Abstract
The current study aimed to investigate the efficacy of dietary thyme essential oil (TEO), Nigella sativa essential oil (NSEO), thyme essential oil nano-emulsion (TEO-NE), and Nigella sativa essential oil nano-emulsion (NSEO-NE) in reducing total ammonia nitrogen (TAN), improving immune response, mitigating stress, and acting as anti-inflammatory agents as well as preventing streptococcosis infection in Oreochromis niloticus (O. niloticus). Fish (N = 330, 14 ± 2 g) were divided into 10 groups of 11 fish each, with three replicates in each group. The negative and positive controls were fed a control diet, while the third group was given 1% TEO. Moreover, the fourth group of fish was given 2% TEO. The fifth and sixth groups were fed 1% TEO-NE and 2% TEO-NE, respectively. The seventh, eighth, ninth, and tenth groups were fed 1% NSEO, 2% NSEO, 1% NSEO-NE, and 2% NSEO-NE, respectively. After dietary intake of TEO, NSEO, and their nano-emulsions for 28 days, the mean values of TAN levels in the water of fish aquaria had a significant reduction in the group fed 2% TEO-NE compared to the control group. On the contrary, NSEO-NE at the same concentration had no significant effect on TAN levels. The levels of lysozyme, complement 5, and IgM increased in all feeding groups compared to the control group. Concerning cortisol level as a stress indicator, it was decreased in all feeding groups compared to the control. Also, the current experiment overall showed a significant decrease in the expression level of pro-inflammatory tumor necrosis factor (TNF-α) gene in the gills of fish groups fed TEO, TEO-NE, NS, and NS-NE relative to the β-actin gene. Oppositely, there was an increase in the expression level of the anti-inflammatory transforming growth factor (TGF-β). In the current study, TEO-NE and NSEO-NE showed a better effect on preventing streptococcosis in O. niloticus with no mortality than 1% TEO and NSEO, respectively. Furthermore, there was a 12.5% mortality rate and an 84.99 RPS in the group fed 1% TEO and injected with Streptococcus inae. On the other hand, the groups fed 1 and 2% NSEO showed 37.5 and 25% mortality rate, respectively and 54.99 and 69.99 RPS. In conclusion, the nano-emulsion either TEO or NSEO had the superior effect. For bulk status, the TEO had superior effect than NSEO. The study needs more investigations for ammonia, either on the mode of action or over a longer period.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Indeed, aquaculture is one of the fastest-growing food-producing sectors, accounting for one-third of worldwide fisheries production (Rocha, 2022). Aquaculture can benefit from nanotechnologies in various ways (Huang et al. 2015). It seeks to increase the efficacy of materials by altering their size and allowing the nanoscale to have a more significant impact on biology and medicine (Sahlan et al. 2017).
Nile tilapia, Oreochromis niloticus (O. niloticus) is the most widely cultured species among a diverse group of aquatic habitats (FAO 2018) because of its rapid development, stress tolerance, and reproduction in both freshwater and saltwater conditions (Gibtan et al. 2008). Water quality management is the most significant component in tilapia farming, and ammonia content is the most critical water quality factor (El-Gendy et al. 2015). Although tilapia intensification at high stocking densities has resulted in significant productivity gains, it also increases ammonia stress (Atle et al. 2009) and the incidence of infectious diseases, particularly those caused by bacterial pathogens ((FAO 2009 and Pękala–Safińska 2018). Furthermore, one of the most common bacterial diseases that cause major economic losses in tilapia production sector is streptococcosis (Gültepe et al. 2014). Streptococcus inae (S. inae) the most common cause of streptococcosis is mostly managed by antibiotics (Gültepe et al. 2014). Since the oral administration of such antibiotics in aqua-feed is prohibited in some countries (Romero et al. 2012), the search for novel natural feed additives to replace antibiotics has become an important and ongoing task (Talbot et al. 1994, Santacruz-Reyes et al. 2012; Li et al. 2014; Engler et al. 2018; Guo et al. 2018). Among those selected natural feed additives are natural essential oils which are categorized as ‘‘generally recognized as safe’’ (GRAS) and are being exploited as natural antimicrobial additives to replace synthetic antimicrobial compounds (Lv et al. 2011). Similarly, thyme essential oil (TEO) and Nigella sativa essentials oil (NSEO) and their nano-emulsions have a high potential for use in aquaculture systems due to a variety of beneficial biological effects reported in aquatic species, such as reducing total ammonia excretion in sea bass and rainbow trout water (Yılmaz et al. 2012; Ozogul et al. 2017). Also, they have immunomodulatory, anti-stress, anti-inflammatory (Mbarek et al. 2007), and antibacterial activities against Gram-positive and negative bacteria (Kooti et al. 2016; Aziz et al. 2017; El-Aarag et al. 2017; Kumari et al 2018; Salam et al. 2021). Therefore, the current study was designed to investigate the efficacy of dietary supplementation with TEO and NSEO and their nano-emulsions in reducing ammonia excretion, improving immune response, mitigating stress, and acting as anti-inflammatory agents in O. niloticus. Also, the prevention of streptococcosis using these natural products was carried out.
Material and methods
Ethical committee
The Faculty of Veterinary Medicine at Beni-Suef University in Egypt’s Institutional Animal Care and Use Committee (number: 022-355) approved all experiments.
Collection of experimental fish
For the experimental studies, 438 healthy O. niloticus with 14±2 g average body weight were gathered alive from the fish hatchery of Abo-Saleh in Beni-Suef, Egypt. The captured fish were taken to the Fish Diseases and Management wet laboratory at Beni-Suef University’s Faculty of Veterinary Medicine in Egypt. Three fiberglass tanks of 500 L capacity each were filled with fish and supplied with tap water without chlorine and consistent aeration. The experimental fish were acclimatized in these tanks for 14 days and fed a 3% of their body weight of fish ration (Table 1).
Management of experimental fish
After acclimation, fish have been added in glass aquaria of 90×25×40 cm with a water capacity of thirty liters. Through an air blower, each tank received continuous aeration. The water rate of exchange in the experimental aquaria was 10% per day. Food was given to the fish at a rate of 3% of body weight. During the trial, water quality was measured twice a week. Water temperature (26 ±1°C) was measured with a water thermometer, dissolved oxygen (D.O; 7 ± 2 mg/L) was measured by a D.O meter (Yellow Spring Instrument Co., USA), and pH (7-8) was determined by pH indicator paper (Fisher Scientific, Denver, CO, USA).
Source of TEO and NSEO
Thyme essential oil (TEO) was purchased from Sigma-Aldrich provided TEO (Chemie GmbH, Steinheim, Germany, containing a pamphlet, supplemented with the oil structure details). However, NS-EO was obtained from a local shop.
Synthesis of TEO-NE and NSEO-NE
Nano-emulsions of TEO and NSEO were prepared according to Pongsumpun et al. (2019) using the ultra-sonication technique. The nano-emulsions were prepared through two stages: the aqueous one, which was prepared by combining tween 80 at a concentration of 3% in distilled water, and the oil phase, which was made up of essential oils diluted to 1.0 and 2 mL/100mL. Then, a coarse emulsion was created by gradually adding the oil phase to the aqueous phase (W/W) and vigorously mixing by a magnetic stirrer (MSH-20D, Wise Stir) at 500 rpm and 25°C for 15 min. An ultrasonic bath was used to combine the two phases, and the result was a nano-emulsion (Ultrasons, P-Selecta). For sonication, a fixed frequency of 43 kHz, output at 210 W, and high levels of oscillation were used. The ultrasound was generated and exposed to a water bath at 25 °C for 10 minutes.
Characterizations of TEO-NE and NSEO-NE
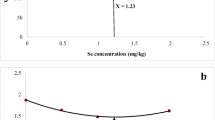
Transmission electron microscopy (TEM) was used to characterize TEO-NE and NSEO-NE. For the TEM procedure, 20 microliters of diluted samples were placed for 10 min on a film-coated 200-mesh copper specimen grid. Next, one drop of 3% phosphotungstic acid was used to stain the grid, and it was left for three minutes to dry. After drying, the coated grid was examined with a TEM microscope (Philips, CM 12). Operating at 120 kV allowed for the observation of the samples. Faculty of Agriculture Central Laboratory at Cairo University in Egypt carried out this work. Also, the samples were characterized by zeta potential at the Faculty of Postgraduate Studies of Advanced Science, Beni-Suef University, Egypt. Zeta potential was operated by adding the emulsion sample (25 ul) into a capillary cell and diluted in 2 ml water. The emulsion was conditioned for 20 minutes before use (Sze et al. 2003).
Formation of diet
The fish ration was powdered into an extremely fine dust using a mortar and pestle. The TEO and NSEO, as well as their nano-emulsion, were combined with the previously made fine powder to produce five fish diets. There were no additives in diet 1 (control), 1% TEO in diet 2, 2% TEO in diet 3, 1% TEO-NE in diet 4, and 2% TEO-NE in diet 5. Diets 6 and 7 each contain 1% NSEO and 2% NSEO and diets 8 and 9 each contain 1% NSEO-NE and 2% NSEO-NE (Salam et al 2021). The components of the fish diet were combined with distilled water to create a homogeneous mixture. To create extruded strings that were dried at room temperature; the mixture was run through a manually operated meat processing machine Rattanachaikunsopon and Phumkhachorn (2010).
Design of experiment and regime of feeding
Three hundred and thirty O. niloticus had been separated into 10 groups of 11 fish each and had three replicates to each group. The negative and positive controls were fed a control diet with no additives, while the third group received 1% TEO. Furthermore, the fish in the fourth group were given 2% TEO. The fifth and sixth groups were fed 1% TEO-NE and 2% TEO-NE. The seventh, eighth, ninth, and tenth groups were fed 1% NSEO, 2% NSEO, 1% NSEO-NE, and 2% NSEO-NE, respectively. During the experiment, each group received 3% of their body weight of their designated food divided into two times a day for 28 days. Total ammonia nitrogen (TAN) was monitored throughout the experiment. Three fish from each group were used to collect serum, plasma, and gills at the end of the feeding period. For the purpose of preventing streptococcosis, the remaining eight fish in each group with their replicates were used.
Measuring TAN in the fish glass aquaria
For four weeks, water samples were collected twice per week. In sterile, colorless glass Stoppard bottles with 1 L capacities, water samples were taken separately from each group of glass aquariums at a depth of 10 cm. To the Department of Hygiene, Zoonoses and Epidemiology, Faculty of Veterinary Medicine, Beni-Suef University, Beni-Suef, the labelled bottles were shipped at 5°C. With the aid of a Multiparameter Photometer, TAN levels were determined (bench, HI83200, HANNA, Romania). The concentration was measured using the Nessler method and reagent kits HI93700A-0 and HI93700B-0. The TAN level was also calculated in parts per million (ppm).
Collection of serum, plasma and gills of experimental feeding groups
Three fish from each group were netted and anesthetized by tricaine methane sulfonate (Sigma-Aldrich Chemical Co., Cairo, Egypt) after 28 days of feeding to collect their blood and gills.
Serum was prepared by collection of blood from caudal veins without anticoagulant while the remaining blood was drawn and put into cooled plastic tubes having 3 mg of Na2EDTA for plasma collection.
To estimate the pro-inflammatory tumor necrosis factor (TNF- -α) and the anti-inflammatory transforming growth factor (TGF- β) genes, the collected pieces of gills were placed in tubes of 2 ml containing RNAlater solution (Merc, Egypt). Following an overnight period at 4 °C in the refrigerator, the samples were then frozen at -80 °C.
Estimation of immune parameters and stress indicators in serum and plasma of feeding groups
Lysozyme, complement 5, and IgM concentrations in the collected serum were determined using test kits from CUSABIO (Fish Lysozyme (LZM) ELISA Kit Catalog Number, CSB-E17296Fh, China), Fish Complement 5 (C5) ELISA Kit Catalog Number, CSB-F13502F, China), and Fish immunoglobulin M ELISA Kit Catalog Number, CSB-E12045Fh, China). Additionally, the plasma cortisol level was measured using a cortisol ELISA kit® (Calbiotech, catalogue No. CO103S, Canada) following manufacturer’s instructions, and according to Schlaghecke et al. (1992) results were calculated using an automatic ELISA reader (SUNRISE®; Tecan, Austria).
Protocol of expression of pro-inflammatory TNF-α and anti-inflammatory TGF-β genes in the gills of the experimental feeding groups
Total RNA was isolated using TransZol Reagent (Transgen Biotech, Beijing, China) following the manufacturer's instructions. The RNA concentration (OD 260nm) and purity (OD 260nm/OD280 nm ratio, range 1.90–2.08) of each sample was measured using NanoDrop2000c (Thermo Scientific, Massachusetts, USA). The integrity of RNA was checked by 1.0% agarose gel electrophoresis. One microgram of total RNA from each sample (4 samples from 10GCI group and 5 samples from every other group) was reverse transcribed to obtain cDNA using Prime Script™RT reagent Kit with gDNA Eraser (TaKaRa, Dalian, China) to remove genomic DNA contamination, following the manufacturer's protocol. Expressions of different immune-related genes were studied by real time PCR. The primers used were listed in Table 2, using β-actin as the reference gene. SYBR green reagent kit used in the real-time PCR experiments was KAPA SYBR® qPCR Kit Master Mix (2X) Universal (KAPA Biosystems, California, USA). Each sample was separately treated in triplicate in a 10μl final volume, including 200nM of each primer (Table 2) and 10ng of cDNA template. PCR amplification cycles were performed using Roche LightCycler®480 system (Roche, Shanghai, China). The cycling profile was as follows: enzyme activation was carried out at 95°C for 3min, followed by 40 cycles of denaturing at 95°C for 3s, and annealing at 60°C for 30s. Results of real-time PCR were analyzed using 2-ΔΔCt method (Livak et al. 2001). The transcript abundance of each target gene was obtained as Ct value, and normalized by that of β-actin gene as an internal reference. The relative expressions of genes were expressed as mean ± SE, and ANOVA with Dunnett test was used to compare the expression differences between experiment groups and control group in IBM SPSS Statistics 22.0 (Table 2).
Determination of the median lethal dose (LD50) of S. inae in O. niloticus
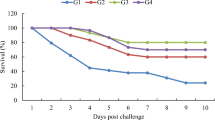
For median lethal dose (LD50) determination of S. inae in O. niloticus, about 108 apparently healthy O. niloticus were divided into 6 groups (6 fish per group) with three replicates. An overnight culture of S. inae was prepared at densities of 1.5 × 108, 1.5 × 107, 1.5 × 106, 1.5 × 105and 1.5 × 10 4 CFU/mL. Each dilution was injected intraperitoneally into a fish group at a dose of 200 μL/fish and the 6th group was injected with 200 μL of physiological saline (control negative). All fish groups were kept for 2 weeks, and mortalities were recorded daily.
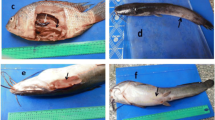
Prevention of streptococcosis in O. niloticus
The S. inae BNS 0014 strain was kindly obtained from the department of Fish Diseases and Management, Faculty of Veterinary Medicine, Beni-Suef University, Beni-Suef, Egypt. The strain was isolated from diseased O. niloticus (Hussein and Hassan 2011). After 28 days of feeding, the final eight fish from each group, along with their replicas, were injected. The negative control and its replicates were injected with 200 µL physiological saline intraperitoneally. S. inae was administered intraperitoneally to fish in the second (positive control), third, fourth, fifth, sixth, seventh, eighth, ninth, and tenth groups at doses of 200 µL of 1.5×107 CFU/mL each. The injected groups were kept in glass aquaria for two weeks (Schlaghecke et al. 1992). The mortality and relative percent survival (RPS) was calculated by the formula proposed by Amend (1981): RPS = 1 (% of mortality in treated groups /% of mortality in control group)×100.
Statistical analyses
All data were assembled and subjected to statistical analysis using the Advanced Models 16.0 software's one-way ANOVA (post hoc test; Dunnet's test) (SPSS, Tokyo, Japan). Statistical significance was defined as p-value < 0.05 was considered statistically significant.
Results
Characterization of TEO-NE and NSEO-NE
The TEM photography of the TEO-NE revealed that it had a spherical shape between 72.4 and 58.9 nm (Fig. 1). TEO-zeta NE’s potential ranged from 606.1 to 789.0 nm (Fig. 2). The droplets in the nano-emulsion appeared dark; however, the TEM micrograph of NSEO-NE shows the actual size and shape. The TEM photography revealed that the NSEO-NE was spherical and measured between 16.4 and 13.3 nm in size (Fig. 3). NSEO-zeta NE’s potential ranged from 324.7 to 574.0 nm (Fig. 4).
Total ammonia nitrogen (TAN) in the fish glass aquaria during feeding period
The effectiveness of dietary intake of thyme oil, Nigella oil, and their nano-emulsion on mitigation of TAN level in different fish groups was determined during the current trial. Adding TEO at a concentration of 2.0% led to slightly alleviate the mean values of TAN during the trial periods at p ≤ 0.05 whereas the TAN level in the 4th week of trial was 0.41±0.0 mg/L compared to control one and pre-experiment level (0.80 ±0.05 and 0.73±0.13 mg/L, respectively. Meanwhile, in the first three weeks, the values were 0.81±0.01, 0.65±0.13, and 0.52±0.01mg/L, respectively. Oppositely, using TEO-NE at the highest concentration 2.0 %) had a significant reduction in the mean values of TAN during the 3rd and 4th week of trial (0.18±0.0 and 0.11±0.0 mg/L) compared to control group (0.71± 0.11, and 0.73± 0.13 mg/L, respectively) besides the level at both 1st and 2nd week was 0.73±0.12 and 0.25±0.01 mg/L, respectively. On the contrary, NSEO at the highest concentration of 2.0 % has no appreciable effect on TAN level during 1st, 2nd, 3rd, and 4th week of trial (0.83±0.15, 0.81±0.02, 0.73±0.21, and 0.67±0.13 mg/L, respectively compared to control group and pretrial concentration (0.75±0.06 mg/L). Concerning using NSEO-NE at the highest concentration of 2.0%, TAN level was significantly alleviated during 3rd and 4th week of trial (0.43±0.02 and 0.35±0.11 mg/L) compared to first week and pre-trial level (0.72±0.23 mg/L and 0.80±1.2 mg/L). Furthermore, in control fish group, the mean values of TAN level were 0.80 ± 0.22, 0.69± 0.01, 0.71 ± 0.11, and 0.73 ±0.13 mg/L, respectively compared to the pretrial level of 0.81 ± 0.16 mg/L as displayed in Table 3.
Immune parameters and stress indicators in serum and plasma of experimental groups
Lysozyme, complement 5, and IgM levels increased in all feeding groups following the administration of various concentrations of TEO, TEO-NE, NSEO, and NSEO-NE compared to control group. Compared to the control, all feeding groups had lower cortisol levels (Table 4).
Expression of pro-inflammatory TNF-α genes and anti-inflammatory TGF-β in gills of feeding groups
The current experiment overall showed a significant decrease in expression of pro-inflammatory TNF-α gene in fish gills fed TEO, TEO-NE, NS and NS-NE relative to the β-actin gene. Oppositely, there was increase in expression level of the anti-inflammatory TGF-β gene in fish fed TEO, TEO-NE, NS and NS-NE relative to the β-actin gene (Table 5).
The LD 50 of S. inae in healthy O. niloticus
The majority of fish mortality occurred during the first week and the LD50 was 1.5×105 CFU/ml (Fig. 5).
Streptococcosis prevention in O. niloticus using TEO and NS and their nano-emulsion
The mortality rate was zero but RPS was 100% in groups fed a high concentration of TEO, as well as high and low concentrations of TEO-NE and NSEO-NE, and then injected with S. inae strains, and control negative group that fed a normal diet and then injected with saline. The group given low concentrations of TEO and subsequently injected with pathogenic S. inae strain had a 12.5% mortality rate and a RPS of 84.99. The groups fed low and high concentrations of NSEO had 37.5 and 25% mortality rates, as well as 54.99 and 69.99 RPS, respectively. The mortality rate in control positive was 83.33%.
Discussion
Improving fish feed is essential for developing efficient and profitable aquaculture production because it is the most expensive component of the aquaculture industry (Ghanawi et al. 2011).
Ammonia is the most harmful aquaculture fish product. It impedes aquaculture intensification and, as a result, fish production El-Gendy et al. (2015) and Tumwesigye et al. (2022). The efficacy of dietary intake of TEO, NSEO, and their nano-emulsion on mitigating TAN levels in different fish groups clarified that a dietary supplement of TEO and its nano-emulsion (TEO-NE) at a concentration of 2.0% had a significant reduction in the mean values of TAN during the 3rd and 4th weeks of the trial compared to first two weeks and pre-experiment. Yılmaz et al. (2012) found a slight decrease in the total amount of ammonia nitrogen excretion in the water of sea bass when adding TEO to fish feed. Parallel findings were also obtained using Yucca schidigera as a feed additive in Nile tilapia diets, resulting in a decreased ammonia excretion rate (El-Saidy & Gaber 2004; Reyes & Chien 2009). The TEO can interact with microbial cell membranes and inhibit the growth of some bacteria, resulting in lower ammonia concentration (Calsamiglia et al., 2007 and Sethiya 2016). There is a lack of information in this direction. Those results agreed with the aforementioned studies that specified that a thyme extract significantly reduced the concentration of ammonia in rabbits compared to a control one (Kandeil et al. and Abd El-Azeem et al 2019). On the opposing, NSEO had no significant effect on TAN levels at the highest concentration of 2.0 % during the feeding period of the current trial. Meanwhile, adding NSEO-NE to fish feed at the same concentration (2.0%) exhibited significant mitigation in TAN levels especially during the 3rd and 4th week of the trial. Ozogul (2017) advised adding NSEO to rainbow trout. Whereas fish that fed NSEO showed a reduction in ammonia during the storage period.
Concerning the role of TEO and NSEO as immune stimulants, the levels of lysozyme, complement 5, and IgM increased significantly (p<0.05) in all feeding groups except the groups fed 1 and 2% NSEO. Increases in immunological responses like lysozyme, complement, and immunoglobulins can thus affect fish health by increasing disease resistance. Salam et al. (2021) demonstrated that feeding TEO and TEO-NE to O. niloticus resulted in significant increases in IgM, and lysozyme, with TEO-NE having a greater effect. Furthermore, Yousefia et al. (2021) discovered that feeding Nigella sativa seeds to common carp increased their lysozyme and immunoglobulin compared to the control. According to Bipul et al. (2020), dietary supplementation with 2% NSEO significantly improved the innate immunity of O. niloticus. Enriched diets with Nigella sativa, either oil or seeds, increased immunity in rainbow trout by increasing lysozyme levels (Awad et al. 2013) and raised haemoglobin, hematocrit, and globulin levels in O. niloticus (Hussein et al. 2021). Regarding cortisol level as a stress indicator, it significantly (p<0.05) decreased in all feeding groups except those fed NSEO, which had a lower cortisol but not significantly lower than the control. These findings were supported by (Alina et al. 2014), who discovered that 1% thyme supplementation resulted in the lowest plasma cortisol levels in O. niloticus when compared to other phytobiotics. Furthermore, Zadmajid and Mohammadi (2017) discovered that cortisol levels in blood were significantly lower in the TEO-added feed groups. Yousefi et al. (2021) demonstrated that feeding 1% NS seeds to carps resulted in a considerable drop in blood cortisol levels. The higher cortisol levels in control groups might be related to higher TAN levels as mentioned by Kuttchantran (2013), Shokr (2015) and Elsayed (2015).
The current study evaluated the anti-inflammatory properties of TEO and NSEO, as well as their nano-emulsion through the expression of pro-inflammatory TNF-α and anti-inflammatory TGF-β genes in the gills of feeding groups. TEO, TEO-NE, NSEO, and NSEO-NE significantly reduced pro-inflammatory TNF-α production and gene expression while they significantly increased the anti-inflammatory TGF-β expression relative to the β -actin gene. These findings were supported by Gulec et al. (2014); Valladão et al. (2019) and Mahboub et al. (2022), who discussed the dietary effect of thyme and Nigella sativa on these two genes of Nile tilapia. Gills were selected based on the findings of Lu et al. (2013), Li et al. (2015), and Liu (2021), who noticed that both of these genes were found in a variety of O. niloticus tissues but with a higher degree in gills. Up-regulated expression of TNF-α has been observed in the gills of goldfish during D. intermedius infection (Lu et al. 2013). The chemical composition of TEO and NSEO might be responsible for the mode of action. Carvacrol and thymol are the two higher chemicals in TEO (Tian et al. 2011) which have anti-inflammatory activity (Braga et al. 2006). Furthermore, thymoquinone is the main active constituent of NSEO, and its effect on decreasing pro-inflammatory TNF-α has been experimentally demonstrated to be an anti-inflammatory agent (Chehl et al. 2009). In our study, the anti-inflammatory gene was significantly down-regulated in the control group. This might be linked to the effect of higher cortisol as indicated by Castro et al. (2011).
For evaluating the antibacterial properties of TEO and NSEO and their nano-emulsions, the current investigation demonstrated that TEO-NE and NSEO-NE had a superior impact on preventing streptococcosis in O. niloticus without mortality than bulk TEO and NSEO. These findings were consistent with the findings of Gultepe et al. (2014), who found that feeding Oreochromis mossambicus at 1% thyme for 45 days had a 22% cumulative mortality rate compared to 61% mortality in the control group. Furthermore, the findings were consistent with those of Salam et al. (2021), who showed that dietary TEO-NE prevented Aeromonas hydrophila infection in O. niloticus without mortality compared to 10% in groups given low concentrations of TEO. These findings might be attributable to the fact that bulk TEO and NSEO have lowered water solubility, which reduces their antibacterial action and restricts their utilization in aqueous media (Pan et al. 2014 and Chang et al. 2015). Furthermore, TEO and NSEO are lipophilic biologically active compounds that are chemically and physically unstable in light and oxygen, lowering their effectiveness (Anton et al. 2008 and Sanguansri & Augustin 2006,). TEO-NE and NSEO-NE might be used to solve these challenges (Rodríguez et al. 2016). Nano-encapsulation of TEO and NSEO is physically and chemically stable in aqueous media (Mason et al. 2006 and McClements et al. 2011). According to Shaaban et al (2015) and Sharif et al. (2017), negatively charged NSEO-NE had a higher bactericidal activity than bulk NSEO due to its greater stability, regulated release, and self-assembly with cell membranes of Gram-positive bacteria followed by destruction of cellular components. Because of the evenly distributed nano-droplets, the nano-emulsion can easily enter and damage the microbial membrane (Xu et al. 2008; Di Pasqua et al. 2007). Furthermore, TEO had stronger antibacterial properties than NSEO, which could be attributed to the higher levels of thymol, p-cymene, carvacrol, eugenol, and 4-allylphenol (Lee et al. 2005; Rota et al. 2008).
Conclusions
The nano-emulsion either TEO or NSEO had the superior effect on ammonia reduction and better prevention of streptococcosis. For bulk status the TEO has the superior effect than NSEO. Also, this study could consider a promising way for application of nano-emulsion or even bulk TEO and NSEO in aquaculture.
Data availability
The data are available with the corresponding author.
Code availability
Not applicable.
References
Abd El-Azeem AE, Al-Sagheer AA, Daader AH, Bassiony SM (2019April) Effect of dietary supplementation with betaine, thyme oil and their mixtures on productive performance of growing rabbits. Zagazig J Agric Res 46(3):8
Alina, M. A. & Cristea, Victor & GRECU, Iulia & Lorena, Dediu & Cretu, Mirela & Bocioc, Elena & Petrea, Stefan Mihai. (2014) Effects of Dietary Supplementation at Nile tilapia with Thymus vulgaris, Trigonela foenum graecum and Azadirachta indica on Welfare Status. Bulletin of University of Agricultural Sciences and Veterinary Medicine Cluj-Napoca. Animal Science and Biotechnologies. 71. https://doi.org/10.15835/buasvmcn-asb:10386.
Amend DF (1981) Potency testing of fish vaccines. Dev Biol Stand 49:447–454
Anton N, Benoit JP, Saulnier P (2008) Design and production of nanoparticles formulated from nano-emulsion templates- a review. J Control Release 128:185–199
Atle F, Imsland AK, Roth B, Schram E, Stefansson SO (2009) Effects of chronic and periodic exposure to ammonia on growth and blood physiology in juvenile turbot (Scophthalmus maximus). Aquaculture 296(1–2):45–50. https://doi.org/10.1016/j.aquaculture.07.013
Awad E, Austin DA, Lyndon A (2013) Effect of black cumin seed oil (Nigella sativa) and nettle extract (Quercetin) on enhancement of immunity in rainbow trout, Oncorhynchus mykiss (Walbaum). Aquaculture 388–391:193–197. https://doi.org/10.1016/j.aquaculture.2013.01.008
Aziz MA, Khan AH, Adnan M, Izatullah I (2017) Traditional uses of medicinal plants reported by the indigenous communities and local herbal practitioners of Bajaur Agency, Federally Administrated Tribal Areas. Pakistan J Ethnopharmacol 198:268–281. https://doi.org/10.1016/j.jep.2017.01.024
Bipul D, Hossain M, Alam M (2020) Effect of black cumin seed oil on growth, innate immunity and resistance against Pseudomonas fluorescens infection in Nile tilapia Oreochromis niloticus. Aquac Int 28. https://doi.org/10.1007/s10499-020-00539-8
Braga PC, Dal Sasso M, Culici M, Bianchi T, Bordoni L, Marabini L (2006) Anti-inflammatory activity of thymol: inhibitory effect on the release of human neutrophil Elastase. Pharmacology 77(3):130–136
essential oils as modifiers of rumen microbial fermentation (2007) Calsamiglia, S.; BUSQUET, M.; CARDOZO, P.W. et al. Invited review. J Dairy Sci 90:2580–2595
Castro RJ, Zou CJ, Secombes SAM, Martin, (2011) Cortisol modulates the induction of inflammatory gene expression in a rainbow trout macrophage cell line, Fish & Shellfish Immunology, 30 1. ISSN 215–223:1050–4648. https://doi.org/10.1016/j.fsi.2010.10.010
Chang Y, McLandsborough L, McClements DJ (2015) Fabrication, stability and efficacy of dual-component antimicrobial nanoemulsions: Essential oil (thyme oil) and cationic surfactant (lauric arginate). Food Chem 172:298–304
Chehl N, Chipitsyna G, Gong Q, Yeo CJ, Arafat HA (2009) Anti-inflammatory effects of the Nigella sativa seed extract, thymoquinone, in pancreatic cancer cells. HPB (oxford) 11(5):373–381
Di Pasqua R, Betts G, Hoskins N, Edwards M, Ercolini D, Mauriello G. Membrane toxicity of antimicrobial compounds from essential oils. J Agric Food Chem. (2007) Jun 13;55(12):4863–70.
El-Aarag B, Hussein WM, Ibrahim WM, Zahran MA (2017) Thymoquinone improves anti-diabetic activity of metformin in streptozotocin-induced diabetic male rats. J Diabetes Metab 8:1–8. https://doi.org/10.4172/2155-6156.1000780
El-Gendy M, Gouda A, Shehab El-Din M (2015) Effect of zeolite on feeding rates and growth performance for Nile tilapia (Oreochromis niloticus). International Journal of Scientific Research in Agricultural Sciences 2:18–24
El-Saidy DMS, Gaber MMA (2004) Effect of yucca (Yucca schidigera) on water quality and growth performances of Nile tilapia (Oreochromis niloticus L) fingerlings. Egypt J Aquat Biol Fish 8:33–50
ElSayed AMS (2015) Effect of ammonia stress on blood constitutes in Nile tilapia. Egypt Acad J Biol Sci 7(1):37–44
Engler P, Caillis P, Voller S, Labrie L (2018) Dietary Supplementation of a Mixture of Saponin-Rich Plants to Reduce Ammonia-Nitrogen Excretion in Juvenile Nile Tilapia (Oreochromis niloticus). Sustainable Food Production 2:6–12
FAO (2009) The State of World Fisheries and Aquaculture 2008. FAO, Rome
FAO (2018) The state of world fisheries and aquaculture 2018—meeting the sustainable development goals. Food and Agriculture Organization of United Nations, Rome, pp 1–210
Ghanawi J, Roy L, Davis DA, Saoud IP (2011) Effects of dietary lipid levels on growth performance of marbled spinefoot rabbitfish, Siganus rivulatus. Aquaculture 310:395–400
Gibtan, A.; Getahun, A. and Mengistou, S. (2008) Effect of stocking density on the growth performance and yield of Nile tilapia [Oreochromis niloticus (L., 1758)] in a cage culture system in Lake Kuriftu, Ethiopia. Aquac Res 39:1450–1460. https://doi.org/10.1111/j.1365-2109.02021.x.
Gulec, A & Kucukgul, Azime & Danabas, D & Ural, Mesut & Seker, E & Arslan, Ali & Serdar, Osman. (2014) Therapeutic effects of thyme (thymus vulgaris linneaus) and fennel (foeniculum vulgare miller) essential oils in infected rainbow trout, oncorhynchus mykiss (Walbaum). Digest Journal of Nanomaterials and Biostructures. 8.
Gultepe, Nejdet & Bilen, Soner & Yilmaz, Sevdan & Güroy, Derya & Aydın, Seyit. (2014) Effects of herbs and spice on health status of tilapia (Oreochromis mossambicus) challenged with Streptococcus iniae. Acta Veterinaria Brno. 83. https://doi.org/10.2754/avb201483020000.
Guo H, Lin W, Hou J, Wang L, Zhang D, Wu X, Li L, Li D (2018) The Protective Roles of Dietary selenium yeast and tea polyphenols on growth performance and ammonia tolerance of juvenile Wuchang Bream (Megalobrama amblycephala). Front Physiol 9:1371
Harms G, Layton AC, Dionisi HM, Gregory IR, Garrett VM, Hawkins SA, Robinson KG, Sayler GS (2003Jan 15) (2003) Real-time PCR quantification of nitrifying bacteria in a municipal wastewater treatment plant. Environ Sci Technol 37(2):343–351. https://doi.org/10.1021/es0257164. (PMID: 12564907)
Huang S, Wang L, Liu L, Yuxuan Hou Lu, Li., (2015) Nanotechnology in agriculture, livestock, and aquaculture in China. A Review Agron Sustain Dev 35:369–400. https://doi.org/10.1007/s13593-014-0274-x
Hussein; S.Y. Metwally M.A; Asmaa, A.M. (2021) Effect of vitamin a andd3 supplementaion on the growth performance of nile tilapia (Oreochromis niloticus). assiut veterinary Medical Journal . 10, 67, 169, P 152–164 XMLPDF (871.78 K) DOI: https://doi.org/10.21608/avmj.2021.188845.
Hussein A, Hassan WH (2011) (2011) Efficacy of fosfomycin in controlling streptococcosis in Nile tilapia (Oreochromis niloticus) M. M BS VET MED J JULY 21(1):59–66
Kandeil MA, Mohamed AH, Abdel Gabbar M, Ahmed RR, Ali SM (2019Sep) Ameliorative effects of oral ginger and/or thyme aqueous extracts on productive and reproductive performance of V-line male rabbits. J Anim Physiol Anim Nutr 103(5):1437–1446
Kooti W, Hasanzadeh-Noohi Z, Sharafi-Ahvazi N, Asadi-Samani M, Ashtary-Larky D (2016) Phytochemistry, pharmacology, and therapeutic uses of black seed (Nigella sativa). Chin J Nat Med 14:732–745. https://doi.org/10.1016/S1875-5364(16)30088-7
Kumari S, Kumaraswamy RV, Choudhary RC, Sharma SS, Pal A, Raliya R, Biswas P (2018) Thymol nanoemulsion exhibits potential antibacterial activity against bacterial pustule disease and growth promotory effect on soybean. Sci Rep 8:6650
Kuttchantran M (2013) Managing ammonia in fish ponds. Universiti Putra Malaysia, Malaysia, Aquatic animal health unit
Lee S, UMANO, K & Shibamoto, Takayuki & Lee, Sangwon. (2005) Identification of volatile components in basil ( L.) and thyme leaves ( L.) and their antioxidant properties. Food Chemistry - FOOD CHEM 91:131–137
Li M, Yu N, Qin JG, Li E, Du Z, Chen L (2014) Effects of ammonia stress, dietary linseed oil and Edwardsiella ictaluri challenge on juvenile darkbarbel catfish Pelteobagrus vachelli. Fish Shellfish Immunol 38(1):158–165
Li M, Sun Y, Zhao J, Shi HJ, Zeng S, Ye K, Jiang DN, Zhou LY, Sun L, Tao WJ, Nagahama Y, Kocher TD, Wang DS, Kiyoshi N (2015) A Tandem Duplicate of Anti-Müllerian Hormone with a Missense SNP on the Y Chromosome Is Essential for Male Sex Determination in Nile Tilapia. Oreochromis Niloticus Plos Genet 11(11):e1005678
Liu M-J, Guo H-Y, Liu B, Zhu K-C, Guo L, Liu B-S, Zhang N, Yang J-W, Jiang S-G, Zhang D-C (2021) Gill oxidative damage caused by acute ammonia stress was reduced through the HIF-1α/NF-κb signaling pathway in golden pompano (Trachinotus ovatus). Ecotoxicol Environ Saf 222. https://doi.org/10.1016/j.ecoenv.2021.112504
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using realtime quantitative PCR and the 2− ΔΔCT method. Methods 25:402–408
Lv F, Liang H, Yuan Q, Li C (2011) In Vitro antimicrobial effects and mechanism of action of selected plant essential oil combinations against four food related microorganisms. Food Res Int 44:3057–3064. https://doi.org/10.1016/j.foodres.2011.07.030
Lu C, Ling F, Ji J, Kang YJ, Wang GX (2013) Expression of immune-related genes in gold fish gills induced by Dactylogyrus intermedius infections. Fish Shellfish Immunol 34:372–377
Mahboub HH, Elsheshtawy HM, Sheraiba NI, Fahmy EM, Masoud SR, Mohamed EAA, Abdelnaeim NS, Mohamed DI, Ismail TA, Ahmed SAA (2022) Dietary black cumin (Nigella sativa) improved hemato-biochemical, oxidative stress, gene expression, and immunological response of Nile tilapia (Oreochromis niloticus) infected by Burkholderia cepacia. Aquaculture Reports 22:100943. https://doi.org/10.1016/j.aqrep.2021.100943
Mason T, Wilking J, Meleson K, Chang C, Graves S (2006) Nanoemulsions: Formation, structure, and physical properties. J Phys Condens Matter 18:R635
Mbarek LA, Mouse HA, Elabbadi N, Bensalah M, Gamouh A et al (2007) Anti-tumor properties of blackseed ( Nigella sativa L.) extracts. Braz J Med Biol Res 40:839–847
McClements DJ (2011) Edible nanoemulsions: Fabrication, properties, and functional performance. Soft Matter 7:2297–2316
Ozogul, Y., İ Yuvka, Y Ucar, M Durmus, AR Kösker, M Öz… - Lwt, (2017) Evaluation of effects of nanoemulsion based on herb essential oils (rosemary, laurel, thyme and sage) on sensory, chemical and microbiological quality of rainbow trout (Oncorhynchus mykiss) fillets during ice storage. LWT, 75, 677–684, ISSN 0023–6438, https://doi.org/10.1016/j.lwt.2016.10.009.
Pan K, Chen H, Davidson PM, Zhong Q (2014) Thymol nanoencapsulated by sodium caseinate: Physical and antilisterial properties. J Agric Food Chem 62:1649–1657
Pękala –Safińska A. Contemporary threats of bacterial infections in freshwater fish J. Vet. Res, 62 (2018), pp. 261–267.
Pongsumpun, P., Iwamoto, S. and Siripatrawan, U. (2019) Response surface methodology for optimization of cinnamon essential oil nanoemulsion with improved stability and antifungal activity. Ultrasonics – Sonochemistry. 60:1–10.
Rattanachaikunsopon P, Phumkhachorn P (2010) Lactic acid bacteria: their antimicrobial compounds and their uses in food production. Ann Biol Res 1:218–228
Reyes RAS, Chien YH (2009) Efficacy of Yucca schidigera extract for ammonia reduction in freshwater: effectiveness analysis and empirical modeling approach. Aquaculture 297(1–4):106–111
Rota CM, Herrera A, Martínez RM, Sotomayor JA, Jordán MJ (2008) Antimicrobial activity and chemical composition of Thymus vulgaris, Thymus zygis and Thymus hyemalis essential oils, Food Control, 19, 7. ISSN 681–687:0956–7135. https://doi.org/10.1016/j.foodcont.2007.07.007
Rocha, Carolina P., Henrique N. Cabral, João C. Marques, and Ana M. M. Gonçalves. (2022) A Global Overview of Aquaculture Food Production with a Focus on the Activity’s Development in Transitional Systems—The Case Study of a South European Country (Portugal). Journal of Marine Science and Engineering 10, : 417. https://doi.org/10.3390/jmse10030417
Rodríguez J, Martín MJ, Ruiz MA, Clares B (2016) Current encapsulation strategies for bioactive oils: From alimentary to pharmaceutical perspectives. Food Res Int 83:41–59
Romero J, Feijoo C, Navarrete P (2012) Antibiotics in Aquaculture – Use. Abuse and Alternatives. https://doi.org/10.5772/28157
Sahlan M, Dienayati D, Hamdi D, Zahra S, Hermansyah H, Chulasiri M (2017) Encapsulation process of propolis extract by casein micelle improves sunscreen activity. Makara J Technol 21:1–6
Salam, H.S.H., Mohamed, W.M.S., Aziz, S.A.A.A. et al. (2021) Prevention of motile Aeromonas septicemia in Nile tilapia, Oreochromis niloticus, using thyme essential oil and its nano-emlusion. Aquacult Int 29, 2065–2084. https://doi-org.libproxy.viko.lt/https://doi.org/10.1007/s10499-021-00735-0
Sanguansri P, Augustin MA (2006) Nanoscale materials development- a food industry perspective. Trends Food Sci Technol 17:547–556
Santacruz-Reyes AR, Chien Y-H (2012) The potential of Yucca schidigera extract to reduce the ammonia pollution from shrimp farming. Biores Technol 113:311–314. https://doi.org/10.1016/j.biortech.2012.02.132
Sethiya NK (2016) Review on natural growth promoters available for improving gut health of poultry: An alternative to antibiotic growth promoters. Asian J Poult Sci 10(1):1–29
Schlaghecke R, Kornely E, Santen RT, Ridderskamp P (1992Jan 23) (1992) The effect of long-term glucocorticoid therapy on pituitary-adrenal responses to exogenous corticotropin-releasing hormone. N Engl J Med 326(4):226–230. https://doi.org/10.1056/NEJM199201233260403. (PMID: 1309389)
Shaaban HA, Zainab S, Emr EE, Amal S-H (2015) Analysis and antimicrobial activity of Nigella sativa essential oil formulated in microemulsion system. J Oleo Sci 64(2):223–232. https://doi.org/10.5650/jos.ess14177
Sharif HR, Abbas S, Majeed H, Safdar W, Shamoon M, Khan MA, Shoaib M, Raza H, Haider J. (2017) Formulation, characterization and antimicrobial properties of black cumin essential oil nanoemulsions stabilized by OSA starch. J Food Sci Technol. 54(10):3358–3365. doi: https://doi.org/10.1007/s13197-017-2800-8. Epub 2017 Aug 31. PMID: 28974821; PMCID: PMC5603003.
Shokr EAM (2015) Effects of ammonium on blood contents of Oreochromus niloticus. J Chem Pharm Res 7(3):1963–1968
Sze A, Erickson D, Ren L, Li D (2003) Zeta-potential measurement using the Smoluchowski equation and the slope of the current–time relationship in electroosmotic flow. J Colloid Interface Sci 261(2):402–410. https://doi.org/10.1016/S0021-9797(03)00142-5
Tian, J., Ban, X., Zeng, H., Huang, B., He, J. and Wang, Y. (2011) In vitro and in vivo activity of essential oil from dill (Anethum graveolens L.) against fungal spoilage of cherry tomatoes. Food Control 22(12): 1992–1999.
Talbot C, Hole R (1994) Fish diets and the control of eutrophication resulting from aquaculture. J Applied Ichth 10:258–270
Thummabancha K, Onparn N, Srisapoome P (2016) Analysis of hematologic alterations, immune responses and metallothionein gene expression in Nile tilapia (Oreochromis niloticus) exposed to silver nanoparticles. J Immunotoxicol 13:909–917. https://doi.org/10.1080/1547691X.2016.1242673
Tumwesigye Z, Tumwesigye W, Opio F, Kemigabo C, Mujuni B (2022) The Effect of Water Quality on Aquaculture Productivity in Ibanda District. Uganda Aquaculture Journal 2:23–36. https://doi.org/10.3390/aquacj2010003
Valladão GMR, Gallani SU, Kotzent S et al (2019) (2019) Effects of dietary thyme essential oil on hemato-immunological indices, intestinal morphology, and microbiota of Nile tilapia. Aquacult Int 27:399–411. https://doi.org/10.1007/s10499-018-0332-5
Xu J, Zhou F, Ji BP, Pei RS, Xu N (2008) The antibacterial mechanism of carvacrol and thymol against Escherichia coli. Lett Appl Microbiol 47:174–179
Yılmaz S, Ergün S, Çelik ES (2012) (2012) Effects of herbal supplements on growth performance of sea bass (Dicentrarchus labrax): Change in body composition and some blood parameters. J Biosci Biotech 1(3):217–222
Yousefi M, Adineh H, Reverter M, Hamidi MK, Vatnikov YA, Kulikov EV, Hoseinifar SH, Van Doan H (2021) Protective effects of black seed (Nigella sativa) diet supplementation in common carp (Cyprinus carpio) against immune depression, oxidative stress and metabolism dysfunction induced by glyphosate. Fish Shellfish Immunol 109:12–19, 1050–4648. https://doi.org/10.1016/j.fsi.2020.11.032
Zadmajid V, Mohammadi C (2017) Dietary thyme essential oil (Thymus vulgaris) changes serum stress markers, enzyme activity, and hematological parameters in gibel carp (Carassius auratus gibelio) exposed to Silver Nanoparticles. Iran J Fish Sci 16:1063–1084
Zhi T, Xua X, Chena J, Zhenga Y, Zhanga S, Penga J, Brownb CL, Yanga T (2018) Expression of immune- related genes of Nile tilapia Oreochromis niloticus after Gyrodactylus cichlidarum and Cichlidogyrus sclerosus infections demonstrating immunosupression in coinfection. Fish Shellfish Immunol 80:397–404
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Fatma M. M. Korni: applied experimental scheme, management of fish throughout the experimental period, prevention experiments, writing—review & editing. Asmaa N. Mohammed & Usama K. Moawad: measuring TAN, writing—review & editing.
Corresponding author
Ethics declarations
Ethics approval
All experiments were approved by the Beni-Suef Institutional Animal Care and Use Committee (BSU-IACUC, 022–355) of the Faculty of Veterinary Medicine, Beni-Suef University, Egypt.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Handling editor: Brian Austin
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Korni, F.M.M., Mohammed, A.N. & Moawad, U.K. Using some natural essential oils and their nano-emulsions for ammonia management, anti-stress and prevention of streptococcosis in Nile tilapia, Oreochromis niloticus. Aquacult Int 31, 2179–2198 (2023). https://doi.org/10.1007/s10499-023-01076-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-023-01076-w