Abstract
The increasing concern for invertebrate welfare has led to improvements in management procedures and protective legislation; under discussion is the use of anaesthetic agents to improve the welfare of farmed prawns. In parallel, the aquatic environments present changes in their physical and chemical characteristics, like the acidification of waters. With this perspective, our study analysed the effectiveness of Ocimum gratissimum essential oil (EO-OG) as a natural anaesthetic for Macrobrachium rosenbergii at different concentrations and water pHs. Juvenile M. rosenbergii were exposed to five different EO-OG concentrations (0, 100, 200, 300, and 400 μL L−1) and vehicle control (ethanol), at two water pHs (8.08 ± 0.10 and 6.42 ± 0.15). Induction time was EO-OG concentration-dependent regardless of water pH; i.e. the time required for sedation and anaesthesia decreased with the increase of EO-OG concentration. No correlation was found between recovery time and EO-OG concentration. The most effective EO-OG concentration was 400 μL L−1, with prawn sedation in 2.0–2.5 min, anaesthesia in 3.5–4.1 min, and full recovery in 16 min, regardless of water pH. The different tested water pHs influenced sedation time; i.e. sedation time was higher in mild acidic water than in mild alkaline water. Anaesthesia induction and recovery times were similar at both water pHs. Survival rates were 100% in all experimental groups. In summary, EO-OG is effective as a sedative and anaesthetic for juvenile M. rosenbergii in routine procedures, at the recommended concentration of 400 μL L−1, with mild alkaline water.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The giant river prawn (Macrobrachium rosenbergii, De Man 1879) is a tropical species highly valued for freshwater aquaculture. Characteristics such as rusticity and resistance to disease, consistent reproductive performance, and high fecundity under captivity make this species particularly relevant for global prawn farming (Cavallo et al. 2001; Pillai et al. 2022). In 2018, the global production of M. rosenbergii was 234.4 thousand tonnes (FAO 2020). Since 2007, several countries including Brazil have been recovering from negative economic impacts on prawn production and are now expanding their farming of M. rosenbergii (David et al. 2018). The progress demands continue high-quality scientific studies to support a sustainable and profitable growing industry. This includes the development of sedatives and anaesthetics to be used during prawn transportation, handling, biometric procedures, and sampling.
In recent years, the Intergovernmental Panel on Climate Change has drawn attention that different anthropogenic activities have contributed to an increase in global temperature, an escalation in the atmospheric concentration of carbonic acid, and a reduction in the pH in both terrestrial and aquatic environments (Collins et al. 2013; IPCC 2014; Abram et al. 2019). With the evidence of water acidification, it is necessary to better comprehend whether different water pHs would influence prawn production and modulate the effect of anaesthetic agents.
Different anaesthetics are used in decapods aquaculture. Many of them, however, may cause adverse effects on both animals and handlers. Studies have proposed natural alternatives to synthetic anaesthetics, for instance, menthol (Li et al. 2018a), eugenol (Jiang et al. 2020), and the essential oils of Lippia alba and Ocimum gratissimum (Becker et al. 2021). Due to their essential oils, aromatic plants are gaining popularity in aquaculture. Essential oils are natural compounds formed by a complex blend of low molecular weight, fat-soluble, and volatile substances; they are secondary metabolites extracted from the secretory glands of herbs and parts of a plant (Ingraham 2018).
The essential oil extracted from the aromatic herb Ocimum gratissimum L., Lamiaceae family (common names: clove basil, African basil, wild basil), has been used in several routine procedures in aquaculture, for example, as antimicrobial and antiparasitic (Bandeira Jr et al. 2017), sedative and anaesthetic (Souza et al. 2019; Boaventura et al. 2020, 2021; Silva et al. 2020; Becker et al. 2021; Ferreira et al. 2021), and anthelmintic (Meneses et al. 2018; Silva et al. 2020). Original from tropical Africa, India, and Southeast Asia, O. gratissimum currently has a pantropical and subtropical distribution across the world (Rojas-Sandoval 2018). The therapeutic properties of O. gratissimum essential oil are attributed to its main compounds (eugenol and 𝛽-caryophyllene). The common Brazilian chemotypes contain the major compounds eugenol and 1,8-cineole (Silva et al. 2012; Ribeiro et al. 2016; Silva et al. 2020). As O. gratissimum is currently consumed as spice and tea by humans (Di Stasi et al. 2002), O. gratissimum is expected to be accepted and approved to be used as an anaesthetic agent for fish and crustaceans used for human consumption.
Thus, our study aimed at evaluating the anaesthetic effect of O. gratissimum essential oil on juvenile giant river prawn (M. rosenbergii) exposed to different water pHs. That is, comparing the anaesthetic effect of the EO using the ideal water pH for M. rosenbergii juveniles’ production (i.e. water pH 7.0–8.5, FAO 2003) and mild acid water simulating water acidification (e.g. water pH 6.02). To the best of our knowledge, this is the first study reporting the potential use of O. gratissimum essential oil in freshwater prawns.
Materials and methods
Plant origin, essential oil extraction, and characterization
Leaves of O. gratissimum were collected from the botanical collection of the Federal University of Santa Maria (UFSM) campus Frederico Westphalen, Rio Grande do Sul, Brazil. The specimen was identified by Adelino Alvares Filho and deposited in the Biology Department Herbarium, Federal University of Santa Maria, Brazil, register number SMDB 11167. The essential oil (EO) was extracted from the leafy material by hydro-distillation using a Clevenger-type apparatus for 2 h (European Pharmacopeia 2007). In order to separate the EO from the hydrolate, both were collected and transported to the UFSM Campus Santa Maria, where the mixture was transferred to a separating funnel, being subjected to liquid-liquid partition with hexane (analytical degree). The hexane fraction was concentrated in a rotary evaporator (40 °C), obtaining the pure EO. The essential oil of Ocimum gratissimum (EO-OG) was then stored at -4° C, in an amber glass bottle, until further analysis and use. The EO-OG composition was analysed by gas chromatography-mass spectroscopy (GC-MS)–total ion chromatogram (TIC), performed with a gas chromatograph (Agilent-7890A) coupled with a mass selective detector (Agilent-5975C), using HP5-MS column and EI-MS of 70eV. Conditions were as described by Bandeira Jr et al. (2017), i.e. split inlet 1:100, program temperature 40–320 °C at 4 °C min−1, carrier gas He, flow rate of 1 mL min−1, injector (2 μL), and detector temperature 250 °C. Kováts retention index and mass spectral library were used for comparative identification of the EO-OG chemical composition (NIST 2010). Table 1 shows the major constituents of the EO-OG.
Experimental design
Juvenile Macrobrachium rosenbergii (weight 0.40 ± 0.03 g; length: 2.43 ± 0.08 cm), regardless of sex, were obtained from the Shrimp Culture Laboratory, Federal University of Paraná, Palotina, Paraná, Brazil. Animals were initially kept for 30 days in a 300-L tank, nursery system, with constant aeration and continuous water flow, and fed with a commercial shrimp diet (Guabitech® Inicial PL, 40% crude protein) twice a day at 08h30 and 16h30 at equal rations. Water parameters were monitored twice a day and kept under optimal conditions for M. rosenbergii farming throughout the trial. The average ± SD observed during the trial were water temperature (20.30 ± 0.82 °C), dissolved oxygen (7.82 ± 1.34 mg L−1), total ammonia nitrogen (0.045 ± 0.035 mg N L−1), nitrite (0.028 ± 0.038 mg L−1), nitrate (4.426 ± 4.339 mg L−1), alkalinity (85.0 ± 7.29 mg CaCO3 L−1), and hardness (83.8 ± 23.2 mg CaCO3 L−1). Animals in the moult stage were not used and no moulting was observed during or after the exposure time to EO-OG. Length measurements occurred once a day with 25 postlarvae (PL) per tank. Mortality was monitored twice a day. Natural photoperiod was set at 06h/18h, light/dark cycle.
After 30 days, prawns were fasted for 24 h before the beginning of the anaesthetic trial. Subsequently, 144 prawns were randomly collected from the nursery system and distributed into glass recipients with 300 mL freshwater content, divided into 24 (3 prawns per unit, per water pH) experimental units. Prawns were then exposed to the following concentrations of EO-OG, previously diluted in ethanol analytical degree (1:10, v/v): 0 (negative control with freshwater only), 100, 200, 300, and 400 μL L−1. A sixth group of animals was exposed to the concentration of ethanol used to dilute the highest concentration of EO-OG, which corresponded to 3600 μL L−1 of ethanol analytical degree (vehicle control). In each of the experimental groups, performed in quadruplicate, three animals were used simultaneously per aquarium, totalling 12 juveniles of M. rosenbergii per concentration of EO-OG divided into four experimental units. Each shrimp was used only once. Additionally, each EO-OG concentration was tested at two different water pHs: alkaline (8.08 ± 0.10) and acidic (6.42 ± 0.15). Figure 1 illustrates the experimental design. The water pH was adjusted by adding sodium bicarbonate (NaHCO3) or hydrochloric acid (1M HCl), respectively.
Sedation and anaesthesia induction and recovery
Sedation and anaesthesia induction and recovery times were evaluated as described by Coyle et al. (2005). The sedation state was characterized as a partial loss of balance and the presence of a positive reaction to touch stimulus. The anaesthesia state was attributed to the complete loss of balance and lack of reaction to touch stimulus. Finally, the recovery stage was determined when the shrimp regained balance and reached the normal, upright position at the bottom of the tank. The maximum observation time of the induction stage was 30 min. A digital stopwatch was used to record the times, expressed in seconds. After a maximum of 30 min, animals were transferred to a recovery aquarium with anaesthetic-free water. Animal survival was observed immediately after reaching the recovery time, and again after 24 h.
Statistical analysis
All data are expressed as mean ± standard error of the mean (S.E.M.). The normality and homoscedasticity of variances were confirmed with Shapiro-Wilk and Levene’s tests, respectively. Comparison of anaesthesia induction and recovery times and different EO-OG concentrations was performed using Nested-ANOVA, and comparison among different water pH was performed using two-way ANOVA, followed by Tukey post hoc test. When necessary, logarithmic transformation was used to make the data satisfy the ANOVA assumptions. Additionally, the evaluation of the anaesthetic activity was performed through exponential regression analysis (concentration × time of induction to anaesthesia). Statistical analyses were performed using Minitab 17, Sigma Plot 14.0, and Statistica 7.1 software, with a significance level of 95% (p < .05).
Results
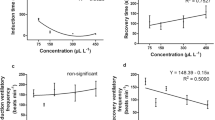
The EO-OG induced sedation and anaesthesia in juvenile M. rosenbergii at all tested concentrations. The increase in the EO-OG concentration proportionally decreased the time required for sedation and anaesthesia, regardless of the water pH; that is, induction times for sedation and anaesthesia with EO-OG were concentration dependent. The significantly highest recovery time was observed in prawns anaesthetised with the highest EO-OG concentration (400μL L−1) when in alkaline water, and 400μL L−1 and 200 μL L−1 when prawns were kept in acidic water (Table 2). Thus, the highest tested concentration of EO-OG (400 μL L−1) resulted in the significantly fastest sedation and anaesthetic effect (2.0–2.5 min and 3.5–4.1 min, respectively). Alkaline water accelerated the EO-OG anaesthesia induction effect, with a recovery time of 16 min, in both tested water pHs. Anaesthesia recovery time did not present a direct relation with the EO-OG concentrations used, particularly in acidic water (Table 2).
There was a significant difference in the anaesthetic effect of EO-OG in the three analysed parameters (i.e. times of sedation, anaesthesia induction, and anaesthesia recovery; p < .05). Our results also showed a significant interaction effect between anaesthetic concentration and water pHs (interaction effect p < .05), suggesting an influence of both factors on the anaesthetic effect of EO-OG (Table 3). Sedation induction time was significantly higher on M. rosenbergii kept in acidic water when exposed to all EO-OG tested concentrations (p < .001), except 300 μL L−1. However, anaesthesia induction and recovery times in mild alkaline and acidic water were not significantly different. Ethanol analytical degree per se (vehicle control) did not present any sedative or anaesthetic effect in the juvenile M. rosenbergii; hence, it is conceivable not to have any synergic effect with EO-OG. Prawns in the negative control group did not show any symptom related to sedation or anaesthesia. No mortality occurred from anaesthesia induction or 24 h after exposure to EO-OG in any of the tested concentrations or different water pHs studied.
Discussion
Our study proposes the use of the EO-OG as an effective natural anaesthetic for the prawn M. rosenbergii. The concentration of 400 μL L−1 proved to be the most appropriate to be used in routine procedures with juveniles of M. rosenbergii. This concentration showed the ideal time for sedation, anaesthesia induction, and recovery for short-duration procedures on shrimp farms, i.e. less than 5 min to induce deep anaesthesia and close to 15 min for full anaesthesia recovery (Keene et al. 1998; Tsantilas et al. 2006; Park et al. 2009). Practices and procedures such as weighting, sampling, transportation, and welfare checks can benefit from sedation and analgesia. Handling, air exposure, transportation, and disturbance cause distress in prawns and impact animal health and performance (Fotedar and Evans 2011; Kamaruding and Abdullah 2021). Therefore, the use of anaesthetics contributes to improve animal welfare and, at the same time, facilitates routine procedures (Saydmohammed and Pal 2009).
Our results demonstrated that the induction times of sedation and anaesthesia were concentration dependent, decreasing with the increase of EO-OG concentration, while the anaesthesia recovery time was independent of the previously applied anaesthetic concentration. Parodi et al. (2012) observed a similar pattern when using the essential oils of Lippia alba and Aloysia triphylla to anaesthetise the Pacific white shrimp (P. vannamei). Becker et al. (2021) also reported a similar result when anaesthetising the São Paulo shrimp (Penaeus paulensis) and P. vannamei with the essential oils of L. alba and O. gratissimum. As such, it is recommended to use the proper effective anaesthetic concentration to control sedation and anaesthesia induction time and depth.
Eugenol is the main component of EO-OG (Bandeira Jr et al. 2017). This compound is one of the most used anaesthetics for decapod crustaceans and has been reported to satisfactory induce anaesthesia in shrimps and prawns, particularly for immersion anaesthesia (de Souza Valente 2022). Eugenol blocks sodium, potassium, and calcium channels, hence decreasing the proprioception (Cowing et al. 2015). Parodi et al. (2012) reported fast, deep anaesthesia of P. vannamei postlarvae in 4 min, using 175 μL L−1 of eugenol, while P. vannamei subadults are anaesthetized in 3 min with 400 μL L−1 of eugenol. Similarly, juveniles of green tiger prawn (Penaeus semisulcatus) can be anaesthetized with 100 and 200 μL L−1 of clove oil (80% eugenol) within 5 and 2 min, respectively (Soltani et al. 2004). Anaesthesia of small grass shrimp (Penaeus sinensis) in less than 10 min can be obtained with 100–200 μL L−1 eugenol, but bigger grass shrimps need higher concentrations (300–500 μL L−1) and mortality occurs at 20–28 °C (Li et al. 2018b). Postlarvae of M. rosenbergii anaesthetized at 28 °C with 60 μL L−1 clove oil in 9.5 min and recovered in 14.5 min, but juveniles needed a much higher concentration (750 μL L−1) to anaesthetize in 10.1 min and all animals died (Vartak and Singh 2006). Saydmohammed and Pal (2009) observed that even with 800 μL L−1 eugenol, M. rosenbergii took 21 min to anaesthetize at 30 °C, while Coyle et al. (2005) noted that at 24 °C, 45 min of exposure to 300 μL L−1 eugenol was needed to anaesthetize this species. Despite the high concentrations of eugenol and long induction time reported to be necessary to anaesthetize M. rosenbergii in these cited studies, in the present research, we found that EO-OG was effective to anaesthetize M. rosenbergii in a shorter time and using a lower concentration. This dissimilarity may be attributable to the synergic effects among the different compounds of EO-OG. On the other hand, ethanol did not lead to any sedative or anaesthetic effect thus acting only as an organic solvent without any isolated or synergic effect with EO-OG. The EO-OG was also more efficient to induce anaesthesia than eugenol in P. paulensis (Becker et al. 2021) and silver catfish, Rhamdia quelen (Silva et al. 2012), but not in other fish species, due to the different composition (1,8-cineole was the main compound) (Silva et al. 2020) or species-specific responses (Boaventura et al. 2020; Ferreira et al. 2021).
The major constituents of the EO-OG used in our study were eugenol and β-caryophyllene, followed by the minor constituents copaene, E-β-ocimene, and germacrene D. Despite eugenol being the major anaesthetic component of the studied EO-OG, the other constituents are worthy of a note. The β-caryophyllene is a sesquiterpene plant volatile reported as a potent natural analgesic for mammals (Fidyt et al. 2016). The proportion of β-caryophyllene found in the essential oil of Aloysia triphylla presents a significant relationship with the time to induce anaesthesia in silver catfish (Parodi et al. 2020). Parodi et al. (2020) observed that the concentration of β-caryophyllene shows a negative relation with the anaesthetic induction; i.e. the increase of β-caryophyllene concentration on the essential oil leads to a faster induction of deep anaesthesia. Notably, the lower β-caryophyllene concentrations found by these authors (5.4 to 12.7% in 2009 and 2.4 to 53.6% in 2010) are comparable to that one identified on the present study (i.e. 5.3%). This compound is also found in other essential oils with anaesthetic effect as Lippia alba in tambaqui (dos Santos Batista et al. 2018) and shrimp (Parodi et al. 2012; Becker et al. 2021), and Syzygium aromaticum on crayfish (in a commercial formulation of clove oil; Ghanawi et al. 2019). It also occurs in essential oils such as Humulus lupulus, Ocimum campechianum, and Origanum vulgare (Tsuchiya 2017). Copaene is a sesquiterpene that can be found in essential oils like Cinnamomum zeylanicum (Plata-Rueda et al. 2018), Decaspermum parviflorum (Khanh et al. 2020), and Vernonia patula (Hoi et al. 2021). Likewise, ocimene is a plant volatile from the terpenoid class and is among the main constituents (> 1%) of several additional essential oils described with anaesthetic effects for aquatic animals and others, such as Piper divaricatum on tambaqui (Colossoma macropomum, Vilhena et al. 2019), Nectandra grandiflora on Nile tilapia (Oreochromis niloticus, Rodrigues et al. 2021), A. triphylla on Nile tilapia (Oreochromis niloticus, Teixeira et al. 2017), and Lavandula angustifolia on chronic pain mice model (Donatello et al. 2020). In like manner, germacrene D is a sesquiterpene hydrocarbon and one of the constituents of essential oils used to anaesthetize aquatic animals, including Nectandra megapotamica on fat snook (Centropomus parallelus, Tondolo et al. 2013) and Hyptis mutabilis on silver catfish (Rhamdia quelen, Silva et al. 2013).
High-quality water parameters are essential for the proper development of aquatic organisms. Parameters such as water pH, hardness, and temperature interfere with the anaesthetic potential of different agents (Gomes et al. 2011). For instance, water temperature and salinity influence the anaesthesia induction and recovery times of the green tiger prawn (Penaeus semisulcatus) exposed to clove oil (Soltani et al. 2004). Thereby, water parameters seem to interact and influence the pharmacokinetic properties of the anaesthetic agents. In the present study, water quality parameters, except pH, were similar between the experimental groups (i.e. no significant difference) thus not influencing the sedative and anaesthetic effect of EO-OG on M. rosenbergii juvenile.
Within the context of the water bodies’ acidification, we investigated whether different water pHs could affect the anaesthesia and recovery of M. rosenbergii exposed to EO-OG. Our results suggest that there is an interaction between EO-OG anaesthetic concentration and water pH, with an influence of water pH on M. rosenbergii sedation time, leading to a significantly longer sedation time in mild acidic water. While water pH did not influence anaesthesia induction and anaesthesia recovery times under the studied conditions; indeed, acidic water has been previously suggested to impact eugenol efficacy as an anaesthetic, inducing higher sedation time in silver catfish kept in acidic water (Gomes et al. 2011). The ideal range of water pH for shrimp and prawns is 7.5 to 8.5, with values out of the range of 6.0 to 9.5 being highly detrimental (Boyd et al. 2014). Mild acidic water (e.g. pH 6.4–6.8) can negatively impact M. rosenbergii survival, growth, moulting frequency, and feeding rate, for both larvae and juvenile stages (Chen and Chen 2003; Liew et al. 2022). Taken together with our results, one recommends the constant monitoring of water pH including when anaesthetising M. rosenbergii, preferring the use of neutral to mild alkaline water. Attention should be paid to situations with a potential reduction of water pH, such as during animal transportation, particularly when associated with high densities (Hong et al. 2019).
In summary, EO-OG presents sedative and anaesthetic effects on the giant river prawn, M. rosenbergii, in a concentration-depended effect. The recommended concentration by our study is 400 μL L−1 of EO-OG in mild alkaline water. This was the effective anaesthetic concentration able to induce smooth sedation, deep anaesthesia, and full recovery in adequate time for farming procedures. The EO-OG sedation effect was influenced by water pH, while anaesthesia induction and recovery times were not modulated by water pH. EO-OG at the effective concentration is suggested to be used during routine procedures, such as animal handling, transportation, biometrics, and sampling.
Data availability
All data that support the findings described in this article are available within the article. Further information shall be directed to Professor Dr Eduardo Luis Cupertino Ballester (elcballester@ufpr.br).
References
Abram N, Gattuso J-P, Prakash A, Cheng L, Chidichimo MP, Crate S, Enomoto H, Garschagen M, Gruber N, Harper S, Holland E, Kudela RM, Rice J, Steffen K, von Schuckmann K (2019) Framing and context of the report. In: Pörtner H-O, Roberts DC, Masson-Delmotte V, Zhai P, Tignor M, Poloczanska E, Mintenbeck K, Alegría A, Nicolai M, Okem A, Petzold J, Rama B, Weyer NM (eds) IPCC Special Report on the Ocean and Cryosphere in a Changing Climate. Cambridge University Press, Cambridge, UK and New York, NY, USA, pp 73–129. https://doi.org/10.1017/9781009157964.003
Bandeira G Jr, Pês TS, Saccol EM, Sutili FJ, Rossi W Jr, Murari AL, Heinsmann BM, Pavanato MA, de Vargdas AC, Silva LL, Baldisserotto B (2017) Potential uses of Ocimum gratissimum and Hesperozygis ringens essential oils in aquaculture. Ind Crops Prod 97:484–491
Becker AJ, Vaz LJ, Garcia LDO, Wasielesky W Jr, Heinzmann BM, Baldisserotto B (2021) Anesthetic potential of different essential oils for two shrimp species, Farfantepenaeus paulensis and Litopenaeus vannamei (Decapoda, Crustacea). Ciência Rural 51(12):e20200793. https://doi.org/10.1590/0103-8478cr20200793
Boaventura TP, Souza CF, Ferreira AL, Favero GC, Baldissera MD, Heinzmann BM, Baldisserotto B, Luz RK (2020) Essential oil of Ocimum gratissimum (Linnaeus, 1753) as anesthetic for Lophiosilurus alexandri: induction, recovery, hematology, biochemistry and oxidative stress. Aquaculture 529:735676
Boaventura TP, Souza CF, Ferreira AL, Favero GC, Baldissera MD, Heinzmann BM, Baldisserotto B, Luz RK (2021) The use of Ocimum gratissimum L. essential oil during the transport of Lophiosilurus alexandri: water quality, hematology, blood biochemistry and oxidative stress. Aquaculture 531:735964
Boyd CE, Boyd CA, Chainark SUWANIT (2014) Shrimp pond soil and water quality management. In: The Shrimp Book. Nottingham Univ. Press, Nottingham, UK, pp 281–303
Cavallo RO, Lavens P, Sorgeloos P (2001) Reproductive performance of Macrobrachium rosenbergii females in captivity. J World Aquac Soc 32(1):60–67. https://doi.org/10.1111/j.1749-7345.2001.tb00922.x
Chen SM, Chen JC (2003) Effects of pH on survival, growth, molting and feeding of giant freshwater prawn Macrobrachium rosenbergii. Aquaculture 218(1-4):613–623
Collins M, Knutti R, Arblaster J, Dufresne J-L, Fichefet T, Friedlingstein P, Gao X, Gutowski WJ, Johns T, Krinner G, Shongwe M, Tebaldi C, Weaver AJ, Wehner M (2013) Long-term climate change: projections, commitments and irreversibility. In: Stocker TF, Qin D, Plattner G-K, Tignor M, Allen SK, Boschung J, Nauels A, Xia Y, Bex V, Midgley PM (eds) Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA
Cowing D, Powell A, Johnson M (2015) Evaluation of different concentration doses of eugenol on the behaviour of Nephrops norvegicus. Aquaculture 442:78–85
Coyle SD, Dasgupta S, Tidwell JH, Beavers T, Bright LA, Yasharian DK (2005) Comparative efficacy of anesthetics for the freshwater prawn Macrobrachiurn rosenbergii. J World Aquac Soc 36(3):282–290
David FS, Fonseca T, Wolff Bueno G, Valenti WC (2018) Economic feasibility of intensification of Macrobrachium rosenbergii hatchery. Aquac Res 49(12):3769–3776
de Souza Valente C (2022) Anaesthesia of decapod crustaceans. Vet Anim Sci 16(1):e100252. https://doi.org/10.1016/j.vas.2022.100252
Di Stasi LC, Oliveira GP, Carvalhaes MA, Queiroz-Junior M, Tien OS, Kakinami SH, Reis MS (2002) Medicinal plants popularly used in the Brazilian Tropical Atlantic Forest. Fitoterapia 73(1):69–91
Donatello NN, Emer AA, Salm DC, Ludtke DD, Bordignon SASR, Ferreira JK, Salgado ASI, Venzke D, Bretanha LC, Micke GA, Martins DF (2020) Lavandula angustifolia essential oil inhalation reduces mechanical hyperalgesia in a model of inflammatory and neuropathic pain: the involvement of opioid and cannabinoid receptors. J Neuroimmunol 340:577145
dos Santos Batista E, Brandão FR, Majolo C, Inoue LAKA, Maciel PO, de Oliveira MR, Chaves FCM, Chagas EC (2018) Lippia alba essential oil as anesthetic for tambaqui. Aquaculture 495:545–549
European Pharmacopeia (2007) European directorate for the quality of medicines, 6th edn. Council of Europe
FAO (Food and Agriculture Organization of the United Nations) 2003. Farming Freshwater Prawns. Available at https://www.fao.org/3/Y4100E/y4100e00.htm#TOC Access 07.Jul.2023
FAO (Food and Agriculture Organization of the United Nations) 2020. The State of World Fisheries and Aquaculture 2020 – SOFIA. Rome, 2020. Available at https://www.fao.org/documents/card/en/c/ca9229en Access 30.Apr.2022.
Ferreira AL, Favero GC, Boaventura TP, de Freitas Souza C, Ferreira NS, Descovi SN, Baldisserotto B, Heinzmann BM, Luz RK (2021) Essential oil of Ocimum gratissimum (Linnaeus, 1753): efficacy for anesthesia and transport of Oreochromis niloticus. Fish Physiol Biochem 47(1):135–152
Fidyt K, Fiedorowicz A, Strządała L, Szumny A (2016) β-caryophyllene and β-caryophyllene oxide—natural compounds of anticancer and analgesic properties. Cancer Med 5(10):3007–3017
Fotedar S, Evans L (2011) Health management during handling and live transport of crustaceans: a review. J Invertebr Pathol 106(1):143–152
Ghanawi J, Saoud G, Zakher C, Monzer S, Saoud IP (2019) Clove oil as an anaesthetic for Australian redclaw crayfish Cherax quadricarinatus. Aquac Res 50(12):3628–3632
Gomes DP, Chaves BW, Becker AG, Baldisserotto B (2011) Water parameters affect anaesthesia induced by eugenol in silver catfish. Rhamdia quelen. Aquac Res 42(6):878–886
Hoi TM, Chung NT, Huong LT, Ogunwande IA (2021) Studies on Asteraceae: Chemical compositions of essential oils and antimicrobial activity of the leaves of Vernonia patula (Dryand.). Merr. and Grangea maderaspatana (L.) Poir. from Vietnam. J Essent Oil-Bear Plants 24(3):500–509
Hong J, Chen X, Liu S, Fu Z, Han M, Wang Y, Gu Z, Ma Z (2019) Impact of fish density on water quality and physiological response of golden pompano (Trachinotus ovatus) flingerlings during transportation. Aquaculture 507:260–265
Ingraham C (2018) Animal self-medication, 2nd edn. Ingraham Trading Limited, Leighterton, UK
IPCC (2014) Climate change 2014: impacts, adaptation, and vulnerability. Part A: Global and Sectoral Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. In: Field CB, Barros VR, Dokken DJ, Mach KJ, Mastrandrea MD, Bilir TE, Chatterjee M, Ebi KL, Estrada YO, Genova RC, Girma B, Kissel ES, Levy AN, MacCracken S, Mastrandrea PR, White LL (eds) . Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, p 1132
Jiang S, Zhou F, Yang W, Wu Z, Le Y, Yang Q, Yu Y, Jiang S (2020) Anaesthetic effect of eugenol at different concentrations and temperatures on black tiger shrimp (Penaeus monodon). Aquac Res 51(8):3268–3273
Kamaruding NAA, Abdullah MI (2021) Effects of handling stress and moulting cycle on reproductive performance and digestive capacity of female giant freshwater prawn. Macrobrachium rosenbergii. Int Aqua Res 13(3):195
Keene JL, Noakes DLG, Moccia RD, Soto CG (1998) The efficacy of clove oil as an anaesthetic for rainbow trout, Oncorhynchus mykiss (Walbaum). Aquac Res 29(2):89–101
Khanh TH, Ban PH, Hoi TM (2020) Constituents of essential oils from the leaf, fruit, and flower of Decaspermum parviflorum (Lam.) J. Scott. Arch Pharm Pract 11(1):88–92
Li Y, Liang S, She Q, Han Z, Li Y, Li X (2018a) Influence of temperature and size on menthol anaesthesia in Chinese grass shrimp Palaemonetes sinensis (Sollaud, 1911). Aquac Res 49(6):2091–2098
Li Y, She Q, Han Z, Sun N, Liu X, Li X (2018b) Anaesthetic effects of eugenol on grass shrimp (Palaemonetes sinensis) of different sizes at different concentrations and temperatures. Sci Rep 8(1):1–9
Liew HJ, Rahmah S, Tang PW, Waiho K, Fazhan H, Rasdi NW, Hamin SIA, Mazelan S, Muda S, Lim LS, Chen YM, Chang YM, Liang LQ, Ghaffar MA (2022) Low water pH depressed growth and early development of giant freshwater prawn Macrobrachium rosenbergii larvae. Heliyon 8(7):e09989
Meneses JO, do Couto MVS, Sousa NC, Cunha FDS, Abe HA, Ramos FM, Chagas EC, Chaves FCM, Martins ML, Maria AN, Carneiro POF, Fujimoto RY (2018) Efficacy of Ocimum gratissimum essential oil against the monogenean Cichlidogyrus tilapiae gill parasite of Nile tilapia. Arquivo Brasileiro de Medicina Veterinária e Zootecnia 70:497–504
NIST - National Institute of Standards and Technology (2010) Mass spectral library and search/analysis programs. John Wiley and Sons, Hoboken
Park MO, Im SY, Seol DW, Park IS (2009) Efficacy and physiological responses of rock bream, Oplegnathus fasciatus to anesthetization with clove oil. Aquaculture 287(3-4):427–430
Parodi TV, Cunha MA, Heldwein CG, de Souza DM, Martins ÁC, Garcia LDO, Wasielesky W Jr, Monserrat JM, Schmidt D, Caron BO, Heinzmann B, Baldisserotto B (2012) The anesthetic efficacy of eugenol and the essential oils of Lippia alba and Aloysia triphylla in post-larvae and sub-adults of Litopenaeus vannamei (Crustacea, Penaeidae). Comp Biochem Physiol C Toxicol Pharmacol 155(3):462–468
Parodi TV, Gressler LT, Silva LDL, Becker AG, Schmidt D, Caron BO, Heinzmann BM, Baldisserotto B (2020) Chemical composition of the essential oil of Aloysia triphylla under seasonal influence and its anaesthetic activity in fish. Aquac Res 51(6):2515–2524
Pillai BR, Ponzoni RW, Das Mahapatra K, Panda D (2022) Genetic improvement of giant freshwater prawn Macrobrachium rosenbergii: a review of global status. Rev Aquac 14(3):1285–1299
Plata-Rueda A, Campos JM, da Silva Rolim G, Martínez LC, Dos Santos MH, Fernandes FL, Serrão JE, Zanuncio JC (2018) Terpenoid constituents of cinnamon and clove essential oils cause toxic effects and behavior repellency response on granary weevil, Sitophilus granarius. Ecotoxicol Environ Saf 156:263–270
Ribeiro AS, Batista EDS, Dairiki JK, Chaves FCM, Inoue LAKA (2016) Anesthetic properties of Ocimum gratissimum essential oil for juvenile matrinxã. Acta Scientiarum. Animal Sci 38:1–7
Rodrigues P, Ferrari FT, Barbosa LB, Righi A, Laporta L, Garlet QI, Baldisseroto B, Heinzmann BM (2021) Nanoemulsion boosts anesthetic activity and reduces the side effects of Nectandra grandiflora Nees essential oil in fish. Aquaculture 545:737146
Rojas-Sandoval, J. (2018). Ocimum gratissimum (African basil). CABI – Invasive species compendium. Available at https://www.cabi.org/isc/datasheet/115839 Access 30.Apr.2022.
Saydmohammed M, Pal AK (2009) Anesthetic effect of eugenol and menthol on handling stress in Macrobrachium rosenbergii. Aquaculture 298(1–2):162–167. https://doi.org/10.1016/j.aquaculture.2009.10.020
Silva LL, Parodi TV, Reckziegel P, de Oliveira Garcia V, Bürger ME, Baldisserotto B, Malmann CA, Pereira AMS, Heinzmann BM (2012) Essential oil of Ocimum gratissimum L.: Anesthetic effects, mechanism of action and tolerance in silver catfish. Rhamdia quelen. Aquaculture 350:91–97
Silva LL, Garlet QI, Benovit SC, Dolci G, Mallmann CA, Bürger ME, Baldisserotto B, Longhi SJ, Heinzmann BM (2013) Sedative and anesthetic activities of the essential oils of Hyptis mutabilis (Rich.) Briq. and their isolated components in silver catfish (Rhamdia quelen). Braz J Med Biol Res 46:771–779
Silva LA, Martins MA, Santo FE, Oliveira FC, Chaves FCM, Chagas EC, Martins ML, de Campos CM (2020) Essential oils of Ocimum gratissimum and Zingiber officinale as anesthetics for the South American catfish Pseudoplatystoma reticulatum. Aquaculture 528:735595
Soltani M, Marmari GH, Mehrabi MR (2004) Acute toxicity and anesthetic effects of clove oil in Penaeus semisulcatus under various water quality conditions. Aquac Int 12(4):457–466
Souza CDF, Baldissera MD, Baldisserotto B, Heinzmann BM, Martos-Sitcha JA, Mancera JM (2019) Essential oils as stress-reducing agents for fish aquaculture: a review. Front Physiol 10:785
Teixeira RR, de Souza RC, Sena AC, Baldisserotto B, Heinzmann BM, Couto RD, Copatti CE (2017) Essential oil of Aloysia triphylla in Nile tilapia: anaesthesia, stress parameters and sensory evaluation of fillets. Aquac Res 48(7):3383–3392
Tondolo JSM, Amaral LDP, Simões LN, Garlet QI, Schindler B, Oliveira TM, da Silva BF, Gomes LC, Baldisserotto B, Mallmann CA, Heinzmann BM (2013) Anesthesia and transport of fat snook Centropomus parallelus with the essential oil of Nectandra megapotamica (Spreng.) Mez. Neotrop Ichthyol 11:667–674
Tsantilas H, Galatos AD, Athanassopoulou F, Prassinos NN, Kousoulaki K (2006) Efficacy of 2-phenoxyethanol as an anaesthetic for two size classes of white sea bream, Diplodus sargus L., and sharp snout sea bream, Diplodus puntazzo C. Aquaculture 253(1-4):64–70
Tsuchiya H (2017) Anesthetic agents of plant origin: a review of phytochemicals with anesthetic activity. Molecules 22(8):1369
Vartak V, Singh RK (2006) Anesthetic effects of clove oil during handling and transportation of the freshwater prawn, Macrobrachium rosenbergii (de man). Isr J Aquac-Bamidgeh 58(1):46–54. https://doi.org/10.46989/001c.20426
Vilhena CS, do Nascimento LAS, de Aguiar Andrade EH, da Silva JKDR, Hamoy M, Torres MF, Barbas LAL (2019) Essential oil of Piper divaricatum induces a general anaesthesia-like state and loss of skeletal muscle tonus in juvenile tambaqui, Colossoma macropomum. Aquaculture 510:169–175
Funding
Open Access funding provided by the IReL Consortium This research was funded by the National Council of Technological and Scientific Development (CNPq), Brazil, under grant number PQ 311456/2020-0 to Eduardo Luis Cupertino Ballester. Eduardo Luis Cupertino Ballester and Bernardo Baldisserotto are recipients of CNPq productivity research grants. Alexssandro Geferson Becker thanks for Post-doctorate grant obtained from CNPq (Process n° 149133/2018-9) and for research funds from the CNPq (Process n° 432449/2018-3).
Author information
Authors and Affiliations
Contributions
Eduardo Luis Cupertino Ballester conceived and designed the research, provided funding, and supervised the study. Braulio Otomar Caron cultivated the Ocimum gratissimum specimen and extracted its essential oil together with hydrolate used in this study. Bernardo Baldisserotto and Berta Maria Heinzmann performed the essential oil extraction from hydrolate and chemical analysis of the O. gratissimum essential oil used. Alexssandro Geferson Becker and Geovane dos Santos conducted the trial and performed the analyses. Geovane dos Santos and Cecília de Souza Valente performed statistical analyses. Geovane dos Santos and Cecília de Souza Valente wrote the first draft of the manuscript. All authors participated in the data interpretation, manuscript revisions, and approved the final version.
Corresponding author
Ethics declarations
Ethical approval
In Brazil, research with decapod crustaceans does not require authorization from the Ethical and Animal Welfare Committee (Law No 11.794, 2008, Brazil). This study was performed in a research facility laboratory and conformed to scientific and commercial standards for the welfare of shrimp, using a proper shrimp diet, adequate water quality, and the minimum number of animals required for statistical relevance. This research does not contain any study with humans or vertebrate animals.
Competing interests
This study was part of the Geovane dos Santos undergraduate thesis. The authors declare no additional conflict of interest.
Additional information
Handling editor: Gavin Burnell
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
de Souza Valente, C., dos Santos, G., Becker, A.G. et al. Anaesthetic effect of clove basil (Ocimum gratissimum L.) essential oil on the giant river prawn (Macrobrachium rosenbergii, De Man 1879) exposed to different water pHs. Aquacult Int 32, 1493–1505 (2024). https://doi.org/10.1007/s10499-023-01226-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-023-01226-0