Abstract
The present study examined the impacts of varying amounts of selenium nanoparticles (Se-NPs) as a natural antioxidant and metabolic regulator on growth performance, antioxidant capacity, digestive enzymes and immune resistance of Oreochromis niloticus challenged to Aspergillus flavus infection. For 60 days, fish were fed the experimental diet of 30% crude protein up to apparent satiation three times a day. Se-NPs were added to the control diet at different levels of 0.0 (control), 0.5 (T1), 1.0 (T2) and 1.5 (T3) mg/kg diet to fed monosex Nile tilapia fingerlings with average initial weight (4.5 ± 0.5 g). Fish were randomly distributed in 12 tanks 100 L at a density of 20 fish per tank after 2-week acclimation to represent four treatments in triplicates. The results indicated that the growth indices, feed efficiency and survival rate were significantly enhanced (P < 0.05) by incorporating Se-NPs up to 1 mg/kg diet. Furthermore, the haemato-biochemical parameters, digestive enzymes activity and antioxidant capacity of the fish were significantly improved (P < 0.05) at T2. When the fish were challenged with Aspergillus flavus, a decreasing mortality rate was observed, which clearly shows that selenium nanoparticles boosted the fish’s immune response at T2. The intestinal morphology as villus length, villus width, muscular fibres layer thickness and absorption intestinal zone after challenged with A. flavus infection were considerably enhanced (P < 0.05) by incorporating all Se-NP levels. Histopathological score significantly improved (P < 0.05) for the hepatopancreatic, intestinal, gills and muscle tissues at T2. The present study concludes that selenium nanoparticles up to 1.0 mg/kg diet can be efficiently used in tilapia feed to help boost fish production, immune system response and histopathological parameters.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The aquaculture and fisheries industry is one of the most important sources of animal proteins for human consumption (Maulu et al. 2021; Eissa et al. 2022a). Oreochromis niloticus is one of the highest cultivated fish in Egypt and globally (Eissa et al. 2022b; Khalil et al. 2022; Eissa et al. 2022c). Tilapia production is one of the world’s fastest-expanding aquaculture sector (Khalil et al. 2021; Hendam et al. 2023). In 2017, Egypt produced 15.4% of the world’s tilapia production, totalling 6,510,700 tonnes (El Basuini et al., 2021). Due to the growing demand for animal protein, intense agricultural activities are required to obtain the desired level of production (Mansour et al. 2021). Aquaculture is subject to exotic, endemic pathogens (mainly parasites and bacteria) (Eissa et al. 2021; Eissa et al. 2023a; Eissa et al. 2023b), as well as climatic and environmental disruptions (Eissa et al. 2022c; Abu-Elala et al. 2016). As a result, developing resistant tilapia that can fight infectious illnesses and attain better growth rates is of significant importance (Kord et al. 2021a). The concept of functional foods is currently being employed in the food industry for the production of animals since diets must meet nutritional requirements as well as support the health of farmed animals such as fish (El Basuini et al. 2021; Eissa et al. 2022d). A nutrient-rich aqua feed should comprise minerals and vitamins, as well as protein, lipids and carbohydrates (Van Doan et al. 2020).
Trace minerals are also connected to several physiological, metabolic and functional features of an organism’s body (Khan et al. 2017). Immuno-nutrition covers fish additives and/or essential trace elements to boost their growth, antioxidant defences and immunological responses (Eissa et al. 2023a). Selenium is a trace mineral that has recently gained much interest in animal nutrition and health (Khalil et al. 2019). Selenium is a necessary component of the non-enzymatic antioxidant system, as well as glutathione peroxidase, which shields the cell membrane from oxidative damage (Talas et al. 2008).
Nanotechnology provides unique ways to raise the safety, absorption and bioavailability of chemicals in aquaculture due to their small size and large surface area (Khalil et al. 2023). Selenium nanoparticles (Se-NPs) were reported to promote an efficient antioxidant defence system, and growth performance of common carp (Cyprinus carpio) compared to selenomethionine or sodium selenite as it increases absorption and bioavailability (Saffari et al. 2018). In fish, Se-NPs boost biochemical markers (growth hormone levels in serum, total protein content in tissue and glutathione (GSH) peroxidase activity in the muscle and liver as well as haematological parameters as RBC count, haemoglobin concentration, haematocrit values and lysozyme activity (Khan et al. 2016). Se-NPs increased skin mucus, serum total proteins, serum and mucus peroxidase, mucus lysozyme activity, tolerance against low salinity and the alternative complement pathway in red sea bream (Dawood et al. 2019).
The previous research confirmed a link between fish mycoses and Aspergillus spp. (Saleemi et al. 2020). Aspergillosis was thought to be caused by Aspergillus terreus and Aspergillus flavus, and it was discovered in Labeo calbasu. Fish mucus is where Aspergillus spores are principally located. In the mucus of silver carp (Hypophthalmichthys molitrix), spores of Aspergillus niger and Aspergillus flavus were discovered (Balasubramanian et al. 2012). In the current decade, aquatic feeds should be extensively researched to serve as a functional nutritional supplement to enhance growth, immune responses and disease resistance of aquatic organisms (Abdel-Latif et al. 2022). Many researchers determined the effects of selenium nanoparticles on the growth performance and nutritional quality in Nile tilapia, Oreochromis niloticus, focusing on flesh quality and lipid fatty acid stability (Dawood et al. 2019; Saleemi et al. 2020; Balasubramanian et al. 2012; Abdel-Latif et al. 2022; Kokila et al. 2017; Dawit Moges et al. 2022).
Thus, the present work was aimed to investigate the impacts of Nano-selenium particles on performance, digestive enzyme activity, antioxidant status and immune resistance of Nile tilapia Oreochromis niloticus when challenged with Aspergillus flavus infection
Material and methods
Synthesis of selenium nanoparticles
Se-NPs were made following Dawood et al. (Dawood et al. 2019) with some adjustments. The stock solutions of sodium selenite (100 mM) and ascorbic acid (50 mM) were formed. The reacted sodium selenite to ascorbic acid ratio was 1:3 from the primary stock solution. The ascorbic acid solution was added dropwise to the sodium selenite solution for 30 min at laboratory temperature while being magnetically stirred. The combinations were then allowed to react until a light red colour was observed instead of a colourless one. The prepared Se-NP was coated with Dextrin at a level of 5% which was applied after the rise of colour using a magnetic stirrer at laboratory temperature using a single-layer coating approach. The produced nanoparticles were diluted using the dextrin solution instead of utilizing water. Finally, the nanoparticles were dried using a freeze-dryer. The formed nanoparticles were characterized using the transmission electron microscope (TEM) presented in Fig. 1. The TEM Se-NPs were prepared for TEM analysis by placing a drop of the nanoparticle suspension on carbon-coated copper grids according to (Kokila et al. 2017).
Fish rearing and experimental diets
Table 1 represents the chemical composition of the control diet. Four identical diets were formulated except for different levels of Se-NPs, a control diet with 30% crude protein (CP). Se-NPs were added to the control diet at amounts of 0.0 (control), 0.5 (T1), 1.0 (T2) and 1.5 (T3) mg/kg diet have been done according to Dawit Moges et al. (Dawit Moges et al. 2022). Se-NP was suspended in 100 mL distilled water, uniformly sprayed onto diet ingredients, well-mixed for 30 min and pelleted (1–2-mL diameter). Diets were prepared and stored in plastic bags at 4 °C until needed. Nile tilapia fingerlings (4.5 ± 0.5 g) were housed in the lab for 2 weeks to acclimate and adjust to the environment. During that time, they were given the control diet of thirty percent (30%) CP up to apparent satiation three times a day. Fish were randomly distributed in 12 tanks 100 L at a density of 20 fish per tank after acclimation to represent four treatments in triplicates. Compressed air was supplied to the fish tanks using air stones and air pumps. For 60 days, fish were fed the experimental diets three times a day at 9:00 h with 4-h intervals until they appeared satiated. Half of the water in each tank was withdrawn daily, along with faeces, and changed with new well-aerated water from a stock during the adaptation. Using fluorescent light tubes, the light was kept at a 12-h:12-h light and dark cycle throughout the experiment.
Water quality
Water samples were taken for the physicochemical test at 15-day intervals. Dissolved oxygen, water temperature, un-ionised ammonia, salinity, NO2, NO3 and pH were recorded on sites using Hanna HI-9147 automatic probe according to APHA (APHA, 2012). Nile tilapia’s fingerlings water quality parameters were rearing according to Kord et al. (Kord et al. 2021b).
Growth indices and fish survival
All fish in the experimental groups were weighed at the end of feeding period to estimate growth variables, including total length (TL) and standard length (SL). Weight gain (WG), specific growth rate (SGR) and feed conversion ratio (FCR) were computed using the formulas.
where W60 DAY is the final weight (g), W0 DAY is the opening weight (g) and 60 is the trial period (day)
Three fish were randomly chosen from each tank after the final weighing to ascertain the proximate composition using standard methods. Crude protein was estimated by nitrogen analysis (N*6.25) using the Kjeldahl technique. Crude lipid was assessed by the Soxhlet petroleum ether extraction method. Moisture was determined by drying samples at 105 °C to a constant weight. Ash was determined by burning at 550 °C for 24 h according to AOAC (AOAC 2016).
Blood sampling
Feeding was stopped 24 h before blood samples were drawn. Sodium bicarbonate-buffered tricaine methanesulfonate (MS222; Sigma-Aldrich, USA) (30 mg/L) was used to anaesthetize five fish per replicate. After that, blood samples were taken from the caudal fish vessels and divided into two parts. The first one with sodium citrate as an anticoagulant was analysed the same day to determine haematological parameters. The second part was allowed to clot at room temperature in clean, dry centrifuge tubes before being centrifuged at 5000 × g for 15 min at room temperature to obtain sera.
Haemato-biochemical examination
A haemocytometer was used to count WBCs and RBCs, according to Brown (Brown 1980). The cyanmethaemoglobin method, developed by Jain (Jain 1986), was used to calculate haemoglobin (Hb) and haematocrit (Ht) levels, respectively. An Olympus oil-immersion light microscope with ×1000 magnification was also used to count lymphocytes, neutrophils and monocytes. Trinder’s methods assessed blood glucose levels (Trinder 1969). The method of Fossati (Fossati & Prencipe 1982) was used to determine serum cholesterol and triglycerides, respectively. Henry’s methods for determining total protein (TP) and albumin (ALB) in serum were used.
Digestive enzyme activities
Diagnostic reagent kits from Cusabio Biotech Co. Ltd., Wuhan, Hubei, China, were used to assess the activities of digestive enzymes in fish serum according to the producer’s guidelines. Bernfeld (Bernfeld 1955), Shihabi (Shihabi & Bishop 1971) and updated by Zaki et al. (Zaki et al. 2023), as a described method for determining amylase, lipase and protease activity, respectively.
Antioxidants and immunity biomarkers
Using diagnostic reagent kits, the antioxidant biomarkers were determined (Bio-diagnostic Co., Giza, Egypt). The method of Ohkawa et al. (Ohkawa et al. 1979) was applied to evaluate the amount of malondialdehyde (MDA) in the samples. Superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPx) activities were determined using McCord (McCord & Fridovich 1969), Aebi (Aebi 1984) and Paglia, Valentine (Paglia & Valentine 1967) techniques, respectively. The turbidimetric approach was used to assess serum lysozyme (LYZ) in phosphate buffer (pH = 6.2) with Micrococcus luteus as the target (Ellis 1990). IgM was quantified after polyethylene glycol precipitation of IgM, and removal of the initial and final total protein was done.
Challenge test with Aspergillus flavus
Aspergillus flavus strain
Aspergillus flavus strain was provided from the Microbiological Unit of Fish Diseases Department, Animal Health Institute, ARC Dokki, Giza province. The definition was confirmed in the mycology lab., Botany Department, Faculty of Sciences, Arish University.
Preparation of fungal spores suspension
Aspergillus flavus fungal strains cultivated on Sabourauds dextrose agar (SDA, Difco). One hundred (100) UI/mL penicillin and 100 ug/mL streptomycin were added to the medium. The conidia were gathered by adding 20-mL sterile distilled water to each culture plate and collecting the solution in sterile 30-mL autoclave tubes after a 7-day incubation period at 25 °C. To verify that the filtrate contains fungal conidia, the suspensions were filtered through two layers of sterile medical gauze. Thereafter, the concentrations were adjusted to 4 × 103 conidia/mL sterile distilled water using a red blood cell counting chamber (Willoughby & Roberts 1994). Ten fish were taken from each group and injected in I/P with 0.2 mL of concentrations 4 × 103 of Aspergillus flavus conidial suspension and monitored for 15 days. During this period, clinical signs, post-mortem lesions and mortality were recorded.
Histological analysis
At the end of the experiment, all fish fasted for 24 h. Subsequently, terminal anaesthesia (MS222; Sigma-Aldrich, USA; 30 mg/L) was used for five fish taken from each tank (n = 15 fish per treatment). Thereafter, collected tissue specimens were taken from the liver, intestine, gills and skin. The collected samples were fixed 10% buffered formalin for 24 h, dehydrated in the ascending grade of ethyl alcohol (50–99%), cleared in xylene, embedded in Parablast wax, cutting by a rotatory microtome (Leica, UK) at 5 μm in thickness and then used the haematoxylin and eosin (H&E) for staining the prepared slides and examined ten fields for five slides from each group under the light microscope (Leica, UK) (Eissa et al. 2023b).
Statistical analysis
The data were computed and presented as means ± standard error using SPSS version 23 by one-way analysis of variance (ANOVA) followed by Duncan’s multiple comparisons to compare the means between individual treatments at the P level of < 0.05 according to Dytham (Dytham 2011).
Results
Water quality parameters
All estimated water quality parameters were within the normal range. There were no differences in temperature, salinity, pH, DO, NH4, NO2 and NO3 in different treatments (Table 2).
Growth indices and fish survival
The growth performance and feed utilization in terms of final weight (g), wet gain (g), wet gain (%), SGR (%/day), FCR and final length (without tail and with tail) of Tilapia fingerlings recorded were significantly higher (P ≤ 0.05) in T2 followed by T1 and T3 diets than in the control group (Table 3). The survival rate was not significant (P ≥ 0.05) in different treatments with selenium nanoparticles.
Proximate composition of the fish body
The proximate composition of tilapia fingerlings displayed a significant increase in moisture content (Table 4, P ≤ 0.05). Crude protein was recorded considerably higher values (P ≤ 0.05) in T3, followed by control, then T1 and finally T2. In terms of the lipid, there was a significant elevation in T2 followed by T1, T3 and control. Ash content was significantly higher (P ≤ 0.05) in the control than in other treatments. However, there was no major difference between T2 and T3 (P ≥ 0.05). Selenium residues in tissues of O. niloticus increased significantly in selenium treatments than in the control group and the highest value was recorded in T3 (Table 4).
Haemato-biochemical parameters
No significant difference in haemoglobin, HCT, MCH, MCHC, MCV, WBCs, RBCs, total protein, albumin, ALT, AST, ALP, creatinine, glucose, uric acid, cholesterol and triglyceride between different groups. Lymphocytes and neutrophils were significantly raised (P ≤ 0.05) in the control more than in other treatments (Table 5). In terms of the monocytes, there were significantly higher in T3 than in the control group but there were no differences in T1, T2 and T3 as shown in Table 5. Globulin increased significantly in fish fed diets supplemented with NSP versus the control group in Table 6.
Digestive enzyme activities
There were no substantial alterations in amylase and protease among different groups. Lipase was increased significantly (P ≤ 0.05) in diets supplemented with selenium compared to the control group, while there were no differences among selenium groups (Table 7).
Antioxidants and immunity biomarkers
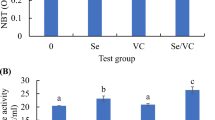
The values of the antioxidants and immunity biomarkers are presented in Table 8. MDA, SOD, CAT, lysozyme and IgM were significantly lower in control than other treatments. There was no difference in GPx measurements between different groups.
Challenge test with Aspergillus flavus
The clinical signs of infection in fish were abnormal swimming behaviour, high mortality rate, dark skin, slight flatulence and exophthalmos with corneal opacity (Fig. 2A). Post-mortem pathological changes include some haemorrhages on the surface of the body in the form of creamy haemorrhage tending to haemorrhagic fluid in the abdominal cavity, congestion and ulceration of the gills, congestion and haemorrhage on the surfaces of the internal organs along with black necrotic foci on the liver, and distention of the gallbladder (Fig. 2B). The recorded mortality was 70%, 60%, 40.33% and 40.67% in control, T1, T2 and T3 groups, respectively (Table 9). Fungal reisolation from all infected fish gills, livers, spleen, kidneys and the muscle of artificially infected fish showed increased resistance.
A A clinical signs: Nile tilapia (O. niloticus) showing skin darkening, slight abdominal inflation, exophthalmia and corneal opacity. B P. M. lesions: Nile tilapia (O. niloticus) showing erosion of gills, congestion and haemorrhages on the surfaces of internal organs with black necrotic foci on the liver
Histological examination
The sections of hepatopancreatic tissues for Nile tilapia are described in Fig. 3. The control group (T0) showed marked degeneration of hepatocytes. The pancreatic islands showed mild congestion, degeneration and dilation (Fig. 3A). T1 is explained the moderate congestion, degeneration, moderate dilation in hepatic sinusoids and inflammatory cells are found in hepatopancreatic tissues (Fig. 3B). T2 and T3 show more marked improvement of hepatopancreatic tissues (Fig. 3C and D). The intestinal tilapia transverse sections are represented in Figs. 4 and 5. The distortion mucosa layer of intestine fish is shown in Fig. 4A. Moderate distortion of apical parts of intestinal villi and absorption zone (Fig. 4B). Markedly improved branched and apical parts of intestinal mucosa in T2 and T3 groups (Fig. 4C and D) according to the nano-selenium levels in the experimental diet (Fig. 5) represented the different significant P < 0.05 that showing the mean of villus (mucosal fold) length is improved as 135.6 μm ± 2.561, 136.8 μm ± 3.917 and 137.4 μm ± 3.295 for T1, T2 and T3 respectively that compared to T0 (control) is 115.2 μm ± 4.684. The mean of intestinal villus width is increased to 42.2 μm ± 3.499, 45.4 μm ± 2.619 and 46.2 μm ± 2.131 for T1, T2 and T3 respectively that compared to T0 (control) is 29.6 μm ± 1.806. The mean intestinal muscular fibres layer thickness is upregulation as 32.8 μm ± 1.655, 35 μm ± 2.121 and 36.4 μm ± 2.676 for T1, T2 and T3 respectively that compared to T0 (control) is 28.6 μm ± 1.208. The mean intestinal absorption zone is ameliorative as 7110.8 μm2 ± 363.94, 7615.4 μm2 ± 364.754 and 8212.2 μm2 ± 488.07 for T1, T2 and T3 respectively that compared to T0 (control) is 4787.4 μm2 ± 395.08.
Liver histological sections of Nile tilapia (O. niloticus) fed with each experimental diet. A control (T0), B T1, C T2 and D T3. HC = hepatic cells, S = hepatic sinusoids, P = hepatopancreatic tissue, D = marked degeneration of hepatocytes and arrows = immune cells and mild congestion in the pancreatic islands (H&E, scale bar = 50 μm)
Intestinal histological sections of Nile tilapia (O. niloticus) fed with the experimental diets. A Control (T0), B T1, C T2 and D T3. S = serosa layer, MF = muscularis fibres layer, SM = submucosal layer, M = mucosal layer, V = intestinal villi and arrows = distortion of some apical parts of intestinal villi (H&E, scale bar = 50 μm)
Column charts representing the intestinal morphology as villus (mucosal fold) length, villus width, muscular fibres layer thickness and intestinal absorption zone for Nile tilapia O. niloticus after being challenged with Aspergillus flavus infection in different experimental groups. Means within the same column with different superscript letters (a–b) are highly significant at villus length, villus width and intestinal absorption zone P ≤ 0.001 and non-significant at muscular fibres layer thickness P > 0.05 compared between experimental groups (mean ± SE, 𝑁 = 5)
Transverse sections of fish gills in Fig. 6A and B show demolition of the gill architectures. Figure 6C and D show some mild remediation of primary and secondary gill lamellae and some laceration of gill arches. Figure 6E and F shows some improvements of primary and secondary gill lamellae and gill arches. Figure 6G and H show markedly ameliorated of the gills architecture with some laceration of gill arches and improvement of fish gills. Figure 6H appears mucoid epidermis with some degenerated, necrosis of the muscle layer and edema with leukocytic cells’ infiltration. The sections of skin and skeletal muscles for Nile tilapia are described in Figs. 7 and 8. The highly significant P > 0.001 that showing the mean of villus length is improved as 8 μm ± 0.70, 6.8 μm ± 0.37 and 6.6 μm ± 0.51 for T1, T2 and T3 respectively that compared to T0 (control) is 12.6 μm ± 0.92. Vertical sections in fish skin and skeletal muscles in Fig. 7A show destruction of the skin structure (control group). Figure 7B shows some remodelling of the skin laceration. Figure 7C shows a decrease in the spacing between skin and skeletal muscle fibres. Figure 7D more ameliorated in the skin layers and least spacing between the skin and skeletal muscle fibres.
Column charts representing the spacing between skin and skeletal muscles for Nile tilapia O. niloticus after being challenged with Aspergillus flavus infection in the different experimental groups. Means within the same column with different superscript letters (a–b) are significantly different at P < 0.001 compared between experimental groups (mean ± SE, 𝑁 = 5)
Discussion
Selenium nanoparticles are characterized and determined by studying their shape and sizes (Durigon et al. 2019). The small size and vast surface of selenium nanoparticles result in high availability and permeability in fish bodies (Hasimuna et al. 2020). The size distribution of the spherical Se-NPs ranges from 8.28 to 58.88 nm (El-Ouny et al. 2023). This makes them be easily absorbed in the fish body tissues.
Growth indices and fish survival
Supplementation of selenium nanoparticles enhanced growth rate in O. niloticus (Adam et al. 2023), Carassius auratus gibelio (Zhou et al. 2009), Oncorhynchus mykiss (Hunt et al. 2011) and Dicentrarchus labrax (Betancor et al. 2012). Likewise, Ibrahim et al. (Ibrahim et al. 2021) found that the inclusion either bulk selenium (Bulk-Se) or nano-selenium (nano-Se) improved growth performance, feed utilization efficiency and biological parameters of Nile tilapia. The improvement of growth performance and feed conversion ratio of the present study could be attributed to different scenario as (i) nano-Se enhanced gastrointestinal tract morphology and histology, then, improved the metabolism and nutrient assimilation, (ii) Se plays a major role as functional cofactors for endogenous digestive enzymes synthesis (Mansour et al. 2017) which consequently improve nutrient digestion and enhanced growth performance and feed efficiency, (iii) selenium enhances protein digestibility and utilization through boosting the quantity and activity of intestinal microbes and digestive protease activity (Dawood et al. 2019), (iv) adding nano-Se to fish diets heightened the mucosal length/width the number of goblet cells in the intestinal epithelium of fish as well as increased cell proliferation and protein synthesis. This consequently improves the surface absorption area of alimentary canal (Ibrahim et al. 2021), (v) Se performs a crucial function as a co-enzyme’s components for the activation of the gut microbiome in the intestine as well as intestinal enzymes, which improves topography of the gut and capacity of absorption, consequently improve performance and feed efficiency of animals (Mansour et al. 2017) and (vi) Se increases the amount of protein in cells of digestive track which stimulates the absorption and assimilation of nutrient (Dawood et al. 2020).
On the contrary to the present findings, Durigon et al. (Durigon et al. 2019) reported no improvement in growth parameters (weight, TL, SL, SGR and TWG) between the different treatments and the control diet. The differences from previous and current results could be attributed to several factors as trial conditions, fish species and differences in chemical forms used as ordinary selenium is not bioavailable as nanoselenium particles.
Water quality parameters
The cornerstone to an aquaculture business is water quality, and it is commonly claimed that before farming an aquatic life, you should first farm the water (Hasimuna et al. 2020). El-Ouny et al. (El-Ouny et al.,2023) reported that physicochemical parameters of fish pond water are crucial indicators of water quality and a culture system’s capacity to maintain the cultivated species. In the present study, there were no variations in all the critical water quality factors such as temperature, DO, NH4, NO2, NO3, pH and salinity. All estimated water quality variables were within the normal ranges for tilapia culture as indicated in previous studies conducted by (El-Ouny et al. 2023; Mechlaoui et al. 2019). The present results are consistent with Zhou et al. (Zhou et al. 2009), who reported that there was no result of different selenium nanoparticle levels on water quality parameters.
Proximate composition of the fish body
The results of this study demonstrated that body chemical compositions and selenium residues in tissues of tilapia fingerlings were affected (P ≤ 0.05) by nano-Se supplementation. These observations are consistent with Zhou et al. (Zhou et al. 2009) and Le and Fotedar (Rathore et al. 2021) for crucian carp and juvenile yellowtail kingfish, respectively. Furthermore, Ibrahim et al. (Ellis 1990) found that chemical composition of fish and flesh quality was considerably altered by the dietary Bulk-Se or Nano-Se, implying that the tilapia flesh composition is susceptible to addition dietary Se. Likewise, the muscle Se concentrations in carp fed 2 mg nano-Se were higher than the control diet and other treatments (Ashouri et al. 2015). In this concern, the inclusion of organic Se increases the deposition of Se in the muscles of rainbow trout (Oncorhynchus mykiss) compared with the inorganic type (Rider et al. 2009).
Haemato-biochemical parameters
The haemato-biochemical parameters are crucial in determining the health status of aquatic animals (Adam et al. 2023). The current investigation showed that the lymphocytes and neutrophils significantly increased (P ≤ 0.05) in the control more than in other treatments. The present findings are in parallel with Rathore et al. (Rathore et al. 2021), who reported the function of Selenium to modify and regulate and expression of immune-regulated selenoprotein in O. niloticus. In addition, Dawood et al. (Dawood et al. 2019) explained that European seabass have strongly cell-mediated immunity stimulated by supplementation with Nano-Se.
Contrary to the present findings, Ibrahim et al. (Ibrahim et al. 2021) reported no major (P ≥ 0.05) differences in biological indices as well as the values of MCH, MCHC, MCV, MPV and OCCB among experimental diets. In addition, the present study showed that the globulin was considerably augmented by the inclusion of nano-Se. This improvement may be due to the role of selenium to increase the high protein level in serum levels in the intracellular intestines of tilapia. The present results are consistent with Ibrahim et al. (Ibrahim et al. 2021) in Nile tilapia who reported that inclusion of nano-selenium increases serum globulin content. Accordingly, diets fortified with nano-Se had positive effects on the health of fish under culture conditions.
Digestive enzyme activities
In the current work, the highest activity of lipase was found in diet enriched with Se compared to the control diets, while amylase and protease activities were not significantly affected with the inclusion of selenium. The present results are consistent with Adineh et al. (Adineh et al. 2021), who noted that the inclusion of nano-Se up to (2 mg kg−1) can substantially enhance digestive enzyme in grass carp. Furthermore, Ibrahim et al. (Ibrahim et al. 2021) found that the inclusion of either nano or bulk selenium in Nile tilapia diets improved digestive enzyme activity. The role of selenium in improving and enhancing digestive enzymes activity could be attributed to Se function as co-enzymes in the synthesis/activation of digestive enzymes (Mansour et al. 2017).
Antioxidants and immunity biomarkers
Selenium nanoparticles are known to act as a foundation for antioxidative enzyme generation leading to high total antioxidative potency. The present work displayed improvement in the activities of MDA, GPX, CAT and SOD. Lysozyme and IgM of fish fed diet augmented with nano-Se than the control group. The current findings are consistent with previous analyses that described supplementation of nano-Se developed antioxidant parameters for common carp (Ashouri et al. 2015) and Nile tilapia (Dawood et al. 2020). Furthermore, European sea bass (Dicentrarchus labrax) fed diet supplemented with selenium nanoparticle decrease the oxidative stress and improve oxidative enzymes activity (Betancor et al. 2012). In addition, inclusion of selenium nanoparticle improved cellular shield against oxidative stress and an increase in antioxidant defences of fish when exposed to disease-causing pathogens (Durigon et al. 2019). Furthermore, Sarkar et al. (Durigon et al. 2019) reported that nano-Se can be fed to fish and livestock to decrease oxidative stress as well as to increase the productivity of the stressed fish and livestock. Likewise, Ibrahim et al. (Ibrahim et al. 2021) found that Nile tilapia fed 0.4 or 0.8 mg nano-Se improved the performance of antioxidant enzymes and lower MDA activity.
Challenge test with Aspergillus flavus
The challenge test of Oreochromis niloticus with Aspergillus flavus revealed a decreasing trend of mortality rates (i.e. 70%, 60%, 40.33% and 40.67% in control, T1, T2 and T3 groups respectively). The present results are consistent Pérez-Valenzuela et al. (Pérez-Valenzuela et al. 2021) who found that Oncorhynchus mykiss fed diets enriched with selenium lowered the cytopathic effect, mortality tare, intracellular bacterial load and cellular mortality of SHK-1 by expanding the abundance and activity of host glutathione peroxidase. Eissa et al. (Eissa et al. 2022b; Eissa et al. 2023b) found an increase in resistance to Aspergillus flavus of Oreochromis niloticus and Red Tilapia (Oreochromis sp.) fed diets supplemented with curcumin nanoparticles due to an increase of antioxidant capacity in fish’s plasma.
Histology findings
The present results are consistent with Mello et al. (Mello et al. 2013), who found that Se-NP addition elevated the quantity and activity of gut microbes and digestive protease potency. Furthermore, the insertion of nano-Se in diets enlarged the villus length/width and the number of goblet cells in intestinal fish as well as amplified cell proliferation and protein synthesis, which consequently improve the surface absorption area of the alimentary canal; Se plays a crucial role as a co-enzyme factor for the activation of the digestive enzymes and the gut flora, which amended topography of gut and capacity of absorption, which subsequently improve performance and feed efficiency of animals. Nano-selenium may increase the content of protein in cells of the intestinal tract, which expands the absorption and assimilation of nutrients. Nano-selenium was able to influence tilapia infection against Aspergillus flavus. The T3 group markedly improved the hepatopancreatic tissue, intestinal layers, gill tissues and fish skin condition. This most likely increased the immune resistance of Nile tilapia and Red Tilapia challenged with Aspergillus flavus infection. This corresponds to the findings in recent studies with fish (Eissa et al. 2022b; Eissa et al. 2023b). Finally, this work has highlighted the potential of nano-mineral delivery in terms of achieving superior Se uptake and assimilation in tilapia. This complies with the recent benefits in aquaculture, as recently reviewed by Nasr-Eldahan et al. (Nasr-Eldahan et al. 2021), to enhance disease resistance and health in farmed fish species.
Conclusion
The supplementation of selenium nanoparticles in diets of O. niloticus significantly improved the fish’s growth performance survival rate, boost immune system response, antioxidant capacity and digestive enzymes with selenium nanoparticles up to 1.0 mg/kg diet that linked to reduce the mortality rate when the fish was challenged with A. flavus.
Recommendation
This is crucial study which recommends more studies be undertaken to investigate the effect of a combination of selenium nanoparticles and vitamin premixes on growth performance, enzymatic activities and immune system response. In addition, more research in the future is recommended to clarify the function of selenium nanoparticles on water quality parameters that could help to achieve aquaculture sustainability of fish farms from different regions (temperate and tropical) should expect in their fish production businesses.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ALB:
-
Albumin
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- ALP:
-
Alkaline phosphatase
- WBCs:
-
White blood cells
- RBCs:
-
Red blood cells
- MCH:
-
Mean corpuscular haemoglobin
- MCHC:
-
Mean corpuscular haemoglobin concentration
- MCV:
-
Mean corpuscular volume
- CAT:
-
Catalase
- FI:
-
Feed intake
- GLO:
-
Globulin
- GPx:
-
Glutathione peroxidase
- GSH:
-
Glutathione content
- LYZ:
-
Lysozyme activity
- MDA:
-
Malondialdehyde
- RBA:
-
Respiratory burst activity
- SGR:
-
Specific growth rate
- SOD:
-
Superoxide dismutase
- TP:
-
Total protein
- WG%:
-
Weight gain percentage
- WG:
-
Weight gain
References
Abdel-Latif HMR, Soliman AA, Khaled AA, Kord M, Abdel-Tawwab M, Darwish S, Grana YS, Zaki M, Nour A, Ali E, Khalil RH, Khalil HS (2022) Growth performance, antioxidant activities, and immunological responses of hapa-reared thinlip mullet (Liza ramada) juveniles fed on diets supplemented with spirulina (Arthrospira platensis). Fish Shellfish Immunol 130:359–367
Abu-Elala NM, Abd-Elsalam RM, Marouf S, Abdelaziz M, Moustafa M (2016) Eutrophication, ammonia intoxication, and infectious diseases: interdisciplinary factors of mass mortalities in cultured Nile tilapia. J Aquat Anim Health 28:187–198
Abu-Elala NM, HO AB, Khattab MS, Mohamed SH, El-Hady MA, Ghandour RA et al (2018) Aquatic environmental risk assessment of chitosan/silver, copper and carbon nanotube nanocomposites as antimicrobial agents. Int J Biol Macromol 113:1105–1115 ISSN 0141-8130
Adam AH, Verdegem M, Soliman AA, Zaki M, Khalil RH, Nour AE et al (2023) Effect of dietary bile acids: growth performance, immune response, genes expression of fatty acid metabolism, intestinal, and liver morphology of striped catfish (Pangasianodon hypophthalmus). Aquac Rep 29:101510
Adineh H, Naderi M, Nazer A, Yousefi M, Ahmadifar E (2021) Interactive effects of stocking density and dietary supplementation with Nano selenium and garlic extract on growth, feed utilization, digestive enzymes, stress responses, and antioxidant capacity of grass carp Ctenopharyngodon idella. J World Aquacult Soc 52:789–804
Aebi H (1984) Catalase in Vitro. Methods Enzymol 105:121–126
AOAC (2016) Official methods of analysis of AOAC Intl. Association of Official Analytical Chemists Gaithersburg
APHA (2012) Standard methods for the examination of water and waste water, 22nd edn. American Public Health Association, American Water Works Association, Water Environment Federation
Ashouri S, Keyvanshokooh S, Salati AP, Johari SA, Pasha-Zanoosi H (2015) Effects of different levels of dietary selenium nanoparticles on growth performance, muscle composition, blood biochemical profiles and antioxidant status of common carp (Cyprinus carpio). Aquac 446:25–29 ISSN 0044-8486
Balasubramanian S, Prakash M, Senthilraja P, Gunasekaran G (2012) Antimicrobial properties of skin mucus from four freshwater cultivable Fishes (Catla catla, Hypophthalmichthys molitrix, Labeo rohita and Ctenopharyngodon idella). Afr J Microbiol Res 6:5110–5120. https://doi.org/10.5897/AJMR11
Bernfeld P (1955) Amylases, α and β. In: Methods in enzymology, pp 149–158
Betancor MB, Caballero MJ, Terova G, Saleh R, Atalah E, Benítez-Santana T et al (2012) Selenium inclusion decreases oxidative stress indicators and muscle injuries in sea bass larvae fed high-DHA microdiets. Br J Nutr 108(12):2115–2128
Brown LJ (1980) A new instrument for the simultaneous measurement of total hemoglobin, % oxyhemoglobin, % carboxyhemoglobin, % methemoglobin, and oxygen content in whole blood. IEEE Trans Biomed Eng 27:132–138
Dawit Moges F, Hamdi H, Al-Barty A, Zaid AA, Sundaray M, Parashar SKS et al (2022) Effects of selenium nanoparticle on the growth performance and nutritional quality in Nile Tilapia, Oreochromis niloticus. PLoS One 17:e0268348
Dawood MAO, Koshio S, Zaineldin AI, Doan HV, Moustafa EM, Abdel-Daim MM et al (2019) Dietary supplementation of selenium nanoparticles modulated systemic and mucosal immune status and stress resistance of red sea bream (Pagrus major). Fish Physiol Biochem 45:219–230
Dawood MAO, Zommara M, Eweedah NM, Helal AI, Aboel-Darag MA (2020) The potential role of nano-selenium and vitamin C on the performances of Nile tilapia (Oreochromis niloticus). Environ Sci Pollut Res 27:9843–9852
Durigon EG, Kunz DF, Peixoto NC, Uczay J, Lazzari R (2019) Diet selenium improves the antioxidant defense system of juveniles Nile tilapia (Oreochromis niloticus L.). Braz J Biol 79(3):527–532
Dytham C (2011) Choosing and using statistics: a biologist's guide. John Wiley & Sons
Eissa EH, Ahmed NH, El-Badawi AA, Munir MB, Abd Al-Kareem OM, Eissa MEH et al (2022a) Assessing the influence of the inclusion of Bacillus subtilis AQUA-GROW® as feed additive on the growth performance, feed utilization, immunological responses and body composition of the Pacific white shrimp, Litopenaeus vannamei. Aquac Res 53:6606–6615
Eissa EH, Che-Zulkifli CI, El-Badawi AA, Ali MAM, Baghdady ES, Abd Al-Kareem OM et al (2021) Growth-promoting and immunomodulatory impacts of commercial stimulants on kuruma shrimp, Penaeus japonicus (Bate, 1888) juveniles. Egypt J Aquat Biol Fish 25(3):607–617
Eissa EH, Elsayed SB, Gaafar AY, El-Badawi AA, Bazina WK, Abd Al-Kareem OM et al (2022d) Assessing the influence of dietary Pediococcus acidilactici probiotic supplementation in the feed of European sea bass (Dicentrarchus labrax L.) (Linnaeus, 1758) on farm water quality, growth, feed utilization, survival rate, body composition, blood biochemical parameters, and intestinal histology. Aquac Nutr 2022:5841220 11 pages, 2022
Eissa E-S, Ezzo OH, Khalil HS, Tawfik WA, El-Badawi AA, Abd Elghany NA et al (2022b) The effect of dietary nanocurcumin on the growth performance, body composition, haemato-biochemical parameters and histopathological scores of the Nile tilapia (Oreochromis niloticus) challenged with Aspergillus flavus. Aquac Res 53:6098–6111
Eissa ESH, Abd El-Hamed NNB, Ahmed NH, Badran MF (2022c) Improvement the hatchery seed production strategy on embryonic development and larval growth performance and development stages of green tiger prawn, Penaeus semisulcatus using environmental aspects. Thalassas: Int J Mar Sci 38:1327–1338
Eissa E-SH, Ahmed RA, Abd Elghany NA, Elfeky A, Saadony S, Ahmed NH et al (2023a) Potential symbiotic effects of -1,3 glucan, and fructooligosaccharides on the growth performance, immune response, redox status, and resistance of Pacific white shrimp, Litopenaeus vannamei to Fusarium solani infection. Fishes 8:105
Eissa E-SH, Alaidaroos BA, Jastaniah SD, Munir MB, Shafi ME, Abd El-Aziz YM et al (2023b) Dietary effects of nano curcumin on growth performances, body composition, blood parameters and histopathological alternation in red tilapia (Oreochromis sp.) challenged with Aspergillus flavus. Fishes 8:208
El Basuini MF, Teiba II, Shahin SA et al (2021) Dietary Guduchi (Tinospora cordifolia) enhanced the growth performance, antioxidative capacity, immune response and ameliorated stress-related markers induced by hypoxia stress in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol 120:337–344
Ellis AE (1990) Lysozyme assays. In: Stolen JS, TC Fletcher DP, Anderson BSR, Van Muiswinkel WB (eds) Techniques in fish immunology, vol 1
El-Ouny YM, Maulu S, Zaki MAA, Helaly AA, Nour AAM, El Basuini MF, Labib EMH, Gouda AH, Hessein AAA, Verdegem M, Khalil HS (2023) Effect of fishmeal replacement with dried red wigglers (Eisenia fetida) worm meal on growth and feed utilization, production efficiency, and serum biochemistry in Nile tilapia (Oreochromis niloticus) fingerlings. Aquac Rep 29:101518
Fossati P, Prencipe L (1982) Serum triglycerides determined colorimetrically with an enzyme that produces hydrogen peroxide. Clin Chem 28:2077–2080
Hasimuna OJ, Maulu S, Mphande J (2020) Aquaculture health management practices in Zambia: status, challenges and proposed biosecurity measures. J Aquac Res Dev 11:584
Hendam BM, Munir MB, Eissa ME, El-Haroun E, van Doan H, Chung TH, Eissa ESH (2023) Effects of water additive probiotic, Pediococcus acidilactici on growth performance, feed utilization, hematology, gene expression and disease resistance against Aspergillus flavus of Nile tilapia (Oreochromis niloticus). Anim Feed Sci Technol 303:115696
Hunt AO, Berkoz M, Ozkan F, Yalin S, Ercen Z, Erdogan E et al (2011) Effects of organic selenium on growth, muscle composition, and antioxidant system in rainbow trout. Isr J Aquacult Bamidgeh 63:10
Ibrahim MS, El-gendy GM, Ahmed AI, Elharoun ER, Hassaan MS (2021) Nanoselenium versus bulk selenium as a dietary supplement: effects on growth, feed efficiency, intestinal histology, haemato-biochemical and oxidative stress biomarkers in Nile tilapia (Oreochromis niloticus Linnaeus, 1758) fingerlings. Aquac Res 52:5642–5655
Jain NC (1986) Schalm's veterinary hematology, 4th edn. Lea & Febiger
Khalil HS, Mansour AT, Goda AMA, Omar EA (2019) Effect of selenium yeast supplementation on growth performance, feed utilization, lipid profile, liver and intestine histological changes, and economic benefit in meagre, Argyrosomus regius, fingerlings. Aquac 501:135–143 ISSN 0044-8486
Khalil HS, Momoh T, Al-Kenawy D, Yossa R, Badreldin AM, Roem A et al (2021) Nitrogen retention, nutrient digestibility and growth efficiency of Nile tilapia (Oreochromis niloticus) fed dietary lysine and reared in fertilized ponds. Aquac Nutr 27:2320–2332
Khalil HS, Maulu S, Verdegem M, Abdel-Tawwab M (2023) Embracing nanotechnology for selenium application in aquafeeds. Rev Aquac 15(1):112–129
Khalil HS, Momoh T, Al-Kenawy D, Yossa R, BadrEldin AM, Roem A et al (2022) Metabolic growth, plankton selectivity, haemato-biochemical and intestinal morphometry of Nile tilapia (Oreochromis niloticus) fed a lysine-deficient diet in earthen ponds. Aquac Rep 24:101122 ISSN 2352-5134
Khan KU, Zuberi A, Nazir S, Fernandes JBK, Jamil Z, Sarwar H (2016) Effects of dietary selenium nanoparticles on physiological and biochemical aspects of juvenile Tor putitora. Turk J Zool 40(5):7
Khan KU, Zuberi A, Nazir S, Ullah I, Jamil Z, Sarwar H (2017) Synergistic effects of dietary nano selenium and vitamin C on growth, feeding, and physiological parameters of mahseer fish (Tor putitora). Aquac Rep 5:70–75 ISSN 2352-5134
Kokila K, Elavarasan N, Sujatha V (2017) Diospyros montana leaf extract mediated synthesis of selenium nanoparticles and their biological applications. New J Chem 41(15):7481–7490
Kord MI, Maulu S, Srour TM, Omar EA, Farag AA, Nour AAM et al (2021b) Impacts of water additives on water quality, production efficiency, intestinal morphology, gut microbiota, and immunological responses of Nile tilapia fingerlings under a zero-water-exchange system. Aquac 547:737503
Kord MI, Srour TM, Omar EA, Farag AA, Nour AAM, Khalil HS (2021a) The immunostimulatory effects of commercial feed additives on growth performance, non-specific immune response, antioxidants assay, and intestinal morphometry of Nile tilapia, Oreochromis niloticus. Front Physiol 12:627499
Mansour AT, Allam BW, Srour TM, Omar EA, Nour AAM, Khalil HS (2021) The feasibility of monoculture and polyculture of striped catfish and Nile tilapia in different proportions and their effects on growth performance, productivity, and financial revenue. J Mar Sci Eng 9(6):586
Mansour AT, Goda AA, Omar EA, Khalil HS, Esteban MÁ (2017) Dietary supplementation of organic selenium improves growth, survival, antioxidant and immune status of meagre, Argyrosomus regius, juveniles. Fish Shellfish Immunol 68:516–524
Maulu S, Nawanzi K, Abdel-Tawwab M, Khalil HS (2021) Fish nutritional value as an approach to children’s nutrition. Front Nutr 8:780844
McCord JM, Fridovich I (1969) Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem 244:6049–6055
Mechlaoui M, Dominguez D, Robaina L, Geraert P-A, Kaushik S, Saleh R et al (2019) Effects of different dietary selenium sources on growth performance, liver and muscle composition, antioxidant status, stress response and expression of related genes in gilthead seabream (Sparus aurata). Aquac 507:251–259 ISSN 0044-8486
Mello HD, Julieta REM, Niza IG, Moraes FRD, OzórioIII ROA, Shimada MT et al (2013) Beneficial effects of probiotics on the intestine of juvenile Nile tilapia. Pesqui Vet Bras 33(6):724–730
Nasr-Eldahan S, Nabil-Adam A, Shreadah MA, Maher AM, Ali TE (2021) A review article on nanotechnology in aquaculture sustainability as a novel tool in fish disease control. Aquac Int 29:1459–1480
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70:158–169
Pérez-Valenzuela J, Mejías M, Ortiz D, Salgado P, Montt L, Chávez-Báez I et al (2021) Increased dietary availability of selenium in rainbow trout (Oncorhynchus mykiss) improves its plasma antioxidant capacity and resistance to infection with Piscirickettsia salmonis. Vet Res 52:64
Rathore SS, Murthy HS, Girisha SK, Nithin MS, Nasren S, Mamun MAA et al (2021) Supplementation of nano-selenium in fish diet: Impact on selenium assimilation and immune-regulated selenoproteome expression in monosex Nile tilapia (Oreochromis niloticus). Comp Biochem Physiol Part - C: Toxicol 240:108907
Rider SA, Davies SJ, Jha AN, Andrew AF, Knight J, John WS (2009) Supra-nutritional dietary intake of selenite and selenium yeast in normal and stressed rainbow trout (Oncorhynchus mykiss): implications on selenium status and health responses. Aquac 295:282–291 ISSN 0044-8486
Saffari S, Keyvanshokooh S, Zakeri M, Johari SA, Pasha-Zanoosi H, Mozanzadeh MT (2018) Effects of dietary organic, inorganic, and nanoparticulate selenium sources on growth, hemato-immunological, and serum biochemical parameters of common carp (Cyprinus carpio). Fish Physiol Biochem 44(4):1087–1097
Saleemi S, Iqbal Z, Khalid AN (2020) Morphological and pathological effects of Aspergillosis in silver carp, hypophthalmichthys molitrix. Punjab Univ J Zool 35:129–133
Shihabi ZK, Bishop C (1971) Simplified turbidimetric assay for lipase activity. Clin Chem 17:1150–1153
Talas ZS, Orun I, Ozdemir I, Erdogan K, Alkan A, Yilmaz I (2008) Antioxidative role of selenium against the toxic effect of heavy metals (Cd+2, Cr+3) on liver of rainbow trout (Oncorhynchus mykiss Walbaum 1792). Fish Physiol Biochem 34(3):217–222
Trinder P (1969) Determination of glucose in blood using glucose oxidase with an alternative oxygen acceptor. Ann Clin Biochem Int J Lab Med 6:24–27
Van Doan H, Hoseinifar SH, Ringø E, Esteban MÁ, Dadar M, Dawood MAO et al (2020) Host-associated probiotics: a key factor in sustainable aquaculture. Rev Fish Sci Aquac 28(1):16–42
Willoughby LG, Roberts RJ (1994) Improved methodology for isolation of the Aphanomyces fungal pathogen of epizootic ulcerative syndrome (EUS) in Asian fish. J Fish Dis 17:541–543
Zaki MAA, Khalil HS, Allam BW, Khalil RH, Basuini MFE, Nour AEM, Labib EMH, Elkholy ISE, Verdegem M, Abdel-Latif HMR (2023) Assessment of zootechnical parameters, intestinal digestive enzymes, haemato-immune responses, and hepatic antioxidant status of Pangasianodon hypophthalmus fingerlings reared under different stocking densities. Aquac Int:1–24
Zhou X, Wang Y, Gu Q, Li W (2009) Effects of different dietary selenium sources (selenium nanoparticle and selenomethionine) on growth performance, muscle composition and glutathione peroxidase enzyme activity of crucian carp (Carassius auratus gibelio). Aquac 291:78–81
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study equally. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Institutional Animal Treatment and Use Committee (IACUC) at Cairo University (Vet CU 2022/604), Egypt.
Competing interests
The authors declare no competing interests.
Additional information
Handling Editor: Amany Abbass
Highlights
1. Growth indices, feed efficiency and survival rate were significantly enhanced (P < 0.05) by incorporating Se-NPs up to 1 mg/kg diet.
2. 1 mg Se-NPs/kg diet improved (P < 0.05) the haemato-biochemical parameters, digestive enzymes activity and antioxidant capacity of Nile tilapia.
3. Selenium nanoparticles boosted the fish’s immune response and intestinal morphology after challenged with A. flavus infection.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Eissa, ES.H., Bazina, W.K., Abd El-Aziz, Y.M. et al. Nano-selenium impacts on growth performance, digestive enzymes, antioxidant, immune resistance and histopathological scores of Nile tilapia, Oreochromis niloticus against Aspergillus flavus infection. Aquacult Int 32, 1587–1611 (2024). https://doi.org/10.1007/s10499-023-01230-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-023-01230-4