Abstract
The present study investigated the potential use of defatted black soldier fly (Hermetia illucens) meal as a protein source in the formulated feed for juvenile lobsters, Panulirus ornatus. A total of 160 heads of juvenile lobsters with initial weight of 0.24 ± 0.01 g and initial length of 21.77 ± 0.32 mm were distributed evenly into 16 rectangular fiberglass aquaria and kept individually for 8 weeks of the feeding experiment. Four formulated feeds containing defatted BSF meal as fishmeal replacements at 0%, 25%, 35%, and 50% and total dietary protein to energy ratio of ~ 26 g CP MJ−1 were prepared and assigned as BSF0, BSF25, BSF35, and BSF50, respectively. Fishmeal is technically a suitable aquafeed ingredient in terms of crude protein, moisture, and amino acid compositions. The partially substituted defatted BSF meal has higher crude lipid and ash content than fishmeal. At the end of week 8 of feeding trial, the specific growth rate, length increment, survival rate, and moulting rate of juvenile lobster were not significantly (P > 0.05) affected by the fishmeal replacement. The fishmeal replacement with the defatted BSF meal did not significantly (P > 0.05) alter the whole-body proximate composition of the juvenile lobster. However, it significantly (P < 0.05) affected the superoxidase dismutase of juvenile lobsters. The cytokine cell expression analysis indicated that up to 35% of fishmeal replacement increased the inflammatory cytokine cells (Interleukin 8 and 17) of juvenile lobster, while an adverse impact was observed on juvenile lobster receiving 50% of fishmeal replacement by BSF meal. Histopathological analysis showed that the hepatopancreas cells of juvenile lobsters were damaged following fishmeal replacement beyond 35%. The present study indicated that up to 35% of defatted BSF meal can be used as fishmeal replacement in the formulated feed of juvenile lobster.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since the 1980s, dietary insect meal has been recognized as an alternative to fishmeal in livestock food and aquaculture industries. With the increase in awareness of sustainability related to the use of fishmeal in aquaculture, research on insect meal as an aquadiet has progressed. The potential use of insect-based ingredients in aquadiets has been reviewed by many authors and one of the most researched insect species that has emerged is black soldier fly (BSF), Hermetia illucens (Alfiko et al. 2022; Mohan et al. 2022; Priyadarshana et al. 2021; Sánchez-Muros et al. 2018). Insects in form of live or processed have been widely used as an alternative protein resource for farmed crustaceans. In crustacean aquaculture, the use of BSF meal was taken into consideration because of the nutritional properties of those ingredients. Black soldier fly meal is known as the second largest insect which have been used as protein sources in aquafeeds (Alfiko et al. 2022; Riddick 2014) and have been successfully reported as an alternative protein source to replace the fishmeal in the diet of seawater crustacean such as shrimp L. vannamei (Chen et al. 2021; Choi et al. 2018; Cummins et al. 2017; Richardson et al. 2021; Rios et al. 2019), and Australian freshwater lobster, C. cainii (Foysal et al. 2019). The BSF meal has sufficient nutritional content that qualifies as a substitute for the fishmeal protein in fin fish and shrimp-formulated feeds. The amino acids and fatty acids in BSF have comparable nutritional values to fishmeal (Mohan et al. 2022) and can be used as the substitute for fishmeal in combination with several other protein sources other than fishmeal (Cummins et al. 2017; Richardson et al. 2021). However, the dietary inclusion of BSF as a protein source is dependent on the life cycle stages of the BSF as the nutritional profile varies according to its life stage also the culture medium used to rear the BSF (Priyadarshana et al. 2021). In general, processed BSF used as a dietary ingredient of the formulated diets in fish species is invariably in the forms of maggot meal that is sun-dried, oven-dried, or frozen, and then powdered (Henry et al. 2018).
The quality of BSF meal highly depends on the culture substrates of BSF maggot which include common organic waste (Shumo et al. 2019) and/or industrial waste (Ravi et al. 2020). Such different culture systems produced a distinctive different nutritional composition particularly with respect to crude protein and fat. The protein content in BSF is expected to be a similar protein content compared to fishmeal, while the high lipid content in BSF meal surpasses that of fishmeal and must be limited to avoid problems in the aquafeed production. Several methods have been incorporated to reduce the fat content in the BSF meal (Ewald et al. 2020; Smets et al. 2021). In some crustaceans, the optimum lipid requirements are between 8 and 13% of dry matter ingredients (Takeuchi and Murakami 2007). The content of lipid outside of that range is reported to impair the growth of animals, emphasizing the importance of the correct selection of BSF meal type in the aquafeed formulation (Zozo et al. 2022).
The use of BSF meal in finfish diets is widely studied (Abdel-Tawwab et al. 2020; Belghit et al. 2019; Hender et al. 2021; Hu et al. 2020; Weththasinghe et al. 2021), whereas there are only several nutritional studies involving BSF meal as the protein source for crustaceans (Chen et al. 2021; Cummins et al. 2017; Richardson et al. 2021). The increase of BSF meal inclusion from 0 to 36% was ineffective in promoting the growth of L. vannamei, suggesting that 7% inclusion of BSF meal is required for the optimum growth of the shrimp juveniles (Cummins et al. 2017). Similarly, the optimum fishmeal replacement with BSF meal for L. vannamei is around 7.5 to 10% (Chen et al. 2021; Richardson et al. 2021). In freshwater lobster, the combination of BSF meal and poultry by-product meal is also found to be suitable for improving the growth and immunological gene performances of Australian native freshwater crayfish, Cherax cainii (Foysal et al. 2019). Those studies showed some successful practical uses of BSF meal in formulated diet for crustaceans.
The general parameters of physiology and immunology of crustaceans have been used to evaluate the effect of feed treatments in nutritional research, and the said parameters have evolved rapidly in recent years reaching to the gene level (Miao et al. 2018). A classical histopathological method of examining the immunological response of the lobster tissues can provide reliable information in the assessment of assessing the presence of diseases and contaminants in the environment (Shields et al. 2012). For scalloped spiny lobster (Panulirus homarus), the changes of certain immunological-related genes have been associated with the result of the feeding treatments (Haryanti et al. 2017). In addition, for another crustacean species C. cainii, the expressions of several important inflammatory cytokine cells were also affected by the nutritional changes within the feed (Foysal et al. 2019).
The ornate spiny lobster or P. ornatus has been successfully cultured in some countries including Indonesia and Vietnam using fresh feeds, with significant apparent challenges due to lack of a commercially available formulated feed. Even though the development of formulated diet for P. ornatus is still in its infancy, there is a noticeable improvement in P. ornatus nutritional study (Nankervis and Jones 2022), wherein other formulated diet development has shown an improvement in the growth performances of P. ornatus reared under laboratory and outdoor conditions (Kurnia et al. 2017; Marchese et al. 2018). An unpublished study conducted by Saputra and Fotedar (2022) indicated that P/E ratio of 26.6 g CP MJ−1 was optimal for juvenile lobster growth. There is no research conducted in the potential use of BSF meal as a protein source for juvenile lobster. Therefore, the present study is aimed to evaluate the potential use of defatted BSF meal as a protein source in a formulated diet for juvenile lobster.
Material and methods
Animal and experimental set up
A randomized design was applied with four dietary treatments and four replicates. The juvenile lobsters were cultured in 16 rectangular 60-L acrylic fish tanks (60 cm × 29.5 cm × 35 cm) for 8 weeks. In each tank, a total of 10 juvenile lobsters (initial weight 0.24 ± 0.01 g, initial length 21.77 ± 0.32 mm) were individually kept in 1.5-L plastic baskets with holes (15 cm height; Ø 12.5 cm) and equipped with hides made of polyurethane plastic (4 cm; Ø 1.5 cm) and plastic net (4 cm × 4 cm). All tanks were supplied with water filtration system, and continuous air supply and a natural photoperiod (12 h light: 12 h dark) were maintained. During the feeding experiment, water quality parameters were measured using digital water quality meter (Horiba U50, Japan).
Four formulated feeds containing BSF meal at 0%, 25%, 35%, and 50% replacements of the fishmeal component of the feed were formulated. The protein to energy ratio of the feeds was 26.6 g CP MJ–1; the respective feeds were prepared and assigned as BSF0, BSF25, BSF35, and BSF50 respectively (Table 1).
A proximate and amino acid analysis was carried to determine the composition of the fishmeal and defatted BSF meal (Table 2) and the finished formulated diets (Table 3).
All feed ingredients were mixed and processed into pelleted feed (Ø 2 mm) by using food processor (Oxone OX-861N, Indonesia). Finished feed was then dried in an oven at 60 °C for 16 h. In this study, the juvenile lobsters were given with each type of formulated feed using stainless steel pincers, four times per day until apparent satiation. All uneaten feed was removed after 1 h of feeding to maintain the water quality and to quantify the amount of ingested feed.
Proximate analysis of feed and the whole-body of juvenile lobsters
The proximate composition of the juvenile lobster and the diets were analyzed using the Kjeldahl method according to an established protocol (AOAC 2006). The moisture content was analyzed by using a heating method at 110 °C ± 1 °C for 2 h until constant weight was achieved, using an air forced oven (Carbolite X50, US). The moisture content was calculated by subtracting the weight after drying from the initial wet weight of the sample. The ash content was obtained through the dry aching method at temperature of 600 °C for 4 h using a furnace. The residue of biochemical process of the sample was treated using H2SO4 1.25% and NaOH 1.25% solution to obtain the crude fiber content. The total lipid and the amino acid composition in the formulated feeds and juvenile lobster’s whole body were measured in accordance to AOAC (2006).
Gut and hepatopancreas histopathology
The histopathology analysis was performed according to an established protocol from Bell and Lightner (1988). Juvenile lobsters from each dietary treatment for histopathology analysis were preserved using Davidson’s solution for 24 h prior to the transfer into a 70% ethanol solution. A small section of abdomen and cephalothorax (5 mm thickness) were placed in the cassettes for further dehydration process on automatic tissue processor (Tissue-Tek VIP 5 Jr. 5903, Japan). The dehydrated samples in the cassette then were blocked and embedded in paraffin. Each of the samples was then trimmed and sliced using a microtome (Leica RM2125, US) with a thickness of 4–5 µm. After the completion of slicing, the thin layer of samples ware stretched in a flotation bath at 45 °C and were then placed on a glass slide. The process continues with the deparaffinization of samples which was carried out using a hotplate at 62 °C. The staining process was performed using xylene, absolute ethanol, ethanol solutions, hematoxylin, bluing reagent, and eosin. After the completion of staining process, the glass slides were then covered and analyzed using a light microscope (Olympus CX21, Japan) and documented using a digital camera (OptiLab, Indonesia).
Gene expression analysis
The immunology status of juvenile lobsters were determined by analyzing four cytokine cells including interleukin 1β (IL-1β), IL-10, and IL-17F as previously reported (Miao et al. 2018) and also prophenoloxidase (ProPO), glutathione peroxide (GPO), and anti-lipopolysaccharide factor (ALFHa-1 & ALFHa-1) (Haryanti et al. 2017). In the present study, these target genes were extracted from lobster’s whole body. The RNA extraction was performed according to RNA kit (IQ-2000™ GeneReach, Taiwan). The synthesis of cDNA (complementary DNA) was carried out using the SensiFAST cDNA Synthesis Kit (Bioline, Australia). Analysis of immune status profiles using the expression of gene transcription methods related to immunity quantitatively using qPCR and specific primers (Table 4). The β-actin was used as the internal keeping gene. The qPCR analysis was performed using real-time PCR cycler (Rotor-GeneTM 3000 real-time PCR, USA) with the SensiFAST SYBR® No-ROX kit (Bioline, Australia). The calculation of ΔCt from the PCR cycle threshold (Ct) of the tested gene was normalized relative to the Ct β-actin (internal control). The value of Ct is calculated from ΔCt (tested sample group)—ΔCt (initial expression). Representation of different relative multiples to the initial expression was calculated by 2 ΔΔCt.
Growth and moulting data
The physiological data including survival rate and specific growth rate of the juvenile lobster were collected at the beginning and at the end of the feeding experiment. The total length increment, moult interval, and moulting rate data were based on daily records. All data parameters were calculated as follows:
-
(1)
Survival rate (%) = \(\frac{\mathrm{Final number of survived spiny lobster juvenile }}{\mathrm{Initial number of spiny lobster juvenile}} x 100\)
-
(2)
Specific growth rate (g.%.day−1) = 100 x [ln final weight (g)–ln initial weight (g)]/days
-
(3)
Total length increment (%) = (Wt total length – W0 total length)/ (initial total length) × 100
-
(4)
Moult interval (days) = duration in days between two consecutive molts
-
(5)
Moulting rate (%) = (Number of molted lobster juvenile)/ (Number of total lobsters juvenile) × 100
Statistical analysis
The biological data parameters were analyzed by using SPSS IBM for Windows (version 25.0). One-way analysis of variance was used to evaluate any significant differences among the variables of the parameters of the juvenile lobster and Turkey’s HSD post hoc test analysis were performed when a significant interaction occurred. The statistical significance was evaluated at P < 0.05.
Results
All formulated diets have similar proximate composition (Table 2). The amino acid contents in fishmeal were higher compared to the amino acid in the defatted BSF meal (Table 3). The amino acid content in feeds without defatted BSF supplementation was higher than the amino acid in the supplemented-BSF feeds. The increase of percentage defatted BSF in the diets have a negative correlation with the availability of amino acids in the feeds (Table 5).
Physiological evaluation on juvenile lobster indicated that there were no significant differences in the mean of final weights, specific growth rates, final lengths, length increments, survival rates, moulting rates, and moult interval of juvenile lobster fed dietary defatted BSF meal (P > 0.05) (Table 6).
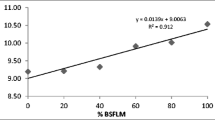
The composition of protein and lipid in the whole-body of juvenile lobster were not significantly different (P > 0.05). The ash and SOD of juvenile lobster’s whole-body fed with the BSF50 dietary were significantly high (P < 0.05) (Table 7).
The relative expression level of cytokine genes in juvenile lobster is shown in Fig. 2. In this study, the gene expression of IL-1β and IL-17 in juvenile lobster fed with BSF25 and BSF35 was higher than those in juvenile lobster fed with BSF0 in terms of fold changes. The IL-17 cells in juvenile lobster given with BSF25 and BSF35 showed a five-fold of gene expression from the control (BSF0). In contrast, those cytokine cells decreased as the juvenile lobster fed the dietary BSF50. The BSF dietary replacement at any level in the formulated diets downregulated the fold of IL-10 cells gene expression in juvenile lobsters. The juvenile lobster given with BSF50 dietary had higher fold of ProPO, GPO, and ALFHa-1 gene expression compared to other dietaries. On the contrary, the ALFHa-2 gene of juvenile lobsters decreases as the inclusion of BSF levels in the formulated diets increases (Fig. 1).
Histology analysis on the hepatopancreas of juvenile lobster shows that necrosis occurred on the lobster fed 50% of fishmeal replacement (Fig. 2). The presence of hemocyte infiltration and sloughing on the hepatopancreas tissue were found in all treatments. The atrophy conditions of hepatopancreas cells were found only in juvenile lobster fed with BSF50 feeds (Table 8).
Histology of hepatopancreas of juvenile lobster fed with dietary of defatted BSF meal inclusion (0%, 25%, 35% and 50%) at 100 × magnification. Hepatopancreas tissue infiltrations and sloughing occur in all of the treatments. Hepatopancreas of juvenile lobster fed with BSF50 experienced necrosis and atrophy tissue. In, infiltration; Sl, sloughing; Ne, necrosis; Ar, atrophy; bar: 300 µm
Discussion
The present study is the first study attempting to use defatted BSF meal as the protein source in a formulated feed for juvenile lobster. The result of the present study indicated that the fishmeal replacement up to 50% with defatted BSF meal did not affect the physiological response and immunological response on juvenile lobster. Measured water salinity and temperature in this study were at optimum level for culturing spiny lobster (Jones 2009) and dissolved oxygen was acceptable for captive environment (Vijayakumaran et al. 2014). Water quality has significant effect on the growth and survival of juvenile lobster and the changes on that parameters can lead to the suboptimal growth (Ratunil 2017) and decrease on the immune response (Verghese et al. 2007). The readings of pH were higher than recommended level (Jones 2009; Vijayakumaran et al. 2014) and it might be caused by the error of the equipment since the physiological response of the lobster is within the normal ranges. The average survival rate in this present study was similar to other reports (Kurnia et al. 2017; Marchese et al. 2018; Sang and Fotedar 2010; Smith et al. 2003; Smith et al. 2005) and was higher to studies conducted in clean water system (Haryanti et al. 2017; Kropielnicka-Kruk et al. 2019; Ratunil 2017). In this study, the replacement of fishmeal with defatted BSF meal up to 50% of protein content did not affect the growth of juvenile lobsters, showing a specific growth rate between 1.77 and 1.82 g % day−1. It is not possible to compare the growth rate of the juvenile lobster in this study with any other study as this is the first study testing defatted BSF meal as protein source for juvenile lobster. However, that growth rate is in agreement with the said nutritional studies on juvenile lobster (Saputra and Fotedar 2022; Smith et al. 2003; Smith et al. 2005) and somewhat lower than other reports (Haryanti et al. 2017; Kropielnicka-Kruk et al. 2019). In addition, any level of defatted BSF diets that resulted to the same growth rate as to the BSF0 or control diet indicated that those diets are suitable for juvenile lobster where fishmeal is used as protein source. The data suggested that fishmeal replacement by defatted BSF meal up to 50% will increase the protein and lipid content of juvenile lobster’s whole body.
Studies evaluating fishmeal replacement using defatted BSF meal in spiny lobster are scant. In the present study, the replacement of fishmeal with defatted BSF meal at any level had no effect on the final total length, final weight, length increment, moulting rate, and moult interval of juvenile lobster. Results of the present study in juvenile lobster are similar to a crustacean study (Richardson et al. 2021). For L vannamei, shrimp fed with a diet of 70% defatted BSF inclusion had similar final bodyweight and survival as shrimp fed with a control diet without defatted BSF meal. In addition, the increase of defatted BSF meal amount to fishmeal replacement in the practical diet improved growth rate and survival rate (Richardson et al. 2021). On the contrary, on a study involving the same species with the said study, the shrimp fed with dietary of BSF meal > 7% on dry weight basis exhibited the decrease in their specific growth rate, final weight and feed conversion ratio (Cummins et al. 2017). The results of the present study were contradicted to study on Cummins et al. (2017) but in line with Richardson findings. The different nutritional compositions of BSF meal used in those two studies might be the ones which are responsible for the different growth and survival responses of the shrimp. The defatted BSF meal has approximately 60% crude protein and 7% of lipid which is used in the experiment conducted by Richardson et al. (2021), while BSF meal used by Cummins et al. (2017) has approximately 52% crude protein and 15.1% lipid. The different growth and survival response of treated animal not only occurred in crustaceans, but also in finfish (Fasakin et al. 2003; Karapanagiotidis et al. 2023). A set of feeding experiments conducted on gilthead seabream (Sparus aurata) indicated that the defatted BSF is more suitable in terms of promoting growth for the fish. While full-fat BSF replacement at any level would experience a decrease in the growth of the fish, only fish that received feeds containing more than 30% of fishmeal replacement with defatted BSF exhibited a suboptimal growth (Karapanagiotidis et al. 2023). The said findings indicate that with similar replacements, the defatted BSF meal is more suitable as fishmeal replacement than the full-fat BSF meal.
In the present study, the survival rate of juvenile lobster ranges from 50.00 to 62.50% without any statistical significance differences. That survival rate was higher than the survival rate of the same juvenile lobster species reared using the similar rearing system reported by Kropielnicka-Kruk et al. (2019) and somewhat beyond the survival rate of juvenile lobster in the research conducted by Marchese et al. (2018). In a study on crustaceans, the mortality rate was reported to be influenced by the moulting rate (Taugbøl and Skurdal 2008) and moulting rate itself has strong association with the availability of supporting moulting hormone (Hosamani et al. 2017). In the present study, the mortality rate was mainly caused by insufficient energy to complete the moulting process which could be observed from an incomplete moult of the juvenile lobster, and it is in agreement with the findings from Irvin and Williams (2009). The survival rate of spiny lobster P. ornatus is influenced by the rearing system both on juvenile (Kropielnicka-Kruk et al. 2019; Marchese et al. 2018) and adult lobster (Vijayakumaran and Kumar 2010). Indeed, the availability of the feed in closed compartment will provide an ease for juvenile lobster to access food. Also, the rearing system affects the feed intake. In addition, the growth rate, final carapace length, and moult rate of the juvenile lobster were also higher than juvenile lobster kept in individual compartment (Marchese et al. 2018).
The amino acids pose an important role in supporting growth and some of them are known as limiting factors in L. vannamei (Cummins et al. 2017; Richardson et al. 2021). In this study, the availability of amino acids in defatted BSF meal was lower than the amino acids in the fishmeal. Among others, amino acids which were analyzed, serine, cysteine, and phenylalanine in the defatted BSF meal, were holding the closest value of amino acids in the fishmeal. In addition, the sum squared calculation indicated that there were variations in the amino acids between formulated diets. The amino acid values in this study were decreasing as the increase of defatted BSF meal inclusion, and similar results were also reported from other crustacean feeding trials (Cummins et al. 2017; Richardson et al. 2021). For instance, the formulated diets with 36% of fishmeal replacement had a higher value of total amino acids sum squared of 140.66 than formulated diets containing 7% of fishmeal replacement with value of total amino acids sum squared as much as 6.87 (Cummins et al. 2017). It is impossible to get same nutrition from particular BSF with others because the nutritional composition of each BSF is highly dependent on the substrate of its culture (Shumo et al. 2019), life stage (Liu et al. 2017), and the form of BSF (Irawan et al. 2020). In the present study, the amino acid values might not have any impact to physiological response of juvenile lobster. However, it can be a foundation for future research in spiny lobster nutritional studies.
The body composition of crustaceans has been reported to be affected by the different dietary ingredients in the formulated diets. While some studies reported that the ash content in the body of crustaceans was lower compared to the one in the experimental diets (Cummins et al. 2017; Goda 2008; Hu et al. 2008; Huo et al. 2014), another study with contradicted results reported that the ash content in the body was higher than the one in the experimental diet (Xu et al. 2013). The body composition analysis of juvenile lobster in this study indicated that the ash content in the whole-body juvenile lobster was higher than the one in the formulated diet, and it is in agreement with the said latter study (Xu et al. 2013). These results argued to a consideration that different ash analysis is the highest possible consequence on the said mixed results. In this study, analyzed juvenile lobster whole body with shell included resulted in high ash content. In crustaceans, any non-edible and edible body part have different ash value with non-edible body part with shell included having a higher ash content than the edible body part (Ali et al. 2018). In terms of the BSF meal replacement within this study, the ash content increased as the defatted BSF meal inclusion in the formulated diet increased, whereas protein, lipid, and moisture content were the same with formulated diet without BSF included. Those results on ash content in the present study is similar to Richardson et al. (2021) which reported that the ash content in the diet with 70% fishmeal replacement is higher than the ash content of the diets with 30% and 50% replacement of BSF meal. In the present study, high ash content was found in the juvenile lobster fed with defatted BSF meal inclusion at 35% and 50%. This result was considerably affected by the nutritional composition of defatted BSF meal, where those inclusions have slightly higher ash content compared to fishmeal. In addition, the inclusion would directly influence the ash content in the whole body of juvenile lobster (Table 2), although physiologically it did not cause a significant difference in growth rate and survival rate. It might come into consideration that ash content on the juvenile lobster whole body relates to a high level of hepatopancreas cell damage. The damage occurred to juvenile lobsters fed with defatted BSF meal replacement at 50% only. The growth rate and survival rate of P. ornatus juvenile reared in sea cage fed with high-ash formulated diet are lower than those of lobster fed with dietary low-ash diet (Kurnia et al. 2017). Ash content is an important source of minerals for fish. Nevertheless, an excessive ash amount in feed may reduce the digestible ability of other ingredients which resulted to a poor growth of the fish (Shearer et al. 1992). There is no exact information of optimum ash content amount required by spiny lobster to achieve their maximum growth. However, it is found on the wild-caught lobster Panulirus japonicus that the ash content in their body is 8–12%, and those values can be used as an acceptable reference (Takeuchi and Murakami 2007). In this study, the ash content of the formulated diets ranged from 8.21 to 8.80 g/100 g, while in juvenile lobster whole body, it ranged from 11.26 to 13.59 g/100 g. In addition, it was found that ash content in the juvenile lobster whole body fed with 50% defatted BSF meal inclusion was significantly different. The differences of ash content amount affected the immunology of juvenile lobster by the occurrence of severe hepatopancreas cell damage evidence but did not affect to physiological parameters of juvenile lobsters.
Histopathology has been used in analyzing the effect of feeding treatments in crustaceans (Ambasankar et al. 2022; Chen et al. 2021; Duan et al. 2018; Jatobá et al. 2018; Li et al. 2022) and also to determine the tissue of certain organs changes due to disease infection (Shields et al. 2012; Zha et al. 2018). The effect of BSF meal inclusion on gut health has been demonstrated in crustaceans (Chen et al. 2021; He et al. 2022), while the effect of defatted BSF meal inclusion on hepatopancreas of spiny lobster is not yet available. However, the histopathology of hepatopancreas has been used as the reference of nutritional status in some crustaceans (Chen et al. 2017; Fernández Gimenez et al. 2004; Sukor et al. 2016). In the present study, the histopathology analysis of intestine failed due to the intestinal tissue sample damage. Therefore, only the histopathology result of hepatopancreas was used for further discussion. In the histopathology analysis, juvenile lobster fed with defatted BSF meal fishmeal replacement at 50% experienced a more severe hepatopancreas cell damage than juvenile lobster fed with control diets (BSF0) and other treatments (BSF25 & BSF35). The histopathology of hepatopancreas juvenile lobster fed with fishmeal replacement at 50% exhibited severe sloughing of cells tissue, hemocyte infiltration, necrotizing, and atrophy cells. Meanwhile, there was only decent sloughing of cells tissue and hemocyte infiltration found on hepatopancreas cells of juvenile lobster fed with other dietary treatments. Those detrimental effects on hepatopancreas cells in this study might be accounted by the overuse of defatted BSF meal inclusion at 50%. This result, however, contradicted with the physiological response of shrimp receiving similar defatted BSF meal dietary which has no differences in shrimp growth. The optimum growth performance is achieved on L. vannamei juvenile receiving 20% of BSF meal inclusion, and beyond that level, the shrimp juvenile experienced a reducing growth rate (Chen et al. 2021). A similar result was also reported by Richardson et al. (2021), with limiting BSF meal inclusion to 25%, which is in converse with a recent study in 2022 on the same species which reported that higher than 25% inclusion of BSF level can be used to promote growth and immunology status of the L. vannamei juvenile (He et al. 2022). The different responses of animals used in the said research which uses BSF meals as fishmeal replacement were considerably caused by the diverse nutritional value particularly the amino acids of used BSF (Chen et al. 2021; Cummins et al. 2017; Richardson et al. 2021) which also occurred as result in the present study.
Study on the search for alternative ingredients in aquafeeds does not only aim to find suitable ingredients supporting fish growth, but also the ones which are able to improve the immunity of the animals. In this study, the fold of gene expression level of juvenile lobster exhibited different responses with respect to the defatted BSF meal replacement in the experimental formulated diets. The replacement of defatted BSF meal at any level did not affect the fold of gene expression of inflammatory cytokines of juvenile lobster. However, juvenile lobster fed with the formulated diets containing defatted BSF meal replacement at 25% and 35% had higher inflammatory cytokine IL-1β and inflammatory cytokine IL-17 expression than juvenile lobster fed with the control diet. In contrast, the fold of gene expression of inflammatory cytokines on the juvenile lobster fed with the formulated diets containing defatted BSF meal replacement at 50% was lower than juvenile lobster fed with the control diet. The result of this study is similar with a study on freshwater lobster C. cainii conducted by Foysal et al. (2019). In that lobster study, the supplementation of 30% BSF in the diet improved the expression level of cytokine cells primarily IL-7, IL-10, and IL-17, which coincide with the high availability of lactic acid bacteria from the Firmicutes phylum. In another study on northern snakehead (Chana argus), the abundance of certain Firmicutes is responsible for the increase of the availability of some inflammatory cytokines (Miao et al. 2018). The inclusion of defatted BSF meal as substitutes on fishmeal in this study might have role to provide Firmicutes phylum affecting the increase of cytokine cells of juvenile lobster. However, it requires further investigation.
The changes of several immune-related gene expression have been used as parameters in assessing the immune status of crustaceans (Duan et al. 2018; Haryanti et al. 2017; Li et al. 2018; Miandare et al. 2017; Wongsasak et al. 2015). In the present study, juvenile lobster receiving high inclusion level of defatted BSF meal in the diet had higher gene expression of prophenoloxidase, glutathione, and anti-lipopolysaccharide factor-1 than others. That finding indicated that a high inclusion level of defatted BSF meal would improve those immune-related genes of juvenile lobster by providing required nutrition. The increase of gene expression fold on prophenoloxidase was also reported for juvenile lobster P. homarus fed with probiotic dietary. The essentials nutrition in the yeast combined with the function of the probiotics have responsibilities in promoting immunological status of juvenile lobster (Haryanti et al. 2017). The prophenoloxidase is a part of innate immunity system in crustacean and plays an important role in the bacterial infection process (Wang et al. 2010). A rigorous study conducted by Vogel et al. (2018) showed that BSF is an insect with 53 of potential antimicrobial peptides and ranked them as the highest insect with potential antimicrobial peptides amongst common insects used in feed. In addition, BSF also have higher potential immunomodulatory factors lauric acid and chitin (Koutsos et al. 2022). These superior attributes of BSF are considered contributions to the results of the present study.
Conclusions
The present study shows that the defatted BSF meal replacement of fishmeal at between 0 and 50% in formulated feeds for juvenile lobster has no effect on growth, survival rate, moulting rate, moult interval, and body composition compared to the standard feed. However, it considerably changes the cytokine cells and some immune-related gene expression juvenile lobster. The hepatopancreas of the juvenile lobster fed with defatted BSF meal dietary at 50% shows a severe cell damage. An increasing amount of defatted BSF meal will increase the availability of amino acid contents in the formulated diets and the superoxidase dismutase availability in juvenile lobster whole body. Further study is required to evaluate the findings as well as other potential ingredients as supplement in defatted BSF meal fishmeal replacement on the formulated diets of juvenile lobster.
Data availability
The data are available from the corresponding author based on reasonable requests.
References
Abdel-Tawwab M, Khalil RH, Metwally AA, Shakweer MS, Khallaf MA, Abdel-Latif HMR (2020) Effects of black soldier fly (Hermetia illucens L.) larvae meal on growth performance, organs-somatic indices, body composition, and hemato-biochemical variables of European sea bass, Dicentrarchus labrax. Aquaculture 522:735136. https://doi.org/10.1016/j.aquaculture.2020.735136
Alfiko Y, Xie D, Astuti RT, Wong J, Wang L (2022) Insects as a feed ingredient for fish culture: status and trends. Aquac Fish 7(2):166–178. https://doi.org/10.1016/j.aaf.2021.10.004
Ali SSR, Ramachandran M, Chakma SK, & Sheriff MA (2018) Proximate composition of commercially important marine fishes and shrimps from the Chennai coast India. International Journal of Fisheries and Aquatic Science 5(5):113–119. https://doi.org/10.22271/fish
Ambasankar K, Dayal JS, Vasagam KPK, Sivaramakrishnan T, Sandeep KP, Panigrahi A, ..., Vijayan KK (2022) Growth, fatty acid composition, immune-related gene expression, histology and haematology indices of Penaeus vannamei fed graded levels of Antarctic krill meal at two different fishmeal concentrations. Aquaculture 553:738069. https://doi.org/10.1016/j.aquaculture.2022.738069
AOAC (2006) AOAC Official Method 990.03 Protein (Crude) in Animal Feed, Combustion Method. Gaithersburg, MD: AOAC International
Belghit I, Liland NS, Gjesdal P, Biancarosa I, Menchetti E, Li Y, ..., Lock E-J (2019) Black soldier fly larvae meal can replace fish meal in diets of sea-water phase Atlantic salmon (Salmo salar). Aquaculture 503:609–619. https://doi.org/10.1016/j.aquaculture.2018.12.032
Bell TA, Lightner DV (1988) A handbook of normal penaeid shrimp histology. Baton Rouge, Louisiana: World Aquaculture Society
Chen WW, Romano N, Ebrahimi M, Natrah I (2017) The effects of dietary fructooligosaccharide on growth, intestinal short chain fatty acids level and hepatopancreatic condition of the giant freshwater prawn (Macrobrachium rosenbergii) post-larvae. Aquaculture 469:95–101. https://doi.org/10.1016/j.aquaculture.2016.11.034
Chen Y, Chi S, Zhang S, Dong X, Yang Q, Liu H, Tan B, Xie S (2021) Evaluation of the dietary black soldier fly larvae meal (Hermetia illucens) on growth performance, intestinal health, and disease resistance to Vibrio parahaemolyticus of the Pacific white shrimp (Litopenaeus vannamei). Front Mar Sci 8. https://doi.org/10.1016/j.aquaculture.2016.11.034
Choi IH, Kim JM, Kim NJ, Kim JD, Park C, Park JH, Chung Th (2018) Replacing fish meal by mealworm (Tenebrio molitor) on the growth performance and immunologic responses of white shrimp (Litopenaeus vannamei). Acta Sci Anim Sci 40. https://doi.org/10.4025/actascianimsci.v40i1.39077
Cummins VC, Rawles SD, Thompson KR, Velasquez A, Kobayashi Y, Hager J, Webster CD (2017) Evaluation of black soldier fly (Hermetia illucens) larvae meal as partial or total replacement of marine fish meal in practical diets for Pacific white shrimp (Litopenaeus vannamei). Aquaculture 473:337–344. https://doi.org/10.1016/j.aquaculture.2017.02.022
Duan Y, Wang Y, Zhang J, Sun Y, Wang J (2018) Dietary effects of succinic acid on the growth, digestive enzymes, immune response and resistance to ammonia stress of Litopenaeus vannamei. Fish Shellfish Immunol 78:10–17. https://doi.org/10.1016/j.fsi.2018.04.008
Ewald N, Vidakovic A, Langeland M, Kiessling A, Sampels S, Lalander C (2020) Fatty acid composition of black soldier fly larvae (Hermetia illucens) – Possibilities and limitations for modification through diet. Waste Manage 102:40–47. https://doi.org/10.1016/j.wasman.2019.10.014
Fasakin EA, Balogun AM, Ajayi OO (2003) Evaluation of full-fat and defatted maggot meals in the feeding of clariid catfish Clarias gariepinus fingerlings. Aquac Res 34(9):733–738. https://doi.org/10.1046/j.1365-2109.2003.00876.x
Fernández Gimenez AV, Fenucci JL, Petriella AM (2004) The effect of vitamin E on growth, survival and hepatopancreas structure of the Argentine red shrimp Pleoticus muelleri Bate (Crustacea, Penaeidea). Aquac Res 35(12):1172–1178. https://doi.org/10.1111/j.1365-2109.2004.01142.x
Foysal J, Fotedar R, Tay CY, Gupta S (2019) Dietary supplementation of black soldier fly (Hermetica illucens) meal modulates gut microbiota, innate immune response and health status of marron (Cherax cainii, Austin 2002) fed poultry-by-product and fishmeal based diets. PeerJ 7:e6891. https://doi.org/10.7717/peerj.6891
Goda AMAS (2008) Effect of dietary protein and lipid levels and protein–energy ratio on growth indices, feed utilization and body composition of freshwater prawn, Macrobrachium rosenbergii (de Man 1879) post larvae. Aquac Res 39(8):891–901. https://doi.org/10.1111/j.1365-2109.2008.01947.x
Haryanti, Sembiring SBM, Sudewi, Widiastuti D, Giri NAG, Sugama K (2017) Immunity responses of lobster juvenile, Panulirus homarus with the use of probiotic in the moist diet. J Ris Akuakultur 12(1):85–97
He Y, Liu X, Zhang N, Wang S, Wang A, Zuo R, Jiang Y (2022) Replacement of commercial feed with fresh black soldier fly (Hermetia illucens) larvae in Pacific white shrimp (Litopenaeus vannamei). Aquac Nutr 2022:9130400. https://doi.org/10.1155/2022/9130400
Hender A, Siddik MAB, Howieson J, Fotedar R (2021) Black soldier fly, hermetia illucens as an alternative to fishmeal protein and fish oil: impact on growth, immune response, mucosal barrier status, and flesh quality of juvenile barramundi, Lates calcarifer (Bloch, 1790). Biology 10(6). Retrieved from https://doi.org/10.3390/biology10060505
Henry MA, Gai F, Enes P, Perez-Jimenez A, Gasco L (2018) Effect of partial dietary replacement of fishmeal by yellow mealworm (Tenebrio molitor) larvae meal on the innate immune response and intestinal antioxidant enzymes of rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol 83:308–313. https://doi.org/10.1016/j.fsi.2018.09.040
Hosamani N, Srinivasa RB, Ramachandra RP (2017) Crustacean molting: regulation and effects of environmental toxicants. Journal of Marine Science Research Development and Change 7(5):1–8. https://doi.org/10.4172/2155-9910.1000236
Hu Y, Tan B, Mai K, Ai Q, Zheng S, Cheng K (2008) Growth and body composition of juvenile white shrimp, Litopenaeus vannamei, fed different ratios of dietary protein to energy. Aquac Nutr 14(6):499–506. https://doi.org/10.1111/j.1365-2095.2007.00555.x
Hu Y, Huang Y, Tang T, Zhong L, Chu W, Dai Z, ..., Hu Y (2020) Effect of partial black soldier fly (Hermetia illucens L.) larvae meal replacement of fish meal in practical diets on the growth, digestive enzyme and related gene expression for rice field eel (Monopterus albus). Aquac Rep 17:100345. https://doi.org/10.1016/j.aqrep.2020.100345
Huo Y-W, Jin M, Zhou P-P, Li M, Mai K-S, Zhou Q-C (2014) Effects of dietary protein and lipid levels on growth, feed utilization and body composition of juvenile swimming crab, Portunus trituberculatus. Aquaculture 434:151–158. https://doi.org/10.1016/j.aquaculture.2014.08.011
Irawan AC, Astuti D, Wibawan I, Hermana W (2020) Supplementation of black soldier fly (Hermetia illucens) on productivity and blood hematology. J Ilmu-Ilmu Peternakan 30:50–68. https://doi.org/10.21776/ub.jiip.2020.030.01.06
Irvin SJ, Williams KC (2009) Comparison of the growth and survival of Panulirus ornatus seed lobsters held in individual or communal cages. In K. C. Williams (eds), Spiny lobster aquaculture in the Asia-Pacific regionProceedings of an international symposium held at Nha Trang, Vietnam, 9–10 December 2008 (vol 132, pp 162). Canberra: Australian Centre for International Agricultural Research. Retrieved from https://www.aciar.gov.au/sites/default/files/legacy/node/11536/ACIAR_PR132%28online%29.pdf
Jatobá A, Moraes K, Rodrigues-Soares J, Jesus G, do Nascimento Vieira F (2018) Effect of protein source and probiotic on the intestinal tract of pacific white shrimp Litopenaeus vannamei. Bol Inst Pesca 44:371. https://doi.org/10.20950/1678-2305.2018.44.4.371
Jones C (2009) Temperature and salinity tolerances of the tropical spiny lobster, Panulirus ornatus. J World Aquac Soc 40(6):744–752. https://doi.org/10.1111/j.1749-7345.2009.00294.x
Karapanagiotidis IT, Neofytou MC, Asimaki A, Daskalopoulou E, Psofakis P, Mente E, Rumbos CI, Athanassiou CG (2023) Fishmeal replacement by full-fat and defatted hermetia illucens prepupae meal in the diet of gilthead seabream (Sparus aurata). Sustainability 15(1). Retrieved from https://doi.org/10.3390/su15010786
Koutsos E, Modica B, Freel T (2022) Immunomodulatory potential of black soldier fly larvae: applications beyond nutrition in animal feeding programs. Transl Anim Sci 6(3):txac084. https://doi.org/10.1093/tas/txac084
Kropielnicka-Kruk K, Trotter AJ, Fitzgibbon QP, Smith GG, Carter CG (2019) The effect of conspecific interaction on survival, growth and feeding behaviour of early juvenile tropical spiny lobster Panulirus ornatus. Aquaculture 510:234–247. https://doi.org/10.1016/j.aquaculture.2019.05.017
Kurnia A, Yusnaini Y, Muskita WH, Astuti O, Hamzah M (2017) Replacement of fish meal with fish head meal in the diet on the growth and feed efficiency of spiny lobster, Panulirus Ornatus under reared in sea net cage. Int J Eng Sci 06:34–38. https://doi.org/10.9790/1813-0601033438
Li Y, Liu H, Dai X, Li J, Ding F (2018) Effects of dietary inulin and mannan oligosaccharide on immune related genes expression and disease resistance of Pacific white shrimp, Litopenaeus vannamei. Fish Shellfish Immunol 76:78–92. https://doi.org/10.1016/j.fsi.2018.02.034
Li X, Yao X, Zhang X, Dong X, Chi S, Tan B, ..., Xie S (2022) Effects of dietary chenodeoxycholic acid supplementation in a low fishmeal diet on growth performance, lipid metabolism, autophagy and intestinal health of Pacific white shrimp, Litopenaeus vannamei. Fish Shellfish Immunol 127:1088–1099. https://doi.org/10.1016/j.fsi.2022.07.045
Liu X, Chen X, Wang H, Yang Q, Rehman K, Li W, ..., Zheng L (2017) Dynamic changes of nutrient composition throughout the entire life cycle of black soldier fly. PloS One 12:e0182601. https://doi.org/10.1371/journal.pone.0182601
Marchese G, Fitzgibbon Q, Trotter A, Carter C, Jones C, Smith G (2018) The influence of flesh ingredients format and krill meal on growth and feeding behaviour of juvenile tropical spiny lobster Panulirus ornatus. Aquaculture 499:128–139. https://doi.org/10.1016/j.aquaculture.2018.09.019
Miandare HK, Mirghaed AT, Hosseini M, Mazloumi N, Zargar A, Nazari S (2017) Dietary Immunogen® modulated digestive enzyme activity and immune gene expression in Litopenaeus vannamei post larvae. Fish Shellfish Immunol 70:621–627. https://doi.org/10.1016/j.fsi.2017.09.048
Miao S, Zhao C, Jinyu Z, Hu J, Dong X, Sun L (2018) Dietary soybean meal affects intestinal homoeostasis by altering the microbiota, morphology and inflammatory cytokine gene expression in northern snakehead. Sci Rep 8. https://doi.org/10.1038/s41598-017-18430-7
Mohan K, Rajan DK, Muralisankar T, Ganesan AR, Sathishkumar P, Revathi N (2022) Use of black soldier fly (Hermetia illucens L.) larvae meal in aquafeeds for a sustainable aquaculture industry: a review of past and future needs. Aquaculture 553:738095. https://doi.org/10.1016/j.aquaculture.2022.738095
Nankervis L, Jones C (2022) Recent advances and future directions in practical diet formulation and adoption in tropical Palinurid lobster aquaculture. Rev Aquac. https://doi.org/10.1111/raq.12675
Priyadarshana K, Ruwandeepika C, Naveenan M, Magamage M, Ruwandeepika D (2021) Substitution of fishmeal with black soldier fly Hermetia illucens Linnaeus, 1758 larvae in finfish aquaculture -a review. Asian Fish Sci 34. https://doi.org/10.33997/j.afs.2021.34.2.001
Ratunil V (2017) Growth and survival of ornate spiny lobster (Panulirus ornatus) in nursery under laboratory condition. Philipp J Agric Econ 1:16. https://doi.org/10.7719/pjae.v1i1.483
Ravi H, Degrou A, Costil J, Trespeuch C, Chemat F, Vian M (2020) Larvae mediated valorization of industrial, agriculture and food wastes: biorefinery concept through bioconversion, processes, procedures, and products. Processes 8:857. https://doi.org/10.3390/pr8070857
Richardson A, Dantas-Lima J, Lefranc M, Walraven M (2021) Effect of a black soldier fly ingredient on the growth performance and disease resistance of juvenile Pacific white shrimp (Litopenaeus vannamei). Animals (Basel) 11(5). https://doi.org/10.3390/ani11051450
Riddick EW (2014) Chapter 16 - Insect protein as a partial replacement for fishmeal in the diets of juvenile fish and crustaceans. Morales-Ramos JA, Rojas MG, Shapiro-Ilan DI (eds), Mass production of beneficial organisms (pp 565–582). San Diego: Academic Press. https://doi.org/10.1016/B978-0-12-391453-8.00016-9
Rios A, Lefebvre T, Do H, Henry M, Jintasataporn O (2019) Replacing fish meal with defatted insect meal (yellow mealworm Tenebrio molitor) improves the growth and immunity of Pacific white shrimp (Litopenaeus vannamei). Animals 9:258. https://doi.org/10.3390/ani9050258
Sánchez-Muros MJ, Renteria P, Vizcaino A, Barroso FG (2018) Innovative protein sources in shrimp (Litopenaeus vannamei) feeding. Rev Aquac 12(1):186–203. https://doi.org/10.1111/raq.12312
Sang HM, Fotedar R (2010) Effects of mannan oligosaccharide dietary supplementation on performances of the tropical spiny lobsters juvenile (Panulirus ornatus, Fabricius 1798). Fish Shellfish Immunol 28(3):483–489. https://doi.org/10.1016/j.fsi.2009.12.011
Saputra I, Fotedar R (2022) Investigating the effect of various dietary protein to energy ratios on juvenile spiny lobsters, Panulirus ornatus (Fabricius, 1798). Unpublished Work. Research Square. Retrieved from https://www.researchsquare.com/article/rs-1798071/v1
Shearer KD, Maage A, Opstvedt J, Mundheim H (1992) Effects of high-ash diets on growth, feed efficiency, and zinc status of juvenile Atlantic salmon (Salmo salar). Aquaculture 106(3):345–355. https://doi.org/10.1016/0044-8486(92)90266-N
Shields J, Wheeler K, Moss J (2012) Histological Assessment of the Lobster ( Homarus americanus ) in the “100 Lobsters” Project. J Shellfish Res 31:439–447. https://doi.org/10.2983/035.031.0204
Shumo M, Osuga IM, Khamis FM, Tanga CM, Fiaboe KKM, Subramanian S, ..., Borgemeister C (2019) The nutritive value of black soldier fly larvae reared on common organic waste streams in Kenya. Sci Rep 9(1):10110. https://doi.org/10.1038/s41598-019-46603-z
Smets R, Goos P, Claes J, Van Der Borght M (2021) Optimisation of the lipid extraction of fresh black soldier fly larvae (Hermetia illucens) with 2-methyltetrahydrofuran by response surface methodology. Sep Purif Technol 258:118040. https://doi.org/10.1016/j.seppur.2020.118040
Smith DM, Williams KC, Irvin S, Barclay M, Tabrett S (2003) Development of a pelleted feed for juvenile tropical spiny lobster (Panulirus ornatus): response to dietary protein and lipid. Aquac Nutr 9(4):231–237. https://doi.org/10.1046/j.1365-2095.2003.00248.x
Smith DM, Williams KC, Irvin SJ (2005) Response of the tropical spiny lobster Panulirus ornatus to protein content of pelleted feed and to a diet of mussel flesh. Aquac Nutr 11(3):209–217. https://doi.org/10.1111/j.1365-2095.2005.00344.x
Sukor SA, Sofea T, Fariborz E, Aziz A, Wing-Keong N, Nicholas R (2016) Effects of different dietary organic acids on the survival, growth, and hepatopancreatic histopathology of the blue swimmer crab (Portunus pelagicus). J Shellfish Res 35(2):555–561. https://doi.org/10.2983/035.035.0228
Takeuchi T, Murakami K (2007) Crustacean nutrition and larval feed, with emphasis on Japanese spiny lobster, Panulirus japonicus. Bull Fish Res Agency 20:15–23
Taugbøl T, Skurdal J (2008) Growth, mortality and moulting rate of noble crayfish, Astacus astacus L., juveniles in aquaculture experiments. Aquac Res 23:411–420. https://doi.org/10.1111/j.1365-2109.1992.tb00785.x
Verghese B, Radhakrishnan EV, Padhi A (2007) Effect of environmental parameters on immune response of the Indian spiny lobster, Panulirus homarus (Linnaeus, 1758). Fish Shellfish Immunol 23(5):928–936. https://doi.org/10.1016/j.fsi.2007.01.021
Vijayakumaran M, Kumar TS (2010) Moulting and growth in communal and individual rearing of the spiny lobster, Panulirus homarus. J Mar Biol Assoc India 52:274–281
Vijayakumaran M, Maharajan A, Rajalakshmi S, Jayagopal P, Remani MC (2014) Early larval stages of the spiny lobster, Panulirus homarus, Panulirus versicolor and Panulirus ornatus under laboratory conditions. Int J Dev Res 4(2):377–383
Vogel H, Müller A, Heckel DG, Gutzeit H, Vilcinskas A (2018) Nutritional immunology: diversification and diet-dependent expression of antimicrobial peptides in the black soldier fly Hermetia illucens. Dev Comp Immunol 78:141–148. https://doi.org/10.1016/j.dci.2017.09.008
Wang KH-C, Tseng CW, Lin HY, Chen IT, Chen YH, Chen YM, ..., Yang HL (2010) RNAi knock-down of the Litopenaeus vannamei Toll gene (LvToll) significantly increases mortality and reduces bacterial clearance after challenge with Vibrio harveyi. Dev Comp Immunol 34(1):49–58. https://doi.org/10.1016/j.dci.2009.08.003
Weththasinghe P, Hansen JØ, Nøkland D, Lagos L, Rawski M, Øverland M (2021) Full-fat black soldier fly larvae (Hermetia illucens) meal and paste in extruded diets for Atlantic salmon (Salmo salar): effect on physical pellet quality, nutrient digestibility, nutrient utilization and growth performances. Aquaculture 530:735785. https://doi.org/10.1016/j.aquaculture.2020.735785
Wongsasak U, Chaijamrus S, Kumkhong S, Boonanuntanasarn S (2015) Effects of dietary supplementation with β-glucan and synbiotics on immune gene expression and immune parameters under ammonia stress in Pacific white shrimp. Aquaculture 436:179–187. https://doi.org/10.1016/j.aquaculture.2014.10.028
Xu W-N, Liu W-B, Shen M-F, Li G-F, Wang Y, Zhang W-W (2013) Effect of different dietary protein and lipid levels on growth performance, body composition of juvenile red swamp crayfish (Procambarus clarkii). Aquacult Int 21(3):687–697. https://doi.org/10.1007/s10499-012-9603-8
Zha H, Jones B, Lewis G, Dong Y, Jeffs A (2018) Pathology of tail fan necrosis in the spiny lobster, Jasus edwardsii. J Invertebr Pathol 154:5–11. https://doi.org/10.1016/j.jip.2018.03.010
Zozo B, Wicht MM, Mshayisa VV, van Wyk J (2022) The nutritional quality and structural analysis of black soldier fly larvae flour before and after defatting. Insects 13(2). https://doi.org/10.3390/insects13020168
Acknowledgements
This study was undertaken as part of the Curtin Malaysia Postgraduate Research Studentship program by Curtin University Malaysia. The authors would like to thank Dr. Dody Hermawan for ingredients supply and Putu Eka Sudaryatma Ph.D. for assisting with the gene expression analysis.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions This work is supported by the Curtin Malaysia Postgraduates Research Studentship program from Curtin Malaysia for the first author.
Author information
Authors and Affiliations
Contributions
Ishaaq Saputra and Ravi Fotedar contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Ishaaq Saputra. The draft of the manuscript was written by Ishaaq Saputra and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
The authors confirm that the ethical policies of the journal, as noted on the journal’s author guidelines page, have been adhered to. No ethical approval was required as this is research involving crustaceans.
Conflict of interest
The authors declare no competing interests.
Additional information
Handling Editor: Gavin Burnell
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Saputra, I., Fotedar, R. The effect of defatted black soldier fly meal (Hermetia illucens) inclusion in the formulated diet on the growth, gene expression, and histopathology of juvenile lobster (Panulirus ornatus Fabricius, 1798). Aquacult Int 32, 11–29 (2024). https://doi.org/10.1007/s10499-023-01151-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-023-01151-2