Abstract
Generally, one of the most significant bottlenecks of land-based abalone aquaculture is the high cost of the feed, which is associated with the use of wild fish stocks to produce fish meal. Fish meal is a key ingredient used in aquaculture feeds due to its nutritional profile and palatability. Alternative ingredients, such as insect meal and grape marc, are potential candidates to be included in aquafeeds due to their suitable nutritional profile and more sustainable production. This study aimed to evaluate the effect of insect meal and grape marc on growth and nutritional profile using abalone as an animal model in a feeding trial for 165 days. Proximate analyses were used to identify nutritional variations in abalone tissue and faecal matter, and liquid chromatography-mass spectrometry and gas chromatography mass-spectrometry analyses were utilised to characterise amino acid and fatty acid composition. Results showed a 95% survival for all treatments. The inclusion of insect meal and grape marc did not affect the protein proportions of abalone tissues, but differences were found in the carbohydrate and lipid proportions. The inclusion of insect meal and grape marc significantly affected levels of amino acids L-histidine, L-methionine, and L-phenylalanine, and fatty acids oleic acid, linoleaidic acid, palmitic acid, palmitoleic acid, and α-linolenic suggesting a correlation between the feed source and fatty acid tissue composition. This work provides promising results for future optimisation of abalone diets including more affordable and sustainable alternative ingredients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Abalone (Haliotis iris) represent a valuable export product in New Zealand, contributing between NZD $50–60 million annually (Prime Minister’s Chief Science Advisor 2021) towards the country’s export economy. Most of this production is from wild harvest, mainly collected from Fiordland in the Southland and the Chatham Islands. A small aquaculture production in New Zealand facilities is projected to reach NZD 1 billion in annual sales by 2035 (The New Zealand Government 2019).
Generally, one of the most significant bottlenecks of land-based abalone farming is the high cost of the feed, which can be up to 50% of the production cost (Fleming et al. 1996). The high price has been attributed to fish meal (FM), which is a key ingredient used in aquaculture feeds due to its excellent amino acid profile and palatability. However, the use of FM in aquafeeds has been questioned since it usually requires 4–5 kg of forage fish input to generate 1 kg of FM (Péron et al. 2010). Thus, the use of wild fish to feed farmed fish is thought to be unsustainable with dramatic effects on already depleting marine fish resources. New trends in aquaculture practices intend to develop feed formulations that use more sustainable replacements for FM, thus reducing the proportion of FM in the final feed. Currently, aquaculture uses almost 60% of the total world production of FM (Péron et al. 2010). Examples of successful alternative ingredients incorporated in abalone trials are steam distilled grape marc meal (Currie et al. 2019), tuna by product meal (Jung et al. 2016), seaweed meal (Viera et al. 2015), soy meal, and silkworm pupae meal (Cho 2010).
Insect sources have recently been considered to be potential candidates for FM replacement due to their excellent nutritional profile with 42–63% crude protein content (Fasolin et al. 2019), rich in amino acids, lipids, vitamins, and minerals (Makkar et al. 2014; Nogales-Mérida et al. 2018). The most used insect species used in feeds are black soldier flies (Hermetia illucens), the house flies (Musca domestica), and mealworm beetles (Tenebrio molitor). Out of these species, T. molitor larvae contain all essential amino acids (EAA) in sufficient quantities to meet the dietary requirements of most aquatic species. The essential amino acid (EAA) composition of the mealworm larval meal is comparable to those of fish and soy meals (Azagoh et al. 2016), and most of the EAA contents are higher than the requirements suggested by the FAO/WHO/UHU (Li et al. 2013). Insect meal (IM) is considered to be a more sustainable ingredient for aquafeeds due to the lower requirements for land and water resources compared to soybean meal (Ferrer et al. 2019). In addition, IM contributes to a substantial reduction in greenhouse emissions since insect rearing has lower waste and waste products can be used for insect rearing (Nugroho and Nur 2018). Some examples of successful inclusion of insects into aquafeeds include houseflies (M. domestica) as partial replacement, which promotes growth performance for tilapia (Oreochromis niloticus) (Wang et al. 2017). Mealworm (T.molitor) inclusion in rainbow trout (Oncorhynchus mykiss) diets has promoted an increase in antioxidant intestinal activity and a reduction of lipid peroxidation (Henry et al. 2018), and black soldier fly (H. illucens) inclusion has shown to enhance gut microbial diversity and the amount of beneficial lactic acid bacteria in rainbow trout (O. mykiss) microbiota (Terova et al. 2019). To our knowledge, no study has yet been conducted to use mealworm meal as a replacement for FM in abalone diets.
Another alternative ingredient for aquafeeds is grape marc (GM), which is an underutilised biowaste product from the viticulture industry comprising the skins and seeds remaining after pressing. Due to the large amount of waste produced annually in vineyards throughout New Zealand, GM disposal has become problematic. It has been estimated that almost 18–20% of all harvested grapes used for wine production end up as GM (Spanghero et al. 2009), and the Marlborough region alone produced approximately 50,000 tonnes of GM in 2020 (Massey University 2020). Grape marc is commonly used as fertiliser, but no use has been assigned to the aquaculture feed industry yet. Grape marc nutritional properties include low levels of digestible carbohydrates, polyphenols (anthocyanins, catechins, flavonols, and phenolic acids), large amounts of resveratrol present in the skin and pulp of red grapes, and high levels of polyunsaturated fatty acids (over 60%), with more than 2% being omega-3 fatty acids (Habeanu et al. 2015). These properties might positively influence the glycaemic load, energy intake, and antioxidant capacity in abalone. Grape marc has been mainly included in feeds for ruminants, showing no detrimental effects in growth, with an improved environmental impact (Moate et al. 2014). However, the dietary application of GM in aquatic species is very limited. To our knowledge, GM derivatives have been only used in feeds for Haliotis laevigata with great success (Currie et al. 2019). Steam distilled grape marc (Acti-Meal®) inclusion has resulted in better growth rates and feed conversion ratios of H. laevigata compared to commercial diets and feed without GM supplement (Currie et al. 2019).
The aim of the present study is to evaluate the effect of different inclusion levels of IM and GM on aquafeeds to improve growth performance, feed utilisation, proximate composition, amino acid, and fatty acid composition of juvenile New Zealand farmed abalone.
Materials and methods
Experimental animals
This study was conducted within a commercial abalone (Halioris iris) farm (The New Zealand Abalone Company) in Bluff, Invercargill, New Zealand. Healthy juveniles with initial mean weight and shell length of 1.1 ± 0.5 g and 21.5 ± 3.3 mm, respectively, were used in the feeding trial. The animals were 17-months old, and they were randomly selected from the farm stock. A total number of 3000 animals were used for the feeding trial.
Feeding trial and sample collection
Fifteen plastic tanks containing 90 L of filtered seawater (100-micron filter) were stocked with 200 abalone juveniles each. From the 200 animals, 50 were tagged for allometric measurements. For tagging, abalone were removed from the tanks, measured in shell length, width, and weight (baseline), and tagged with one colour plastic tags (numbered) which were adhered to the shells with cyanoacrylate glue. The tagging process was performed on 1 day, and the water temperatures during this process fluctuated from 12.1 to 13 °C.
Five dietary treatments were randomly allocated to three replicate tanks per treatment. A flow-through water system was maintained at a rate of 1.5 L/min, which equates to a total water exchange of 40 times per day. The tanks were drained and cleaned as per farming procedure every other day. Briefly, the stopper at the bottom of the tank was pulled off from the tank, and the tanks were completely drained. Faeces, debris, and uneaten food residues were flushed out with the help of water supply. Animals remained undisturbed in the tank, while cleaning was performed. Animals were kept in darkness except when feeding and cleaning were performed. Water temperature and dissolved oxygen were measured electronically before and after cleaning and fluctuated from 12.4 to 19.7 °C. Water quality dissolved oxygen was measured before and after cleaning using a dissolved oxygen meter (Handy Polaris TCP, Denmark). The dissolved oxygen meter was calibrated before use in ‘air-saturated’ seawater according to manufacturer’s instructions. The dissolved oxygen was maintained between 86.3 and 104.1% oxygen saturation.
Abalone were fed 1.2–2.2% of their body weight per day. The daily ration was given once in the late afternoon (about 1600 h). The experiment lasted 165 days. Abalone were evaluated for proximate analyses, fatty acid, and amino acid composition before (baseline) and after the feeding trial. Before the feeding trial, 105 abalone were randomly collected from the cohort for proximate analyses, and 20 abalone for fatty acid and amino acid composition analyses. After the feeding trial, 70 abalone were collected from each tank for proximate composition and 15 abalone for fatty acid and amino acid composition analyses. The whole soft bodies were placed into 2 mL cryovial (Biostor™) and quenched in liquid nitrogen for 10 min and then stored in dry ice (−80 °C) for transportation to the −80 °C freezer located at the Auckland University of Technology (Auckland, New Zealand) where samples were stored until further analyses.
Diet preparation
Four experimental diets were formulated to contain different levels of fish meal (FM), corn meal (CM), insect meal (IM), and grape marc (GM). The source of energy came from carbohydrates either in the CM or GM and the source of protein FM or IM or both. Seaweed and starch served as attractant and binder, respectively. The composition of the experimental diets is shown in Table 1. The commercial feed Marifeed® was used as the control. The procedures for diet preparation were as follows:
Experimental diets were prepared by mixing pre-weighed finely ground (200 μm) ingredients in a commercial food mixer. All ingredients were mixed, except the starch (native maize flour). Starch syrup at 10% was prepared and sequentially added to the dried mixture to form a dough with even consistency, which was then placed on a flat tray to be dried at 65 °C for 16 h. The dried dough was ground using a commercial blender (Nutri Bullet 600 household mixer) for 30 s, and the powder was sieved twice using a kitchen sieve (200 μm).
Encapsulation of experimental diets
Encapsulation was performed using calcium chloride solution (0.1M) and alginate solution (1% w/v). Alginate solution (1%) was prepared overnight to promote good incorporation of the powder. Briefly, for encapsulation, 70 g of feed powder were mixed with 250 mL of distilled water until hydrated completely. A total of 750 mL alginate solution (1% w/v) was added and mixed right before encapsulation. For encapsulation, 400 mL of calcium chloride were placed in a 1-L beaker to encapsulate 100 mL of alginate solution (which contained the feed powder). The formed beads were rinsed with 100–200 mL distilled water and placed in an oven tray covered with aluminium foil. Beads were air-dried in a commercial oven (Piron PF8906, Italy) at 65 °C for 16 h. The dried beads were then packed in a vacuum-sealed bag to avoid contamination. Dried beads were hydrated in seawater for 8–12 h before abalone feeding.
Growth parameters
Growth measurements of tagged animals were recorded on animals collected at the start of the experiment (baseline) and after 165 days of treatment. This was done by removing the animals from the water with a blunt knife. Then, the animals were dried with paper towels, and their maximum shell lengths and widths (mm) and total animal wet weights (g) were recorded. Lengths were measured with a vernier calliper (Mitutoyo 0–125 mm, Warwickshire, UK) to the nearest 0.1 mm, and weights were measured with a digital balance to the nearest 0.1 g.
Abalone specific growth rate (SGR, in % day−1) was calculated according:
Specific growth rate Total weight
Specific growth rate Shell length
Specific growth rate Shell width
where lnf is the natural log of the final total weight (TW), final shell length (SL), and final shell width (SW) and lni is the natural log of the initial total weight (TW), initial shell length (SL), and initial shell width (SW).
Daily increment in shell length (DISL, in μm day−1) was calculated according to Dlaza et al. (2008):
condition factor
The condition factor (CF, in g mm−1), which is an index to account for the relationship between the weight of abalone per unit shell length, was calculated following Britz (1996)
where BW is the mean body weight (g) and SL the mean shell length (mm); 2.99 and 5.575 are constants.
Muscle yield (%):
Faecal sample collection
Faecal collection occurred at week 12 of the feeding trial and was performed for 1 week. All samples were pooled together according to the treatment due to insufficient biomass for analysis. For the faecal collection, the feeding regime changed from nighttime to daytime to facilitate faecal matter collection not contaminated with feed particles. Briefly, animals were fed in the early morning and tanks were completely cleaned of feed residues in the afternoon. After the cleaning, animals deposited faeces until the next day when tanks were cleaned, faeces collected, and animals fed again. The faecal matter was dried for 16 h at 50 °C and weighed after. Dried faecal matter was stored in air-tight container in the freezer at 2–8 °C until proximate and fibre analyses.
Proximate analyses
Proximate composition analyses, including crude protein, crude lipid, ash, and moisture content were conducted on the experimental diets, animal soft tissues, and faecal samples following AOAC (1985). Moisture was determined in three replicates using a convection oven at 135 °C for 3 h for feeds and faecal samples. For abalone soft tissues, 105 animals were collected at the beginning of the trial and 70 animals at the end of the trial. Abalone soft tissues were freeze-dried (Christ alpha series freeze dryer, Osterode am Harz, Germany) for 48 h and moisture calculated as the weight difference. After moisture determination, dried diets, abalone tissues, and faecal samples were pulverised using a grinder (IKA A11 model analytical mill, Germany) with the addition of liquid nitrogen to avoid denaturation/oxidation of metabolites.
Protein analyses
Ground samples (2–4 mg) were weighed, and nitrogen content was determined by a CE-440 Elemental Analyser (Exeter, Chelmsford, Massachusetts, USA). The combustion and reduction temperatures were 980 and 700 °C, respectively, with pure oxygen as the combustion gas and pure helium as the carrier gas. The purge and combustion times were 15 and 20 s, respectively. The percentage of protein was calculated multiplying the total nitrogen in the samples by the conversion factor 5.8 (faecal matter and abalone tissues) according to the marine organisms described by Gnaiger and Bitterlich (1984), and 6.25 for nitrogen conversion (for feed samples).
Lipid analyses
Two different methods were used for lipid determination. For feeds, Soxhlet extraction with petroleum ether for 2.5 h was used. For animal tissues and faecal samples, crude fat extraction was performed according to Bligh and Dyer (1959) for small samples. Briefly, 0.1 g of dried samples were hydrated in 1 mL chloroform and 2 mL methanol and 800 μL distilled water. The mixture was vortexed for 2 min, and 1 mL chloroform was added. The solution was vortexed again for 30 s, and 1 mL distilled water was added to separate layers. The mixture was vortexed again for 30 s and centrifuged at 3000 rpm for 5 min. The lower organic solvent layer was collected into a pre-weighted 8-mL glass tube. A nitrogen stream was used until all solvent was vaporised. Finally, the net weight of the lipids in the sample was recorded.
Ash analyses
The ash content was obtained by complete combustion in a furnace at 550 °C for 6 h.
Carbohydrate analyses
Carbohydrate contents were determined using the Anthrone method (Yemm and Willis 1954). Briefly, 30 mg of sample (diets, abalone tissue, or faecal samples) were digested with 2.5 N HCl for 3 h. The homogenate was centrifuged at 15,000 rpm for 15 min at 4 °C. The supernatant was collected and diluted 50 times for better determination. Four millilitres of Anthrone reagent were added to each tube having 1 mL sample (diluted if needed for better determination). All the tubes were capped and incubated at 100 °C for 15 min. After cooling down, the colour developed was read against glucose standard and blank at 620 nm in a UV–visible spectrophotometer using a quartz cuvette. The values obtained were expressed as mg of glucose/g wet weight of tissue.
Digestibility of feeds
Acid insoluble ash
Acid insoluble ash analyses were performed on the experimental diets and faeces as described by Montaño-Vargas et al. (2002) using small samples due to low faecal matter biomass (0.2 g or less) as described by Liu (2022). Briefly, ashes were obtained after using a furnace at 550 °C for 16 h. Then, 0.1 g ash sample from either feed or faeces were weighed and digested in 20 mL 2N HCl. The mixture was heated in a water bath at 85 °C for 30 min and centrifuged at 4000 rpm × 10 min. The supernatant was separated and washed twice with distilled water by using a centrifugation method (4000 rpm × 10 min). The final precipitate was dried in a forced air oven at 95 °C for 2 h, and the residues weighed over a blank.
The feed digestibility coefficient of diets using acid insoluble ash (apparent digestibility) was calculated as follows:
Marker: acid insoluble ash. AD is also called apparent digestibility coefficient (in dry matter).
Amino acid composition
The total amino acid extraction of diets and animal bodies were conducted in accordance with Paramás et al. (2006). Three replicates of 50 mg dried powdered samples of abalone soft bodies and diets were mixed with 1.5 mL ultra-pure water in a 4-mL screw-top glass tube. Each tube was placed in a sonicator (Elma sonicator S30H, Elmasonic, Germany) for 1 min to promote dispersion of loads. To each sample, 1.5 mL 12M concentrate hydrochloric acid was added. Air was removed with the help of nitrogen stream and tubes were capped tightly and heated using a heating block at 110 °C for 22 h. Once the samples were digested, the tube content was filtered using an ashless filter paper grade 50 (Whatman, UK) and rinsed with 10 mL ultra-pure water. Each solution was neutralised to pH 4–6 with NaOH 1 M. Once neutralised, all volumes were adjusted to 20 mL with ultra-pure water. Each solution was centrifuged at 3000 rpm × 5 min and kept frozen until further analysis.
The amino acid standard for these analyses contained 37 amino acids (A9906 amino acid standard Sigma 485845). After extraction, samples were derivatised using the AccQ-Tag method for liquid chromatography–mass spectrometry (LC–MS). Briefly, 40 μL of samples were placed in a 1.5-mL centrifuge tube and 40 μL of Internal standard was added. The internal standard was prepared using 1 mg d-4 alanine in 100 mL methanol. Samples were vortexed and centrifuged at 10,000 rpm × 5 min at 4 °C. The supernatant collected was derivatised with AccQ-Tag reagent (6-aminoquinolyl-N-hydroxysuccinimidyl carbamate): In a plastic autosampler, 70 μL of borate buffer (7.63 g of sodium tetraborate decahydrate-borax in 90 mL miliQ water and 10 mL acetonitrile) were placed along with 10 μL of sample or standard. Samples were vortexed and subsequently 10 μL of AccQ-Tag reagent was added. Samples were vortexed immediately after AccQ-Tag addition, capped, and incubated at 55 °C for 15 min in a heating block. After this time, 400 μL of neutralising solution (10 mL formic acid in 90mL miliQ water) was added to each vial.
Samples were processed on a LC-MS system using an Agilent 1260 Infinity Quaternary LC System (Santa Clara, CA 95051 USA). The system consisted of the following components: 1260 quaternary pump (model number: G1311B), 1260 infinity ALS sampler (model number: G1329B), 1200 series autosampler thermostat FC/ALS/Therm (model number: G1330B), 1260 infinity TCC column component (model number: G1316A), and 1260 infinity diode array detector (DAD) (model number: G4212B), connected to a 6420 triple quadruple LC/MS system operating in positive electrospray ionisation mode. Multiple reaction monitoring (MRM) was used for quantification.
Phenomenex Kinetex evo C18 (2.1 × 150 mm, 1.7 μm) was used for this analysis. The mobile phase was composed of water containing 0.1% (v/v) formic acid (A) and acetonitrile containing 0.1% (v/v) formic acid (B). The initial gradient condition was 99:1 (A:B). From 3 to 8 min, solution B was increased to 13%, from 8 to 15 min was increased to 17%, from 15 to 16 min was increased to 80%, and from 16 to 17.5 min was decreased to 1%. The total run time was 30 min.
Fatty acid composition
The fatty acid extraction of diets and animal bodies were conducted in accordance with Lepage and Roy (1986). Briefly, 20 mg of samples was weighed into 8 mL screw-cap glass culture tubes (Kimax). To each sample, 2 μL of the surrogate solution (containing 20 mg tridecanoic acid in 50 mL toluene, analytical grade, Merck), and 200 μL of the extraction solution (2 mL of methanol:toluene 1:1, analytical grade, Merck) containing the internal standard (nonadecanoic acid, reference number: 72332, Sigma Aldrich). Due to the reactive nature of acetyl chloride, the solution was added slowly in a dropwise manner over a period of 1 min to avoid excessive heating. Each tube was closed tightly and sealed with Teflon tape around the cap to avoid leakage. Tubes were incubated at 100 °C for 1 h. Once incubated, the tubes were cooled before the addition of 4 mL of 6% potassium carbonate (K2CO3). Tubes were vortexed and centrifuged at 2500 rpm for 5 min at room temperature to separate the clear upper toluene phase. This phase was transferred to a 4-mL culture tube, and a scoop (0.1–0.2 g) of sodium sulphate was added to remove remaining water. The remaining liquid on the vial was pipetted with a glass pipette and placed in an amber autosampler vial. Toluene was used to top up the final solution to 1 mL.
The samples were analysed using the Agilent 6890N GC equipped with 5973 mass spectrometer detector (MSD) with an electron impact ionisation source. An Agilent DB-Fast FAME column (20 m × 0.18 mm × 0.2 μm) was used. The inlet temperature was set at 250 °C, and the samples were injected in split mode with a split ratio of 20:1. The initial column temperature was held at 85 °C for 0.74 min, then ramped at 43.84 °C /min to 175, at 6.75 °C /min to 185 °C, held for 3 min, ramped at 10 °C /min to 230 °C, held for 1.5 min, then ramped 40 °C /min to 28 °C and held for 2 min. The total run time was 13.39 min. Mass spectrometer detector transfer line was held at 280 °C, ion source at 250 °C, and the quad at 150 °C, and the solvent delay was 1.12 min. The data were acquired using selective ion monitoring (SIM) mode, and the SIM ion used for quantification are listed in supplementary table S1.
Statistical analyses
Growth and nutritional composition data (dietary treatments and abalone) were analysed by one-way analysis of variance (ANOVA) when a normal distribution was found (Kolmogorov-Smirnov test, p < 0.05) followed by pairwise comparisons with Tukey’s post hoc test. Non-parametric Kruskal Wallis test was used when data did not follow a normal distribution (p < 0.05) followed by Dunn post hoc test for multiple comparison. Homogeneity of variances was analysed using Levene’s test when samples followed a normal distribution (p < 0.05). The baseline measurements (before feeding trial) were not considered in statistical computation. The fixed factor was dietary treatment (diet F, FI, FG, FIG, and commercial feed), and the random factor was tank. Principal component analysis (PCA) was further applied to the dataset of one dependent variable (fatty acid) to further explore the relationship between the means of the samples. All univariate and multivariate analysis were carried out using MetaboAnalysit 5.0 (California, USA) and XLSTAT 2022.3.1 (Addinsoft, New York, USA).
Results
Proximate composition, amino acid, and fatty acid composition of dietary treatments
Proximate compositions of the experimental diets are shown in Table 2. The estimated energy content was in the range of 15,900–20,400 J/g for all diets. The experimental diets were isonitrogenous with no significant protein differences. Significant higher levels of protein (%) were detected in commercial feed (32.4 ± 2.7%) compared to experimental diets FI and FIG. Commercial feed had the highest carbohydrate-reducing sugars levels (39.1 ± 4.7%), whereas diet FIG and FG had the lowest values (9.1 ± 1.1% and 8.9 ± 1.9%, respectively). Diet FIG had the highest lipid level (7.2 ± 0.3%) followed by diet FI (7.0 ± 0.6%). The lowest lipid level was in the commercial feed (1.4 ± 0.3%). Diet FG had the highest ash level (1.4 ± 0.3%) followed by diet F (13.5 ± 0.1%). The commercial feed had the highest level of moisture (10.7 ± 0.1%) and diet FI the lowest (3%). The acid insoluble ash was the highest in diet FG (8.4 ± 0.7%) and lowest in diet F (1.6 ± 0.2%) and FI (1.9 ± 0.1%).
Amino acid composition of the dietary treatments are shown in Table 3. Significant differences were detected in hydroxy-proline, taurine, hydroxy-lysine, and cysteine. Fatty acid composition of the dietary treatments showed a significant variation of 20 fatty acids among the diets (Table 4). The inclusion of IM and GM promoted higher levels of myristic acid, palmitic acid, palmitoleic acid, heptadecanoic acid, oleic acid, linoleaidic acid, α-Linolenic acid (ALA), arachidic acid, behenic acid, and tricosanoic acid. The decreased levels of fish meal in diets FI and FIG reduced the contents of fatty acids cis-11-eicosenoic acid, arachidonic acid (ARA), 5,8,11,14,17-eicosapentaenoic acid (EPA), and 4,7,10,13,16,19-docosahexaenoic acid (DHA).
Feeding trial
Survival
Abalone survival was high during the feeding trial with an overall average of 99% among all dietary treatments.
Growth parameters
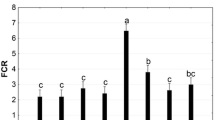
Specific growth rates determined from allometric measurements prior and post to the feeding trial revealed that the inclusion of IM and GM did not significantly affect the growth performance compared to the IM/GM free diet (diet F). A significant increased growth in abalone fed with the commercial feed compared to the experimental diets was observed (Fig. 1).
Specific growth rate for total weight (SGR TW), specific growth rate for shell length (SGR SL), and specific growth rate for shell width (SGR SW) of juvenile Haliotis iris fed on four experimental diets and a commercial feed. Data represents means ± standard deviation (n = 750). Significant differences are represented by different superscripts in the error bar after Dunn post hoc pairwise comparison (p < 0.05). Abbreviations: Diet F (fish meal based), FI (fish meal + insect meal), FG (fish meal + grape marc), FIG (fish meal + insect meal + grape marc), and commercial feed (CF)
The growth parameters showed that the inclusion of IM and GM did not significantly affect the condition factor, muscle yield, and soft body/shell ratio (Table 5). The inclusion of IM and GM significantly affected the shell length increment showing higher shell growth in abalone fed diet FG compared to diet FI. Significant differences in shell length and condition factor were also observed between experimental diets and commercial feed.
Abalone proximate composition
On a dry basis, animal soft bodies showed protein levels from 57.3 to 59.1% (Table 6). There were no significant differences in protein levels among dietary treatments. The overall protein mean was 58.1 ± 0.2 (SE). There was a significant difference in carbohydrate content in the soft body of abalone among all diets with the highest value found in animals fed on commercial feed (4.9 ± 0.5%) followed by FI (3.3 ± 0.2%), FG (3.3 ± 0.5%), F (3.1 ± 0.2%), and FIG (3.0 ± 0.3%). Animals in the baseline showed 4.6 ± 0.3% carbohydrate content. Lipid content was significantly lower in animals fed diets containing grape marc (FG and FIG) compared to diets that did not contain grape marc (F and FI). The lowest lipid value was found in animal fed commercial feed (5.7 ± 0.9%). Moisture and ash content was not significantly different among treatments. The overall mean for moisture was 81.8 ± 0.2% (SE) and for ash content 13.3 ± 0.1% (SE).
Amino acid composition
In our study, 27 amino acids were identified in abalone soft tissues, including 11 essential amino acids (valine, leucine, isoleucine, threonine, phenylalanine, lysine, histidine, methionine, tryptophan, taurine, and arginine) and 16 non-essential amino acids (hydroxyproline, ethanolamine, serine, glycine, aspartic acid, alanine, glutamic acid, citrulline, GABA, proline, hydroxylysine, ornithine, anserine, cysteine, and tyrosine).
After the feeding trial, the amino acid composition of abalone soft tissues was not greatly impacted by the experimental feeds or commercial diet (Table 7). Significant differences were only seen in eight amino acids including L-histidine, ethanolamine, glycine, L-aspartic acid, taurine, L-threonine, and L-methionine. The inclusion of IM (diet FI) did not produce significant differences in abalone compared to the IM/GM free diet (diet F). The inclusion of GM (diet FG) produced abalone with significant lower levels of L-histidine. The inclusion of both, IM and GM, produced abalone with significant lower levels of L-histidine, glycine, L-methionine, and L-phenylalanine.
The commercial feed produced abalone with significant higher ethanolamine, glycine, L-aspartic acid, taurine, and threonine compared to animals fed the IM/GM free diet (diet F). The more similar AA composition in abalone soft tissues came from animals fed the commercial diet and diet FIG and the least were the commercial feed and diet F.
Fatty acid composition
The inclusion of IM and GM significantly affected the fatty acid composition of abalone (Table 8). Diet F produced animals with significant lower levels of α-linolenic acid (ALA), linoleaidic acid, cis-11,14-eicosadienoic acid, cis-8,11,14-eicosatrienoic acid, and arachidonic acid. The experimental diet with IM inclusion (diet FI) produced animals with significant higher levels of oleic acid, linoleaidic acid, α-linolenic acid (ALA), and arachidonic acid. The experimental diet with inclusion of both, IM and GM (diet FIG), produced animals with significant higher levels of palmitic acid, palmitoleic acid, heptadecanoic acid, stearic acid, oleic acid, α-linolenic acid (ALA), arachidonic acid and tricosanoic acid. In addition, significant higher levels of EPA, palmitoleic acid, and myristic acid were observed in abalone fed on commercial feed (Fig. 2).
Faeces proximate composition
On a dry basis, the crude protein of faecal matter from animals fed diets F (23.4 ± 1.7%) and FI (25.3 ± 1.9%) was significantly higher to diets FG (21.9 ± 3.1%), FIG (16.6 ± 2.2%), and commercial feed (18.8 ± 2.5%) (Table 9). The lipid proportion of faecal matter from animals fed diets containing GM, such as diet FG (4.1 ± 1.0%) and FIG (4.5 ± 0.8%), was higher than diets F (1.8 ± 0.6%) and FI (1.7 ± 0.6%) and commercial feed (2.5 ± 0.7%). There were significant differences in carbohydrate content in the faeces from abalone receiving diets containing GM compared to those which did not. Faecal matter from animals fed on diet FI (11.7 ± 0.4%) and diet F (10.1 ± 1.1%) contained higher levels of carbohydrates compared to animals fed on diets with GM, diet FG (4.4 ± 0.6%) and FIG (4.8 ± 0.6%). Ash levels indicated higher contents of organic matter in faeces from animals fed on diets F (48.3 ± 0.3%), commercial feed (47.6 ± 1.2%), and diet FI (40.8 ± 0.3%) and lower values in animals fed on diets containing GM, such as diet FG (32.6 ± 0.1%) and FIG (40.8 ± 0.3%). Acid insoluble ash resulted significantly higher in diet FG (13.2 ± 0.2%) and lower in diet FIG (5.4 ± 0.3%).
Apparent nutrient digestibility coefficients
The mean apparent digestibility coefficients calculated using acid-insoluble ash from faecal matter and feeds are shown in Table 10. The digestibility coefficient in dry matter was significantly reduced when GM was included in the diet in diet FG (36.3 ± 0.9%). A similar trend was found in the digestibility coefficient based on organic matter, crude protein, and carbohydrate. The inclusion of IM in diet FI (93.6 ± 0.3%) did significantly affect the digestibility of the lipid proportion compared to commercial feed (39.3 ± 11.4%).
Discussion
Growth performance of abalone was not significantly affected by the inclusion of grape marc (GM) and insect meal (IM) compared to the experimental diet without these ingredients (Diet F). This indicates that GM and IM are potential alternative ingredients in aquafeeds for abalone. Grape marc and IM inclusion did not significantly affect the protein, carbohydrate, and ash levels of abalone after 165 days compared to an IM/GM free diet (diet F). In addition, the inclusion of GM and its combination with IM affected the fatty acid composition of abalone meat showing higher levels of polyunsaturated fatty acids (PUFAs) C18:2n − 6,9, C18:3n − 3 (ALA), C20:2n − 6, and C20:3n − 9. Grape marc has been successfully included in feed for ruminants (Moate et al. 2014), carp (Cyprinus carpio) (Mocanu et al. 2022), and abalone (Currie et al. 2019) providing different results depending on the species trialled. For instance, in carp, GM inclusion did not change the PUFA profile of the animal’s meat and did not affect the growth rate significantly but increased the feed conversion ratio in a feeding trial of 56 days’ duration (Mocanu et al. 2022). In abalone, the inclusion of up to 20% of Acti- Meal® (grape marc steam distilled) resulted in better growth and better feed conversion ratios compared to animals fed control diets (Currie et al. 2019). Furthermore, Acti-Meal® inclusion resulted in higher amounts of n − 3 long chain-polyunsaturated fatty acids by more than 100% (Currie et al. 2019) compared to diets without Acti-Meal®, suggesting the potential role of GM as a supplement of long chain-PUFA relevant for human dietary interest. In another study, GM inclusion has improved the amounts of certain polyunsaturated fatty acids in the liver of pigs, such as C20:5n − 3 (EPA), C22:5n − 3 9(DPA), and C22:6n − 3 (DHA) (Habeanu et al. 2015) which are known for their protective effect against lipid peroxidation and health benefits (Voicu et al. 2017). Grape marc antioxidant properties can also be attributed to large amounts of resveratrol, one of the strongest antioxidants existing mostly in the skin and pulp of red grapes, polyphenols (5–8%), such as anthocyanin, hydroxybenzoic acid, hydroxycinnamic acid, flavonols, and stilbenes (Kammerer et al. 2004; Voicu et al. 2017), and the large amount of PUFAs, mainly C18:2n − 6 (Gómez-Brandón et al. 2019; Tsiplakou and Zervas 2008).
One of the main problems of GM inclusion in feeds is the presence of antinutrients, such as lignin that can interfere with nutrient absorption if the ingredient is not pre-treated. Grape marc is high in lignin (Moate et al. 2014). Lignin is a collection of phenolic polymers, and it is one of the major components found in lignocellulosic biomass, playing a critical role in the rigidity of the cell wall and plant structure. It has been demonstrated that native lignin constitutes a barrier to nutrient digestion, and it is resistant to microbial degradation in monogastric animals (Moate et al. 2014). High lignin content lowers the energy value of animal feed and leads to reduced feed intake (Amyot et al. 2018). However, some purification techniques for lignin (enzymatic and chemical treatments) can offer new alternatives for grape marc inclusion in aquafeeds. Treated lignin, which refers to low molecular weight mix of phenolic monomers, have been successfully used as prebiotic at an inclusion of 1% in feeds for Atlantic salmon (Salmo salar) without affecting body weight, survival, and gut health compared to animals not receiving lignin supplementation (Yossa et al. 2018).
In the present study, abalone fed on commercial feed resulted in significant better growth compared to the experimental diets. Traditional commercial feeds usually incorporate growth enhancers, additional vitamins, and minerals to expedite growth, and therefore, the comparison between commercial feed and experimental diets produced in this study should only be used as a reference. Results showed that both IM and GM inclusion can be included in aquafeeds for abalone and that further optimisation of the feed formulation of the experimental diets can be carried out to promote faster growth. Another possible explanation for the significant higher growth of abalone fed on commercial feed compared to experimental diets may be attributed to the high levels of dietary lipids in experimental diets. Evidence suggests that abalone growth is impaired when fed with dietary lipids above 5–7% (Bautista-Teruel et al. 2011), which occurred in treatments FI and FIG, due to IM. Abalone are herbivores and as such, utilise carbohydrates rather than lipids as an energy source with carbohydrate:lipid ratios of 48:2 and 47:3 as optimal values for growth (Lee et al. 2019). Contrary to other aquatic species, abalone do not possess digestive enzyme lipases to metabolise high levels of dietary lipids, which might deteriorate digestive function and growth performance. The experimental diets with the highest proportion of lipids included insect meals, which have a high crude lipid proportion of 39–48% depending on the larval stage (Dreassi et al. 2017).
Apart from the crude lipid proportion difference found, the fatty acid composition differed significantly among commercial feed and experimental diets which may explain the relevance of these compounds for animal growth. Provision of essential fatty acids and fat-soluble nutrients, such as sterols and polar lipids, are crucial for abalone growth due to their role in cell membrane structure (Bautista-Teruel et al. 2011). The variation of dietary polyunsaturated fatty acids (PUFAs), which are considered to be the most important fatty acids for abalone growth (Bautista-Teruel et al. 2011) can explain the difference in growth performance due to the inclusion of IM and GM. Insect meals have high level of lipids, mainly comprising polyunsaturated fatty acids (PUFAs), such as α-linolenic acid (C18:3n-3) and α-linoleic acid (C18:2n-6); saturated fatty acids (SFA), such as palmitic acid (C16:0) and stearic acid (C18:0); and monounsaturated fatty acids (MUFA), such as oleic acid (C18:1n-9) (Dreassi et al. 2017). Insects normally do not contain eicosapentaenoic acid (C20:5n-3, EPA) and docosahexaenoic acid (C22:6n-3, DHA), which are highly elevated in the marine environment and found specially in diatoms, fish meal, and seaweeds (De la Peña et al. 2016). These two key fatty acids have been associated with defence mechanisms (Kiron et al. 1995), reduced inflammation, and disease resistance in humans (Stenberg et al. 2019).
The role of essential fatty acids in abalone has been barely studied. Previous studies have demonstrated that supplementation of certain fatty acids produce better growth; however, these requirements are species-specific and depend on the developmental stage of the animal. For instance, the supplementation of highly unsaturated fatty acids (HUFA) and polyunsaturated fatty acids (PUFAs) C18:2n − 6 and C18:3n − 3 in Haliotis asinina have produced animals with higher weight gain compared to animals receiving only their essential fatty acid requirements. Results from this study suggest that abalone do not possess fatty acyl desaturases and cannot form the previously mentioned PUFAs from the monoene C18:1n − 9, and therefore, these must be obtained from the diet as essential fatty acids (Bautista-Teruel et al. 2011). Uki et al. (1986) reported that Haliotis discus hannai require n − 3 and n − 6 HUFA as essential fatty acids. Similarly, Mai et al. (1996) reported that C18:2n − 6, C18:3n − 3, C20:4n − 6, and C20:5n − 3 are important fatty acids for the growth of abalone H. discus hannai. Durazo-Beltrán et al. (2004) have shown that HUFAs C20:4n − 6, C20:5n − 3, and C22:5n − 3 are essential in the diet of Haliotis fulgens. The above-mentioned studies showed that under healthy conditions, essential fatty acids specially PUFAs and HUFAs have critical roles in chain elongation and desaturation processes of fatty acids resulting in the production of higher PUFAs for lipid metabolism (Bautista-Teruel et al. 2011). In this study, it was observed that the inclusion of IM promoted significant higher levels of essential fatty acids such as arachidonic acid, oleic acid, and α-linolenic acid, suggesting an increased presence of omega-6 fatty acids precursors and derivatives which may impact abalone growth and fatty acid synthesis. The inclusion of GM promoted significant higher levels of α-linolenic acid. However, the inclusion of both ingredients, IM and GM, promoted a synergistic effect on increasing pentadecanoic acid, palmitic acid, stearic acid, and oleic acid. This finding may suggest that abalone can reaccommodate fatty acid products and metabolites according to the needs, while integrating carbohydrate metabolites (Wang et al. 2009) without affecting growth.
Lipid metabolism is closely involved with that of carbohydrates where a pyruvate resulting from glycolysis is oxidised to a compound called acetyl CoA which is the substrate for synthesising fatty acids. Abalone do not possess enzymes to metabolise fatty acids, and therefore, an excess of dietary lipids might be translated into poor growth (Lee et al. 2019).
In our study, the fatty acid composition of the commercial feed showed higher levels of myristic acid (C14:0), palmitoleic acid (C16:1n − 7), heptadecanoic acid (C17:0), cis-8,11,14-eicosatrienoic acid (C20:3n − 9), and 5,8,11,14,17-eicosapentaenoic acid–EPA (C20:5n − 3) compared to the experimental diets. From all these fatty acids, C20:5n − 3 has been highlighted in abalone nutrition because some species such as H. asinina do not synthesise it de novo, and therefore, it needs to be included in their diet. C20:5n − 3 (EPA) is further elongated to C22:6n − 3 (DHA), which plays an essential role in osmotic regulation and salinity changes (Dunstan et al. 1996). Our results show that C22:6n − 3 (DHA) was not significantly affected by the diet, suggesting that this compound could have been used for growth or other living processes. However, C20:5n − 3 (EPA) levels were affected by feed, having the highest values in animals fed the commercial feed. This finding concurs with previous studies suggesting the importance of C20:5n-3 (EPA) in feeds and its potential inclusion to promote abalone growth. However, further studies to evaluate the role of its inclusion as a supplement will need to be conducted, with special attention to the effects on nutrition, health, and immunity. According to Mai et al. (1995) growth promotion of abalone is collectively affected by the combination of different essential fatty acids rather than a single effect of one particular nutrient. The importance of adding supplements to the final formulation, such as fatty acids to optimise abalone feed, becomes more relevant as terrestrial alternative ingredients are being used for feed formulations for marine animals.
Another possible explanation for the better growth of commercial feed versus the experimental diets is the difference in carbohydrate levels amongst diets. In the wild, abalone consume seaweeds which consist of 40–50% carbohydrates. Abalone metabolism is carbohydrate-based (Bautista-Teruel et al. 2011), and they manage to metabolise complex carbohydrates by excreting amylase, cellulase, laminarinase, carrageenase, and alginase (Lee et al. 2017). The introduction of terrestrial carbohydrate sources, such as IM and GM, may result in the inability of abalone to degrade dietary fibre due to the lack of specific digestive enzymes. For example, IM contains chitin, which would need to be degraded by chitinase and GM degraded by lignin peroxidase to be used as an energy metabolite. To our knowledge, there are no studies on the native chitinase or lignin peroxidase activity on abalone and their use as energy source or prebiotic still needs further research. The diets including GM (Diet FG and FIG) had 16.1% and 15.4% fibre levels, and the diets without GM (diets F and FI) had 9% and 7.5%; both groups with higher fibre levels compared to the commercial feed (3.7%). As fibre compounds are indigestible, abalone increased the faecal output by allocating energy to excretion rather than using the energy in growth. Abalone only use 5% of the ingested energy for growth (Barkai and Griffiths 1988). Hence, if energy expenditure is allocated to additional processes, such as extra excretion, the risk of affecting growth performance is high.
The inclusion of GM and IM significantly affected the digestibility of the feed. Grape marc mainly reduced the digestibility of the carbohydrates and protein, whereas IM had an effect on the lipid digestibility of the diets. This finding indicates that GM and IM can be included in the diet up to 100% and 25%, respectively, and although they have a synergistic effect on reducing digestibility, abalone growth performance is not negatively affected compared to diet F, which did not have IM nor GM. Based on the digestibility coefficient of the commercial feed, which was comparatively less than diet F and diet FI, it is suggested that animals needed less amount of commercial feed to grow compared to diets F and FI. Although diet F had the highest digestibility coefficient, growth in this group of animals was not greatly improved. Further investigation on the digestibility of diets including IM and GM needs to integrate feed intake, to conclude the absolute effect on digestibility in abalone.
The present study delivered the experimental feeds in an alginate matrix due to the well-known effect of alginate as an immunostimulant. However, immunostimulatory properties may be jeopardised by higher doses of alginate. A previous study has shown positive effects of alginate inclusion doses of ≤ 3.0 g per kg−1 in Haliotis diversicolor supertexta showing an improved immune response, increasing resistance to pathogens such as Vibrio parahaemolyticus, increased activity of phenoloxidase (PO), and super oxide dismutase (SOD) (Cheng and Yu 2013). In our study, alginate doses were close to 100 g per kg−1, which is significantly more than the amount of immunostimulants added in the feed for other species, such as tilapia (O. niloticus) (Van Doan et al. 2016), and shrimp (Penaeus monodon) (Liu et al. 2006). The overdose of immunostimulants, such as alginate, may be the cause of slow growth. However, there is not enough evidence to support this claim. Further studies of long-term effects or oral administration of immunostimulants in aquatic animals are required. There are possible negative feedback responses that can be activated once administration of immunostimulants is sustained for long periods of time, depending on species and doses.
This study aimed at providing data on two sustainable ingredients which can be included in aquafeeds to replace fish meal at some extent. Although formulations with IM and GM inclusion did not outperform formulations free of these two ingredients, they do not jeopardise abalone growth or nutritional profile. Insect meal and GM represent cheaper alternatives compared to fish meal by being reared on waste, low energy, and high efficiency (insects), and by contributing to reuse a biowaste that represents and environmental issue (grape marc). The optimisation of these two ingredients to reduce deterrents, antinutrients, and increase digestibility will allow to produce cheaper feed alternatives with less environmental costs.
Conclusion
This study reported the effect of insect meal from Tenebrio molitor and grape marc inclusion on Haliotis iris proximate, fatty, and amino acid composition, and apparent digestibility. The inclusion of insect meal and grape did not produce significant variations in protein and carbohydrate proportions of animal tissues, but differences were observed in the lipid proportion. The amino acid composition of abalone was affected by the inclusion of insect meal and grape marc modifying levels of 8 amino acids, such as taurine, threonine, and methionine. The fatty acid composition was significantly modified due to the inclusion of insect meal and grape marc suggesting a key role of fatty acids in growth. Increased levels of specific fatty acids in abalone were correlated with insect meal and grape marc fatty acid composition. The inclusion of insect meal and grape marc increased the digestibility of diets in the lipid component and decreased in the protein and carbohydrate component. The insights gained from this study suggest that insect meal and grape marc are valuable ingredients for aquaculture feed in terms of producing an acceptable nutritional profile for human consumption. However, further studies with gradual inclusions of insect meal and grape marc are recommended to evaluate correlation of nutrients and presence/absence of specific amino acids and fatty acids.
Data availability
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Amyot L, McDowell T, Martin SL, Renaud J, Gruber MY, Hannoufa A (2018) Assessment of antinutritional compounds and chemotaxonomic relationships between Camelina sativa and its wild relatives. J Agric Food Chem 67(3):796–806. https://doi.org/10.1021/acs.jafc.8b04724
Association of Official Analytical Chemists (AOAC) (1985) Official methods of analysis, 14th edn. Association of Official Analytical Chemists, Washington, DC
Azagoh C, Ducept F, Garcia R, Rakotozafy L, Cuvelier ME, Keller S, Lewandowski R, Mezdour S (2016) Extraction and physicochemical characterization of Tenebrio molitor proteins. Food Res Int 88:24–31. https://doi.org/10.1016/j.foodres.2016.06.010
Barkai R, Griffiths C (1988) An energy budget for the South African abalone Haliotis midae Linnaeus. J Molluscan Stud 54(1):43–51
Bautista-Teruel MN, Koshio SS, Ishikawa M (2011) Diet development and evaluation for juvenile abalone, Haliotis asinina Linne: lipid and essential fatty acid levels. Aquaculture 312(1-4):172–179. https://doi.org/10.1016/j.aquaculture.2011.01.004
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37(8):911–917. https://doi.org/10.1139/o59-099
Britz PJ (1996) The suitability of selected protein sources for inclusion in formulated diets for the South African abalone, Haliotis midae. Aquaculture 140(1-2):63–73. https://doi.org/10.1016/0044-8486(95)01197-8
Cheng W, Yu J-S (2013) Effects of the dietary administration of sodium alginate on the immune responses and disease resistance of Taiwan abalone, Haliotis diversicolor supertexta. Fish Shellfish Immunol 34(3):902–908. https://doi.org/10.1016/j.fsi.2012.12.020
Cho CY, Slinger SJ, Bayley HS (1982) Bioenergetics of salmonid fishes: energy intake, expenditure and productivity. Comp Biochem Physiol B Comp Biochem 73(1):25–41. https://doi.org/10.1016/0305-0491(82)90198-5
Cho SH (2010) Effect of fishmeal substitution with various animal and/or plant protein sources in the diet of the abalone Haliotis discus hannai Ino. Aquac Res 41:e587–e593. https://doi.org/10.1111/j.1365-2109.2010.02561.x
Currie KL, Purvis M, Bansemer M, Harris JO, Stone DAJ (2019) Dietary inclusion of acti-meal improves growth and feed utilisation of greenlip abalone (Haliotis laevigata). Aquaculture 498:364–370. https://doi.org/10.1016/j.aquaculture.2018.08.074
De la Peña MR, Teruel MB, Oclarit JM, Amar MJA, Ledesma EGT (2016) Use of thraustochytrid Schizochytrium sp. as source of lipid and fatty acid in a formulated diet for abalone Haliotis asinina (Linnaeus) juveniles. Aquac Int 24(4):1103–1118. https://doi.org/10.1007/s10499-016-9974-3
Dlaza TS, Maneveldt GW, Viljoen C (2008) Growth of post-weaning abalone Haliotis midae fed commercially available formulated feeds supplemented with fresh wild seaweed. Afr J Mar Sci 30(1):199–203. https://doi.org/10.2989/AJMS.2008.30.1.22.472
Dreassi E, Cito A, Zanfini A, Materozzi L, Botta M, Francardi V (2017) Dietary fatty acids influence the growth and fatty acid composition of the yellow mealworm Tenebrio molitor (Coleoptera: Tenebrionidae). Lipids 52(3):285–294. https://doi.org/10.1007/s11745-016-4220-3
Dunstan GA, Baillie HJ, Barrett SM, Volkman JK (1996) Effect of diet on the lipid composition of wild and cultured abalone. Aquaculture 140(1):115–127. https://doi.org/10.1016/0044-8486(95)01191-9
Durazo-Beltrán E, Viana MAT, D'Abramo LR, Toro-Vazquez JF (2004) Effects of starvation and dietary lipid on the lipid and fatty acid composition of muscle tissue of juvenile green abalone (Haliotis fulgens). Aquaculture 238(1):329–341. https://doi.org/10.1016/j.aquaculture.2004.03.025
Fasolin LH, Pereira RN, Pinheiro AC, Martins JT, Andrade CCP, Ramos OL, Vicente AA (2019) Emergent food proteins — towards sustainability, health and innovation. Food Res Int 125:108586. https://doi.org/10.1016/j.foodres.2019.108586
Ferrer P, Kallas Z, Reig L, Amores de Gea D (2019) The use of insect meal as a sustainable feeding alternative in aquaculture: current situation, Spanish consumers’ perceptions and willingness to pay. J Clean Prod 229:10–21. https://doi.org/10.1016/j.jclepro.2019.05.012
Fleming A, Van Barneveld R, Hone P (1996) The development of artificial diets for abalone: a review and future directions. Aquaculture 140(1-2):5–53. https://doi.org/10.1016/0044-8486(95)01184-6
Gnaiger E, Bitterlich G (1984) Proximate biochemical composition and caloric content calculated from elemental CHN analysis: a stoichiometric concept. Oecologia 62(3):289–298
Gómez-Brandón M, Lores M, Insam H, Domínguez J (2019) Strategies for recycling and valorization of grape marc. Crit Rev Biotechnol 39(4):437–450. https://doi.org/10.1080/07388551.2018.1555514
Habeanu M, Lefter N, Ropota M, Chedea V, Gheorghe A, Toma SM, Ciurescu G, Dragomir C (2015) Dried grape pomace influenced fatty acids composition of Longissimus dorsi muscle and plasma polyphenols spectrum in finishing pigs. Indian J Anim Sci 85(7):786–789
Henry MA, Gai F, Enes P, Peréz-Jiménez A, Gasco L (2018) Effect of partial dietary replacement of fishmeal by yellow mealworm (Tenebrio molitor) larvae meal on the innate immune response and intestinal antioxidant enzymes of rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol 83:308–313. https://doi.org/10.1016/j.fsi.2018.09.040
Jung WG, Kim HS, Lee KW, Kim YE, Choi DK, Jang BI, Cho SH, Choi CY, Kim BH, Joo YI (2016) Growth and body composition effects of tuna byproduct meal substituted for fish meal in the diet of juvenile abalone, Haliotis discus. J World Aquac Soc 47(1):74–81. https://doi.org/10.1111/jwas.12255
Kammerer D, Claus A, Carle R, Schieber A (2004) Polyphenol screening of pomace from red and white grape varieties (Vitis vinifera L.) by HPLC-DAD-MS/MS. J Agric Food Chem 52(14):4360–4367. https://doi.org/10.1021/jf049613b
Kiron V, Fukuda H, Takeuchi T, Watanabe T (1995) Essential fatty acid nutrition and defence mechanisms in rainbow trout Oncorhynchus mykiss. Comp Biochem Physiol A Physiol 111(3):361–367. https://doi.org/10.1016/0300-9629(95)00042-6
Lee KW, Kim HJ, Kim HS, Choi DG, Bok J II, Cho SH, Min BH, Kim KD, Joo YI (2017) Effects of dietary carbohydrate sources on growth and body composition of juvenile abalone (Haliotis discus, Reeve). J Shellfish Res 36(1):151–156. https://doi.org/10.2983/036.036.0115
Lee KW, Kim HS, Jeong HS, Kim J, Yun AY, Cho SH (2019) Effect of dietary carbohydrate-to-lipid ratio on growth and carcass composition of juvenile abalone, Haliotis discus, Reeve 1846. J World Aquac Soc 50(3):604–613. https://doi.org/10.1111/jwas.12485
Lepage G, Roy CC (1986) Direct transesterification of all classes of lipids in a one-step reaction. J Lipid Res 27(1):114–120
Li L, Zhao Z, Liu H (2013) Feasibility of feeding yellow mealworm (Tenebrio molitor L.) in bioregenerative life support systems as a source of animal protein for humans. Acta Astronaut 92(1):103–109. https://doi.org/10.1016/j.actaastro.2012.03.012
Liu C-H, Yeh S-P, Kuo C-M, Cheng W, Chou C-H (2006) The effect of sodium alginate on the immune response of tiger shrimp via dietary administration: activity and gene transcription. Fish Shellfish Immunol 21(4):442–452. https://doi.org/10.1016/j.fsi.2006.02.003
Liu K (2022) New and improved methods for measuring acid insoluble ash. Anim Feed Sci Technol 288:115282. https://doi.org/10.1016/j.anifeedsci.2022.115282
Mai K, Mercer JP, Donlon J (1995) Comparative studies on the nutrition of two species of abalone, Haliotis tuberculata L. and Haliotis discus hannai Ino. III. Response of abalone to various levels of dietary lipid. Aquaculture 134(1-2):65–80. https://doi.org/10.1016/0044-8486(95)00043-2
Mai K, Mercer JP, Donlon J (1996) Comparative studies on the nutrition of two species of abalone, Haliotis tuberculata L. and Haliotis discus hannai Ino. V. The role of polyunsaturated fatty acids of macroalgae in abalone nutrition. Aquaculture 139(1-2):77–89. https://doi.org/10.1016/0044-8486(95)01158-7
Makkar HPS, Tran G, Henze V, Ankers P (2014) State-of-the-art on use of insects as animal feed. Animl Feed Sci Technol 197:1–33. https://doi.org/10.1016/j.anifeedsci.2014.07.008
Massey University. (2020). Repurposing Grape Marc. Retrieved October 1, 2022 from https://www.marlborough.govt.nz/repository/libraries/id:1w1mps0ir17q9sgxanf9/hierarchy/Documents/Services/Recycling%20and%20Resource%20Recovery/Milestone_Report_Template_MDC_Grape_Marc_Milestone_3_%28Year_1%29.pdf
Moate PJ, Williams SRO, Torok VA, Hannah MC, Ribaux BE, Tavendale MH, Eckard RJ, Jacobs JL, Auldist MJ, Wales WJ (2014) Grape marc reduces methane emissions when fed to dairy cows. J Dairy Sci 97(8):5073–5087. https://doi.org/10.3168/jds.2013-7588
Mocanu E, Dima FM, Savin V, Popa MD, Patriche N (2022) The effect of diets with added grape marc on growth parameters and meat quality of carp (Cyprinus carpio). Sci Papers Ser D Anim Sci 65(2): 449-455
Montaño-Vargas J, Shimada A, Vásquez C, Viana MT (2002) Methods of measuring feed digestibility in the green abalone (Haliotis fulgens). Aquaculture 213(1):339–346. https://doi.org/10.1016/S0044-8486(02)00020-0
Nogales-Mérida S, Gobbi P, Dudek K, Józefiak D, Kierończyk B, Mazurkiewicz J, Rawski M, Józefiak A (2018) Insect meals in fish nutrition. Rev Aquac. https://doi.org/10.1111/raq.12281
Nugroho R, Nur F (2018) Insect-based protein: future promising protein source for fish cultured. IOP Confe Ser Earth Environ Sci (144):012002
Paramás AMG, Bárez JAG, Marcos CC, García-Villanova RJ, Sánchez JS (2006) HPLC-fluorimetric method for analysis of amino acids in products of the hive (honey and bee-pollen). Food Chem 95(1):148–156. https://doi.org/10.1016/j.foodchem.2005.02.008
Péron G, François Mittaine J, Le Gallic B (2010) Where do fishmeal and fish oil products come from? An analysis of the conversion ratios in the global fishmeal industry. Mar Policy 34(4):815–820. https://doi.org/10.1016/j.marpol.2010.01.027
Prime Minister’s Chief Science Advisor. (2021). Pāua fisheries and industry-led management. Retrieved January 1, 2022 from https://www.pmcsa.ac.nz/2021/02/21/paua-fisheries-and-industry-led-management/
Spanghero M, Salem A, Robinson P (2009) Chemical composition, including secondary metabolites, and rumen fermentability of seeds and pulp of Californian (USA) and Italian grape pomaces. Anim Feed Sci Technol 152(3-4):243–255. https://doi.org/10.1016/j.anifeedsci.2009.04.015
Stenberg OK, Holen E, Piemontese L, Liland N, Lock E-J, Espe M, Belghit I (2019) Effect of dietary replacement of fish meal with insect meal on in vitro bacterial and viral induced gene response in Atlantic salmon (Salmo salar) head kidney leukocytes. Fish Shellfish Immunol 91:223–232. https://doi.org/10.1016/j.fsi.2019.05.042
Terova G, Rimoldi S, Ascione C, Gini E, Ceccotti C, Gasco L (2019) Rainbow trout (Oncorhynchus mykiss) gut microbiota is modulated by insect meal from Hermetia illucens prepupae in the diet. Rev Fish Biol Fish 29(2):465–486. https://doi.org/10.1007/s11160-019-09558-y
The New Zealand Government. (2019). The government’s aquaculture strategy to 2025. Retrieved September 1, 2022 from https://www.mpi.govt.nz/dmsdocument/15895-the-governments-aquaculture-strategy-to-2025
Tsiplakou E, Zervas G (2008) The effect of dietary inclusion of olive tree leaves and grape marc on the content of conjugated linoleic acid and vaccenic acid in the milk of dairy sheep and goats. J Dairy Res 75(3):270–278. https://doi.org/10.1017/S0022029908003270
Uki N, Sugiura M, Watanabe T (1986) Requirement of essential fatty acids in the abalone Haliotis discus hannai. Bull Japan Soc Sci Fish 52(6):1013–1023
Van Doan H, Tapingkae W, Moonmanee T, Seepai A (2016) Effects of low molecular weight sodium alginate on growth performance, immunity, and disease resistance of tilapia, Oreochromis niloticus. Fish Shellfish Immunol 55:186–194. https://doi.org/10.1016/j.fsi.2016.05.034
Viera MP, Courtois de Viçose G, Robaina L, Izquierdo MS (2015) First development of various vegetable-based diets and their suitability for abalone Haliotis tuberculata coccinea Reeve. Aquaculture 448:350–358. https://doi.org/10.1016/j.aquaculture.2015.05.031
Voicu D, Vasilachi A, Voicu I (2017) Influence of feeding dried grape marc to fattening steers on the fatty acids composition of the hepatic tissue. Indian J Anim Sci 87(9):1141–1144
Wang L, Li J, Jin JN, Zhu F, Roffeis M, Zhang XZ (2017) A comprehensive evaluation of replacing fishmeal with housefly (Musca domestica) maggot meal in the diet of Nile tilapia (Oreochromis niloticus): growth performance, flesh quality, innate immunity and water environment. Aquac Nutr 23(5):983–993. https://doi.org/10.1111/anu.12466
Wang W, Mai K, Zhang W, Xu W, Ai Q, Yao C, Li H (2009) Effects of dietary carbohydrates sources on lipids compositions in abalone, Haliotis discus hannai Ino. J Ocean Univ China 8(3):254–258. https://doi.org/10.1007/s11802-009-0254-y
Yemm E, Willis A (1954) The estimation of carbohydrates in plant extracts by anthrone. Biochem J 57(3):508. https://doi.org/10.1042/bj0570508
Yossa R, Levesque S, Groman DB, Lora JH (2018) Preliminary evaluation of purified lignin and hemicellulose as prebiotics candidates for Atlantic Salmon, Salmo salar L. J Appl Aquac 30(3):256–271. https://doi.org/10.1080/10454438.2018.1439795
Acknowledgements
We would like to thank the New Zealand Abalone company, Bluff, Invercargill, New Zealand, for providing the experimental animals and the location for this study. We especially thank members of the Drug Delivery Research Group and Aquaculture Biotechnology Research Group, especially Sara Masoomi, Shaakila Nordien, Martin Jinchen Guo, Joanna Copedo, Leonie Venter, Tim Young, Shaneel Sharma, and Awanis Azizan at the Auckland University of Technology (AUT) for their assistance with sampling and data collection. This project was performed under a collaborative programme between AUT and The New Zealand Abalone Company.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions The authors received in-kind support from The New Zealand Abalone Co. and financial support from the Aquaculture Biotechnology Research Group, Auckland University of Technology, Auckland, New Zealand.
Author information
Authors and Affiliations
Contributions
Natalia Bullon: conceptualisation (equal); data curation (lead); formal analysis (lead); investigation (lead); methodology (lead); project administration (lead); validation (lead); writing—original draft (lead); writing–review and editing (lead). Nazimah Hamid: writing—review and editing (supporting), data curation (supporting); Ali Seyfoddin: conceptualisation (equal); funding acquisition (equal); resources (equal); supervision (supporting). Moganakumaar Manivannan: methodology and field work (supporting). Andrea C. Alfaro: conceptualisation (equal); funding acquisition (lead); resources (lead); supervision (lead); writing–review and editing (equal).
Corresponding author
Ethics declarations
Ethics approval
Not applicable for this study.
Competing interests
The authors declare no competing interests.
Additional information
Handling editor: Gavin Burnell
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(NUMBERS 180 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bullon, N., Seyfoddin, A., Hamid, N. et al. Effects of insect meal and grape marc in the nutritional profile, growth, and digestibility of juvenile New Zealand farmed abalone. Aquacult Int 32, 1507–1536 (2024). https://doi.org/10.1007/s10499-023-01227-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-023-01227-z