, Valladão GMR, Tamashiro GD, Criscoulo-Urbinati E, Hashimoto DT, Pilarski F (2021) Phenotypic and genotypic characterization of Aeromonas jandaei involved in mass mortalities of cultured Nile tilapia, Oreochromis niloticus (L.) in Brazil. Aquaculture 541:736848
Ayyat MS, Ayyat AM, Naiel MA, Al-Sagheer AA (2020) Reversal effects of some safe dietary supplements on lead contaminated diet induced impaired growth and associated parameters in Nile tilapia. Aquaculture 515:734580
Bailone R, Martins M, Mouriño J, Vieira F, Pedrotti F, Nunes G, Silva B (2010) Hematology and agglutination titer after polyvalent immunization and subsequent challenge with Aeromonas hydrophila in Nile tilapia (Oreochromis niloticus). Arch Med Vet 42:221–227
Biller-Takahashi JD, Takahashi LS, Marzocchi-Machado CM, Zanuzzo FS, Sabioni RE, Urbinati EC (2012) Hemolytic activity of alternative complement pathway as an indicator of innate immunity in pacu (Piaractus mesopotamicus). Rev Bras Zootec 41:237–241
Bıtıs L, Kultur S, Melıkoglu G, Ozsoy N, Can A (2010) Flavonoids and antioxidant activity of Rosa agrestis leaves. Nat Prod Res 24:580–589
Bodnár A, Pajor F, Steier J, Kispál T, Póti P (2014) Nutritive value of paulownia (Paulownia spp.) hybrid tree leaves. Hung Agric Res 23:27–32
Chandrupatla DM, Molthoff CF, Ritsema WI, Vos R, Elshof E, Matsuyama T, Low PS, Musters RJ, Hammond A, Windhorst AD (2018) Prophylactic and therapeutic activity of alkaline phosphatase in arthritic rats: single-agent effects of alkaline phosphatase and synergistic effects in combination with methotrexate. Transl Res 199:24–38
Chiu S-T, Tsai R-T, Hsu J-P, Liu C-H, Cheng W (2008) Dietary sodium alginate administration to enhance the non-specific immune responses, and disease resistance of the juvenile grouper Epinephelus fuscoguttatus. Aquaculture 277:66–72
Clark J, Leland C (1979) The hydrogen peroxide sensing platinum anode as an analytical enzyme electrode, Methods in enzymology. Elsevier, pp 448–479 https://doi.org/10.1016/0076-6879(79)56044-3
Djordjevic B, Škugor S, Jørgensen SM, Øverland M, Mydland LT, Krasnov A (2009) Modulation of splenic immune responses to bacterial lipopolysaccharide in rainbow trout (Oncorhynchus mykiss) fed lentinan, a beta-glucan from mushroom Lentinula edodes. Fish Shellfish Immunol 26:201–209
Egerton S, Culloty S, Whooley J, Stanton C, Ross RP (2018) The gut microbiota of marine fish. Front Microbiol 9:873
El-Hakim YMA, El-Houseiny W, AE EL-M, Ebraheim LL, Moustafa AA, Rahman Mohamed AA (2020) Melamine and curcumin enriched diets modulate the haemato-immune response, growth performance, oxidative stress, disease resistance, and cytokine production in Oreochromis niloticus. Aquat Toxicol 220:105406
El-Moghazy M, Zedan NS, El-Atrsh AM, El-Gogary M, Tousson E (2014) The possible effect of diets containing fish oil (omega-3) on hematological, biochemical and histopathogical alterations of rabbit liver and kidney. Biomed Prev Nutr 4:371–377
El-Rahman GIA, Ahmed SA, Khalil AA, Abd-Elhakim YM (2019) Assessment of hematological, hepato-renal, antioxidant, and hormonal responses of Clarias gariepinus exposed to sub-lethal concentrations of oxyfluorfen. Aquat Toxicol 217:105329
Ezhilan BP, Neelamegam R (2012) GC-MS analysis of phytocomponents in the ethanol extract of Polygonum chinense L. Pharmacogn Res 4:11
FAO (2018) Food and agriculture organization of the united nations. In: 2018 Global aquaculture production. Contributing to food security and nutrition for all, Rome, pp 200. https://www.fao.org/3/I9553EN/i9553en.pdf
Fazio F, Marafioti S, Torre A, Sanfilippo M, Panzera M, Faggio C (2013) Haematological and serum protein profiles of Mugil cephalus: effect of two different habitats. Ichthyol Res 60:36–42
Feng P, Weagant SD, Grant MA, Burkhardt W, Shellfish M, Water B (2002) BAM: Enumeration of escherichia coli and the coliform bacteria. In: 1998 Bacteriological analytical manual, 8th edn. Silver Spring, Berlin
Fritze D, Claus D (2003) Media for Bacillus spp. and related genera relevant to foods. Prog Ind Microbiol 37:61–77
Gobi N, Ramya C, Vaseeharan B, Malaikozhundan B, Vijayakumar S, Murugan K, Benelli G (2016) Oreochromis mossambicus diet supplementation with Psidium guajava leaf extracts enhance growth, immune, antioxidant response and resistance to Aeromonas hydrophila. Fish Shellfish Immunol 58:572–583
Guerreiro I, Serra CR, Coutinho F, Couto A, Castro C, Rangel F, Peres H, Pousão-Ferreira P, Matos E, Gasco L (2021) Digestive enzyme activity and nutrient digestibility in meagre (Argyrosomus regius) fed increasing levels of black soldier fly meal (Hermetia illucens). Aquac Nutr 27:142–152
Harikrishnan R, Balasundaram C, Kim M-C, Kim J-S, Han Y-J, Heo M-S (2009) Innate immune response and disease resistance in Carassius auratus by triherbal solvent extracts. Fish Shellfish Immunol 27:508–515
Hasan N, Yang H (2019) Factors affecting the composition of the gut microbiota, and its modulation. PeerJ 7:e7502
He T, Vaidya BN, Perry ZD, Parajuli P, Joshee N (2016) Paulownia as a medicinal tree: traditional uses and current advances. Eur J Med Plants 14:1–15. https://doi.org/10.9734/EJMP/2016/25170
Henry R (1964) Colorimetric determination of total protein. Clinical Chemistry. Harper and Row Publ, New York, USA, p 181
Hoseini SM, Yousefi M (2019) Beneficial effects of thyme (Thymus vulgaris) extract on oxytetracycline-induced stress response, immunosuppression, oxidative stress and enzymatic changes in rainbow trout (Oncorhynchus mykiss). Aquac Nutr 25:298–309
Ismael NE, Abd El-hameed SA, Salama AM, Naiel MA, Abdel-Latif HM (2021) The effects of dietary clinoptilolite and chitosan nanoparticles on growth, body composition, haemato-biochemical parameters, immune responses, and antioxidative status of Nile tilapia exposed to imidacloprid. Environ Sci Pollut Res 28:1–16
Ismail HTH (2019) Hematobiochemical disturbances and oxidative stress after subacute manganese chloride exposure and potential protective effects of ebselen in rats. Biol Trace Elem Res 187:452–463
Jamala G, Tarimbuka I, Moris D, Mahai S, Adamawa S (2013) The scope and potentials of fodder trees and shrubs in agroforestry. IOSR J Agric Vet Sci 5:1–17
Jung S, Moon H-I, Ohk J, Lee S, Li C, Kim S-K, Lee M-S (2012) Inhibitory effect and mechanism on antiproliferation of isoatriplicolide tiglate (PCAC) from Paulownia coreana. Molecules 17:5945–5951
Kaper J, Lockman H, Colwell R, Joseph S (1981) Aeromonas hydrophila: ecology and toxigenicity of isolates from an estuary. J Appl Bacteriol 50:359–377
Khademi Hamedi M, Adineh H, Harsij M (2019) The effect of some herbal extracts on nutrition and growth performance, digestive enzymes activity and immune parameters of rainbow trout (Oncorhynchus mykiss). ISFJ 28:47–56
Khafaga AF, Naiel MA, Dawood MA, Abdel-Latif HM (2020) Dietary Origanum vulgare essential oil attenuates cypermethrin-induced biochemical changes, oxidative stress, histopathological alterations, apoptosis, and reduces DNA damage in Common carp (Cyprinus carpio). Aquat Toxicol 228:105624
Khalil SR, Abd Elhakim Y, Abd El-fattah AH, Farag MR, Abd El-Hameed NE, Abd Elhakeem E-M (2020) Dual immunological and oxidative responses in Oreochromis niloticus fish exposed to lambda cyhalothrin and concurrently fed with Thyme powder (Thymus vulgaris L.): Stress and immune encoding gene expression. Fish Shellfish Immunol 100:208–218
Kumar S, Raman R, Kumar K, Pandey P, Kumar N, Mallesh B, Mohanty S, Kumar A (2013) Effect of azadirachtin on haematological and biochemical parameters of Argulus-infested goldfish Carassius auratus (Linn. 1758). Fish Physiol Biochem 39:733–747
Lee D-H, Ra C-S, Song Y-H, Sung K-I, Kim J-D (2012) Effects of dietary garlic extract on growth, feed utilization and whole body composition of juvenile sterlet sturgeon (Acipenser ruthenus). Asian Australas J Anim Sci 25:577
Magnadóttir B (2006) Innate immunity of fish (overview). Fish Shellfish Immunol 20:137–151
Mehrotra S, Srivastava AK, Nandi SP (2010) Comparative antimicrobial activities of Neem, Amla, Aloe, Assam Tea and Clove extracts against Vibrio cholerae, Staphylococcus aureus and Pseudomonas aeruginosa. J Med Plants Res 4:2473–2478
Mirghaed AT, Hoseini SM, Hoseinifar SH, Van Doan H (2020) Effects of dietary thyme (Zataria multiflora) extract on antioxidant and immunological responses and immune-related gene expression of rainbow trout (Oncorhynchus mykiss) juveniles. Fish Shellfish Immunol 106:502–509
Monteiro DA, De Almeida JA, Rantin FT, Kalinin AL (2006) Oxidative stress biomarkers in the freshwater characid fish, Brycon cephalus, exposed to organophosphorus insecticide Folisuper 600 (methyl parathion). Comp Biochem Physiol c: Toxicol Pharmacol 143:141–149
Naiel MA, Ismael NE, Negm SS, Ayyat MS, Al-Sagheer AA (2020) Rosemary leaf powder–supplemented diet enhances performance, antioxidant properties, immune status, and resistance against bacterial diseases in Nile Tilapia (Oreochromis niloticus). Aquaculture 256:735370
Naiel MA, Shehata AM, Negm SS, Abd El-Hack ME, Amer MS, Khafaga AF, Bin-Jumah M, Allam AA (2020b) The new aspects of using some safe feed additives on alleviated imidacloprid toxicity in farmed fish: a review. Rev Aquac 12:2250–2267
Naiel MA, Khames MK, Abdel-Razek N, Gharib AA, El-Tarabily KA (2021a) The dietary administration of miswak leaf powder promotes performance, antioxidant, immune activity, and resistance against infectious diseases on Nile tilapia (Oreochromis niloticus). Aquacult Rep 20:100707
Naiel MA, Farag MR, Gewida AG, Elnakeeb MA, Amer MS, Alagawany M (2021b) Using lactic acid bacteria as an immunostimulants in cultured shrimp with special reference to Lactobacillus spp. Aquacult Int 29:219–231
Naiel MA, Gewida AG, Merwad A-RM, Abdel-Hamid EA, Negm SS, Alagawany M, Farag MR (2022) The effects of various organic fertilizers with or without adsorbents on the productivity, antioxidant status and immune responses of Nile tilapia raised in cement ponds. Aquaculture 548:737593
Naiel MA, Negm SS, Ghazanfar S, Shukry M, Abdelnour SA (2023a) The risk assessment of high-fat diet in farmed fish and its mitigation approaches: a review. J Anim Physiol Anim Nutr 107:948–969
Naiel MA, Ghazanfar S, Negm SS, Shukry M, Abdel-Latif HM (2023b) Applications of antimicrobial peptides (AMPs) as an alternative to antibiotic use in aquaculture: a mini-review. Annal Anim Sci. https://doi.org/10.2478/aoas-2022-0090
Naiel MA, El-Kholy AI, Negm SS, Ghazanfar S, Shukry M, Zhang Z, Ahmadifar E, Abdel-Latif HM (2023c) A mini-review on plant-derived phenolic compounds with particular emphasis on their possible applications and beneficial uses in aquaculture. Annal Anim Sci. https://doi.org/10.2478/aoas-2023-0007
Negm SS, Ismael NE, Ahmed AI, Asely AME, Naiel MA (2021) The efficiency of dietary Sargassum aquifolium on the performance, innate immune responses, antioxidant activity, and intestinal microbiota of Nile Tilapia (Oreochromis niloticus) raised at high stocking density. J Appl Phycol 33:4067–4082
Oskay M, Oskay D, Kalyoncu F (2009) Activity of some plant extracts against multi-drug resistant human pathogens. Iran J Pharm Res 8(4):293–300
Panase P, Kamee B, Moungmor S, Tipdacho P, Matidtor J, Sutthi N (2018) Effects of Euphorbia hirta plant leaf extract on growth performance, hematological and organosomatic indices of hybrid catfish, Clarias macrocephalus× C. gariepinus. Fish Sci 84:1025–1036
Paray BA, Hoseini SM, Hoseinifar SH, Van Doan H (2020) Effects of dietary oak (Quercus castaneifolia) leaf extract on growth, antioxidant, and immune characteristics and responses to crowding stress in common carp (Cyprinus carpio). Aquaculture 524:735276
Parrino V, Kesbiç OS, Acar Ü, Fazio F (2019) Hot pepper (Capsicum sp.) oil and its effects on growth performance and blood parameters in rainbow trout (Oncorhynchus mykiss). Nat Prod Res 34:1–5
Pontaza-Licona YS, Ramos-Jacques A, Cervantes-Chavez J, López-Miranda JL, de Jesús R-B, Maya-Cornejo J, Rodríguez-Morales AL, Esparza R, Estevez M, Pérez R (2019) Alcoholic extracts from Paulownia tomentosa leaves for silver nanoparticles synthesis. Result Phys 12:1670–1679
Popova TP, Baykov BD (2013) Antimicrobial activity of aqueous extracts of leaves and silage from Paulownia elongata. Am J Biol, Chem Pharm Sci 1:8–15
Pratheepa V, Sukumaran N (2014) Effect of Euphorbia hirta plant leaf extract on immunostimulant response of Aeromonas hydrophila infected Cyprinus carpio. PeerJ 2:e671
Reitman S, Frankel S (1957) A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol 28:56–63
Roncarati A, Melotti P, Dees A, Mordenti O, Angellotti L (2006) Welfare status of cultured seabass (Dicentrarchus labrax L.) and seabream (Sparus aurata L.) assessed by blood parameters and tissue characteristics. J Appl Ichthyol 22:225–234
Sakr SA, EL-Emam HA, Naiel MA, Wahed NM, Zaher HA, Mohamed Soliman M, Shukry M, Shehata AM, Alkhedaide A, Elghareeb MM (2022) The effects of paulownia (Paulownia tomentosa) leaf extract enriched diets on meat quality, sensory attributes, and the potential economic impact of broilers. Ital J Anim Sci 21:1430–1441
Sakr SA, El-Emam HA, Naiel MA, Wahed NM, Zaher HA, Abougabal MS, Alghamdi YS, Albogami S, Soliman MM, Shukry M (2022) The impact of Paulownia leaves extract on performance, blood biochemical, antioxidant, immunological indices, and related gene expression of broilers. Front Vet Sci 9:882390
Schneiderová K, Šmejkal K (2015) Phytochemical profile of Paulownia tomentosa (Thunb). Steud Phytochem Rev 14:799–833
Sönmez AY, Bilen S, Alak G, Hisar O, Yanık T, Biswas G (2015) Growth performance and antioxidant enzyme activities in rainbow trout (Oncorhynchus mykiss) juveniles fed diets supplemented with sage, mint and thyme oils. Fish Physiol Biochem 41:165–175
Stewart WM, Vaidya BN, Mahapatra AK, Terrill TH, Joshee N (2018) Potential use of multipurpose Paulownia elongata tree as an animal feed resource. Am J Plant Sci 9:1212
Sundeman MFW (1964) Studies of the serum proteins. Am J Clin Path 43:1–12
Sunyer JO, Tort L (1995) Natural hemolytic and bactericidal activities of sea bream Sparus aurata serum are effected by the alternative complement pathway. Vet Immunol Immunopathol 45:333–345
Sutthi N, Panase A, Chitmanat C, Sookying S, Ratworawong K, Panase P (2020) Effects of dietary leaf ethanolic extract of Apium graveolens L. on growth performance, serum biochemical indices, bacterial resistance and lysozyme activity in Labeo chrysophekadion (Bleeker, 1849). Aqua Rep 18:100551
Tan X, Sun Z, Huang Z, Zhou C, Lin H, Tan L, Xun P, Huang Q (2017a) Effects of dietary hawthorn extract on growth performance, immune responses, growth-and immune-related genes expression of juvenile golden pompano (Trachinotus ovatus) and its susceptibility to Vibrio harveyi infection. Fish Shellfish Immunol 70:656–664
Tan X, Sun Z, Chen S, Chen S, Huang Z, Zhou C, Zou C, Liu Q, Ye H, Lin H (2017b) Effects of dietary dandelion extracts on growth performance, body composition, plasma biochemical parameters, immune responses and disease resistance of juvenile golden pompano Trachinotus ovatus. Fish Shellfish Immunol 66:198–206
Tan X, Sun Z, Liu Q, Ye H, Zou C, Ye C, Wang A, Lin H (2018) Effects of dietary ginkgo biloba leaf extract on growth performance, plasma biochemical parameters, fish composition, immune responses, liver histology, and immune and apoptosis-related genes expression of hybrid grouper (Epinephelus lanceolatus♂× Epinephelus fuscoguttatus♀) fed high lipid diets. Fish Shellfish Immunol 72:399–409
Uchiyama M, Mihara M (1978) Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem 86:271–278
Wang ZB, Wang YK, Zhao DM, Zhang ZQ (2013) The effect of flavonoids from Paulownia tomentosa lowers on the ultrastructure and immune function of spleen and thymus in mice. Chin J Anim Vet Sci 44:952–959. https://doi.org/10.11843/j.issn.0366-6964.2013.06.018
Watts M, Munday B, Burke C (2001) Immune responses of teleost fish. Aust Vet J 79:570–574
Xu W, Gao Z, Qi Z, Qiu M, Peng J-q, Shao R (2014) Effect of dietary Chlorella on the growth performance and physiological parameters of gibel carp, Carassius auratus gibelio. Turkish J Fish Aquat Sci 14:53–57
Yadav NK, Vaidya BN, Henderson K, Lee JF, Stewart WM, Dhekney SA, Joshee N (2013) A review of Paulownia biotechnology: a short rotation, fast growing multipurpose bioenergy tree. Am J Plant Sci 4:2070
Yılmaz S, Sebahattin E, Celik E (2012) Effects of herbal supplements on growth performance of sea bass (Dicentrarchus labrax): change in body composition and some blood parameters. Energy 5:21–66
Yilmaz S (2019) Effects of dietary blackberry syrup supplement on growth performance, antioxidant, and immunological responses, and resistance of Nile tilapia, Oreochromis niloticus to Plesiomonas shigelloides. Fish Shellfish Immunol 84:1125–1133
Yousefi M, Ghafarifarsani H, Hoseinifar SH, Rashidian G, Van Doan H (2021a) Effects of dietary marjoram, Origanum majorana extract on growth performance, hematological, antioxidant, humoral and mucosal immune responses, and resistance of common carp, Cyprinus carpio against Aeromonas hydrophila. Fish Shellfish Immunol 108:127–133
Yousefi M, Farsani MN, Ghafarifarsani H, Hoseinifar SH, Van Doan H (2021) The effects of dietary supplementation of mistletoe (Viscum album) extract on the growth performance, antioxidant, and innate, immune responses of rainbow trout (Oncorhynchus mykiss). Aquaculture 536:736385
Yuan X, Wang C, Huang Y, Dai Y, Desouky HE (2020) A comparative study on intestinal morphology and function of normal and injured intestines of Jian carp (Cyprinus carpio). Aquaculture 528:735496
Yuan G, Guan Y, Yi H, Lai S, Sun Y, Cao S (2021) Antibacterial activity and mechanism of plant flavonoids to gram-positive bacteria predicted from their lipophilicities. Sci Rep 11:1–15
Zahran E, El-Gawad EAA, Risha E (2018) Dietary Withania sominefera root confers protective and immunotherapeutic effects against Aeromonas hydrophila infection in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol 80:641–650
Zargar A, Rahimi-Afzal Z, Soltani E, Taheri Mirghaed A, Ebrahimzadeh-Mousavi HA, Soltani M, Yuosefi P (2019) Growth performance, immune response and disease resistance of rainbow trout (Oncorhynchus mykiss) fed Thymus vulgaris essential oils. Aquac Res 50:3097–3106
Zhang Q, Ma H, Mai K, Zhang W, Liufu Z, Xu W (2010) Interaction of dietary Bacillus subtilis and fructooligosaccharide on the growth performance, non-specific immunity of sea cucumber. Apostichopus Japonicus Fish Shellfish Immunol 29:204–211
Zhu Z-H, Chao C-J, Lu X-Y, Xiong YG (1986) Paulownia in China: cultivation and utilization. International Development Research Centre. Chinese Academy of Forestry, Beijing. ISBN9971-84-546-6
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
All authors have an equal contribution to the conceptualization, implementation, and outputs of this research work presented in this manuscript.
Corresponding author
Ethics declarations
Ethics approval
All in vivo studies were approved and followed the general guidelines for the Care and Use of Animals in experimental inquiries. The ethical number (ZU-IACUC/2/F/81/2019) was accepted and approved by the Institutional Animal Care and Use Committee of Zagazig University.
Consent to participate
Not applicable.
Consent for publication
The authors approve processing this manuscript for publication.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Handling editor: Brian Austin
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El-Refiae, N.M., Ayyat, M.S., Mahmoud, H.K. et al. The effects of Paulownia leaf extract dietary administration on growth, redox status, immune responses, and modulate intestinal microbial content in Nile tilapia. Aquacult Int 32, 1857–1877 (2024). https://doi.org/10.1007/s10499-023-01247-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-023-01247-9