Abstract
This study aimed to evaluate the application of synbiotic containing multispecies of probiotics with different cell densities in white shrimp rearing against infectious myonecrosis virus (IMNV) and Vibrio parahaemolyticus coinfection. This study used a completely randomized design with five treatments and three replications. One additional replication of each treatment was provided for the lethal sampling. Pacific white shrimp were fed with three dosages of synbiotic multispecies for 30 days, namely 103 CFU mL−1 (Sin 3), 106 CFU mL−1 (Sin 6), and 109 CFU mL−1 (Sin 9), and the controls without synbiotic administration consisted of the positive control (K +) and the negative control (K −). Pacific white shrimp from all treatments, except for the K − , were challenged with IMNV a dose of 100 µL and 106 CFU mL−1 V. parahaemolyticus, injected intramuscularly. Infected Pacific white shrimp showed clinical signs like anorexia, melanosis, empty gut, colorless hepatopancreas, and white necrotic areas in striated skeletal muscles, especially of the distal abdominal segments and uropod. The results showed that administration of synbiotic for 30 days resulted in higher immune parameters, such as total hemocyte count (THC), phenoloxidase activity (PO), respiratory bursts (RB), and total viable bacterial count (TBC) compared to K + /K − . After coinfection, they showed significantly higher levels for THC, PO, RB, gene expression prophenoloxidase (ProPO), and lipopolysaccharide and β-1.3-glucan-binding protein (LGBP), better clinical signs, and lower mortalities. Sin 9 treatment significantly showed the highest survival rate (SR) compared to the other treatments.
Similar content being viewed by others
Introduction
Indonesia is one of the world’s largest shrimp exporters with an average export value from 2019 to 2021 of 54.3 thousand tonnes. Even during the COVID-19 pandemic, the value of Indonesian Pacific white shrimp exports still increased by 12% from 55.3 thousand tonnes in 2020 to 62.1 thousand tonnes in 2021 (FAO 2021). Pacific white shrimp production cannot be achieved without the application of intensive or super-intensive shrimp farming technology with high density and feed inputs. However, the disease outbreaks dominated by viral and bacterial infections are still one of the factors limiting the success of the shrimp culture.
Infectious myonecrosis virus (IMNV) is one of the viral diseases that can cause huge mortality and huge economic losses in shrimp culture. The target organ attacked by this virus is a skeletal muscle and can attack a variety of shrimp stadia. Pacific white shrimp infected with IMNV show clinical signs of necrosis, especially on the distal abdominal. IMNV was first reported in Brazil in 2002 and spread in Indonesia in 2006 (Senapin et al. 2007).
In addition to viral infections, Pacific white shrimp are also susceptible to vibriosis. Vibriosis is an infectious disease caused by vibrio bacteria and was among the most dominant disease agents. Vibrio is a natural organism that lives in salinity waters (Aguilera-Rivera et al. 2019), so its connection to the environment and host is very important as a disease agent. Vibrio parahaemolyticus is one of the pathogens widely reported to infect Pacific white shrimp (Tran et al. 2013). Disease outbreaks in Pacific white shrimp farming activities are usually not only caused by one type of pathogen but can also occur due to the coinfection, infection of more than one type of pathogen (Pang et al. 2019).
The application of synbiotic is one of the alternatives in controlling disease outbreak on Pacific white shrimp farming. Yao et al. (2021) reported that synbiotic applications increase nonspecific immune response in Pacific white shrimp and are more effective than the use of probiotics and prebiotics separately (Hamsah et al. 2019). In addition, the availability and the application of multispecies probiotic (Yuhana, 2010) could improve the fish health status much better than the use of one type of probiotic (Wang et al. 2019). The number of study on efficacy of multispecies synbiotic application against coinfectious disease in white shrimp is still limited. Therefore, our study aimed to evaluate the effect of adding multispecies synbiotic on feed to improving immune response, and resistance of Pacific white shrimp to IMNV and V. parahaemolyticus coinfection.
Materials and methodology
Materials
The test materials in this study were shrimp, disease agents, and multispecies synbiotic. The shrimp used was a Pacific white shrimp (Litopenaeus vannamei) (10.4 ± 0.96 g) and obtained from the shrimp farmer in Kronjo, Tangerang. Infectious myonecrosis virus (IMNV) isolated from positive infected shrimp that obtained from Situbondo, East Java. The polymerase chain reaction (PCR) method was used for verification of IMNV using primers 5′-CGA-CGC-TGC-TAA-CCA-TAC-AA-3′; 5′-ACT-CGG-CTG-TTC-GAT-CAA-GT-3′ for 1st PCR and primers 5′-GGC-ACA-TGC-TCA-GAG-ACA-3′; 5′-AGC-GCT-GAG-TCC-AGT-CTT-G-3′ for nested PCR (OIE, 2013).
Vibrio parahaemolyticus in this study was also used in previous study (Munaeni et al. 2020). It was confirmed using API 20 NE system (BioMérieux, France) and sequencing method with accession number 7DDBGXP2013, and this V. parahaemolyticus was non AHPND type (negative pyrA/pyrB PCR amplification) by method of Dangtip et al. (2015). The multispecies synbiotic consisted of prebiotic mannan oligosaccharides (MOS) (Bio-MOS Alltech Inc., KY USA) and probiotics was consisting of the bacterium Vibrio alginolyticus (SKT-b), Bacillus cereus (BR2), and Pseudoalteromonas piscicida (1UB). All these bacterial probiotic strains were the Aquatic Organisms Health Laboratory’s collection, Department of Aquaculture, IPB University.
Methodology
Preparation of coinfection agent
IMNV was isolated from the muscle of Pacific white shrimp that have been cleaned from carapace then pulverize until smooth and dissolved with a PBS (Phosphate Buffered Saline) solution with a ratio of weight and volume of 1:10 (w/v) and then homogenized. Furthermore, the mixture was centralized at a speed of 6500 rpm and a temperature of 4 °C for 20 min. The supernatant was filtered with a filter size of 0.45 μm and diluted filtrate (Oktaviana et al. 2014).
V. parahaemolyticus was cultured in 25 mL of liquid seawater completes (SWC) media in water shakers at 28 °C for 18 h. The bacterial cell suspension was then centrifuged with 5000 rpm for 10 min (Thermo Scientific). Total plate count (Bender et al. 2019) was performed to determine the bacterial density. The cells were marked antibiotic rifampicin and diluted to reach a cell density of 106 CFU mL−1.
Preparation of synbiotic
After harvesting probiotics, the bacterial cell suspension was diluted according to the treatment doses of 109 CFU mL−1, 106 CFU mL−1, and 103 CFU mL−1, respectively. Furthermore, the commercial feed (containing 40% protein) was mixed with 2% MOS and 2% multispecies probiotic with the top-dressing method (Oktaviana et al. 2014).
The rearing of experimental animals and feeding administration of multispecies synbiotic
The shrimp used was confirmed as free IMNV (OIE 2003) and free from V. parahaemolyticus (Dangtip et al. 2015) shrimp using reverse-transcription quantitative PCR. The shrimp were divided into five groups and four replicates with 10 shrimp container−1 (one replication was used to lethal sampling). The rearing container was aquaria sizing 60 × 30 × 30 cm3 and each was filled with 30 L sea water equipped with filtration and recirculating water systems (Munaeni et al. 2020). The rearing medium was sea water with a salinity of 25 g L−1.
The feeding multispecies synbiotic pellet was given five times a day, at 06:00 am, 10:00 am, 02:00 pm, 06:00 pm, and 10:00 pm. Water quality monitoring was conducted 3 days once with ranges during the rearing period were within a proper range for Pacific white shrimp based on National Standard No. ref. 8008 (2014), temperatures were ranging from 26.5 up to 28.5 °C, salinity ranges were between 26.0 and 28.0 g L−1, dissolved oxygen level was 3.5‒6.3 mgL−1, and the total amonia nitrogen (TAN) value was less than 0.156 mgL−1.
The co-infection challenge test of the Pacific white shrimp
The challenge test was conducted on day 30th after the shrimp were fed with multispecies synbiotic. IMNV infection (10−3) (SID 50) was performed by 100-µl intramuscular injection between the second and third segments and 100 μl bacterial infection of V. parahaemolyticus (106 CFUmL−1) (SID 50) was performed by intramuscular injection 24 h after IMNV infection (Rubio-Castro et al. 2016).
The measurement of parameters
The immune responses of Pacific white shrimp
The parameters observed in the study were clinical sign, total hemocyte count (THC) (Blaxhall and Daisley 1973), respiratory burst (RB) (Song and Hsieh 1994), phenoloxydase (PO) (Liu and Chen 2004), and immune-related gene expression in the form of prophenoloxidase (ProPO), lipopolysaccharide, and β-1.3-glucan-binding proteins (LGBP) (Zokaeifar et al. 2012a), as well as post-coinfection mortality and monitoring of intestinal bacterial cell populations using total plate count method (Madigan et al. 2019).
Data analysis
Data of THC, PO, RB, survival rate, and TBC of Pacific white shrimp were performed with variant analysis (ANOVA). If there were differences among treatments, those were further analyzed through Duncan’s test using SPSS version 22. Clinical sign data were analyzed descriptively.
Results
Clinical signs
The clinical signs showed on the control treatment (K +) and synbiotic treatment group of Pacific white shrimp showed the rate from low to severe infection with clinical signs in the form of anorexia, inactive movement, and in some parts of the body, there is a reddish-color, melanization, and necrosis. The rate of severe infection is at least showed by the Sin 9 treatment (Fig. 1) of 30%. The clinical signs that can be observed in severely infected Pacific white shrimp are abnormalities in the form of pink color or necrosis of distal abdominal as many as two or more segments (Fig. 2).
The PCR result for the test Pacific white shrimp after coinfection. Shrimp samples showing clinical signs of white to pink necrosis on distal abdominal muscle. Lane 1: A IMNV Positif; Lane 2: Sin 3; Lane 3: Sin 6; Lane 4: Sin 9; Lane 5: (K −); Lane 6: Marker: Lane 7: NTC. The band at 314 bp for sample indicates a light IMNV infection according to the OIE, 2013
Survival rate
Observations of Pacific white shrimp survival rates over 10 days showed that the highest survival rate value obtained by Sin 9 (70.00 ± 0.08%) is significantly different (p < 0.05) to those of other treatments (Fig. 3).
The immune response of Pacific white shrimp
The results of the immune response examination of THC, RB, and PO during maintenance are displayed sequentially in Figs. 4, 5, and 6 respectively. The Pacific white shrimp reared for 30 days with the application of a multispecies synbiotic on the day 30th showed an increase in THC, RB, and PO values compared to the controls. At the 3rd hour of examination after coinfection, Pacific white shrimp THC in the multispecies synbiotic treatment group was higher than the controls and significantly different (p < 0.05). THC began to decrease at 6th to 120th hours after coinfection. In addition, the timing of these observations, the control treatment, and multispecies synbiotic treatment group were no more noticeable, except for the Sin 9 treatment at the 6th and 24th hours.
RB activity increased on the day 30th and the multispecies synbiotic treatment group had higher RB activity and was significantly different from the control treatment (p < 0.05). After coinfection, RB activity decreased in all treatments but the treatment of the synbiotic group remained higher and more distinctly real (p < 0.05) than the controls.
The PO activity of Pacific white shrimp during maintenance is shown in Fig. 6. Prior to coinfection, control treatment and synbiotic groups showed no differences (p > 0.05). At the 3rd-hour PO activity check after the coinfection, the Sin 9 treatment was higher compared to Sin 6 and Sin 3. The multispecies synbiotic treatment group was significantly different from the controls. In general, the PO value decreases at each examination until the end of sampling (120 h).
Immune-related genes of Pacific white Pacific white shrimp
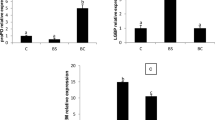
Feeding with synbiotic consortiums with different doses showed that rising expression of lipopolysaccharide and β-1.3-glucan-binding protein (LGBP) and gen prophenoloxidase (ProPO) expression differently. At the beginning and the end of maintenance (30-day modulation) before the coinfection, there was no increase in the expression of the LGBP gene among treatment (p > 0.05). The increase in LGBP was found at the 6th hour after treatment, and Sin 3 showed higher value (p < 0.05) than the control and the multispecies treatment group (Fig. 7). In the Sin 9 treatment, the expression of the gene increased at the 12th hour and the 24th hour and then the return decreased at the 48th hour and the 72nd hour. For the Sin 3 treatment, although no different (p > 0.05), there was an increase in gene expression that begins on the 30th day before the coinfection and returns to decline at the next check after the coinfection.
ProPO gene expression began to show an increasing in the multispecies synbiotic treatment group 6 h after coinfection. The Sin 6 treatment showed the highest value (p < 0.05) compared to other treatments in the same hour (Fig. 8). At the 12th hour, the expression of the return gene decreased until the end of the examination at the 72nd hour.
The number of bacteria in the Pacific white shrimp’s intestines
The results of calculating the abundance of bacterial cells in the Pacific white shrimp intestine including the total viable bacterial count (TBc) and the number of each of the bacteria multispecies SKT-b RifR, 1UB RifR, and BR2 CipR after 30 days of maintenance with supplemented feed synbiotics multispecies with different doses could be seen in Fig. 9.
Discussion
Pacific white shrimp infected with IMNV and V. parahaemolyticus showed gross clinical signs (Fig. 2). The infection rate of shrimp was categorized as various, from low infection to severe infection (Fig. 1). In the Sin 9 treatment, it showed a lower infection rate than all treatments that were coinfected. Tang et al. (2019) reported clinical symptoms of shrimp infected with IMNV, namely necrosis in the distal abdominal muscle, and showed the reddish in color. Then, infection of bacteria V. parahaemolyticus in shrimp according to Ananda Raja et al. (2017) was characterized by lesions on carapace such as reddish tail fins and melanization. Thitamadee et al. (2016) reported that shrimp infected with V. parahaemolyticus bacteria show clinical sign, such as anorexia and colorless hepatopancreas, and the intestines look empty. The survival rate of shrimp was directly proportional to the dose of treatment given. Yao et al. (2021) reported that synbiotic administration increase the Pacific white shrimp resistance to pathogenic infections.
The immune responses of shrimp, THC, RB, and PO increased along with the supplementation treatment of multispecies synbiotic in the feed. This is in line with the study of Huynh et al. (2018) that synbiotics were influential in increasing the immune response of shrimp. THC played an important role in the body of shrimp because it could initiate a host to respond to various kinds of infections. These results in THC values begin to decrease after coinfection (Fig. 4). Referred to research by Chiu et al. (2007), hemocyte cell phagocytosis response for incoming pathogens. RB is the process of destroying foreign particles by releasing degradative enzymes into phagosomes and the production of reactive oxygen intermediate (ROI) that is bactericidal. RB was produced by phagocyte cells that serve to fight pathogens that invade shrimp (Wang et al. 2013). The increase in RB value after being given synbiotic treatment is in line with Prabawati et al. (2022). The value of RB decreases after coinfection (Fig. 5) because THC also decreases due to the phagocytosis process of hemocyte cells against foreign object being reduced. In shrimp immune systems, PO acts as an identifier and effector component through communication between cells and eliminating pathogens. PO was an enzyme in the proPO system that is closely related to THC activity (Amparyup et al. 2013). The decrease in PO value after coinfection is thought to be due to the decreased amount of THC. This indicates the activity of resistance of shrimp immune system by hemolymph to bacteria invading shrimp.
The expression of immune-related genes described by the ProPO and LGBP genes was known to be one of the keys in shrimp’s immune response in recognizing and responding to infection at the beginning of the invasion of the bacteria, and then activating the ProPO system (Cheng et al. 2005). LGBP was usually active in the first 24 h after infection (Zokaeifar et al. 2012). In this study, LGBP gene expression was also found at the same time as gene ProPO expression at the 6th hour after coinfection. This proves that the regulation of LGBP gene expression and ProPO expression is interrelated.
Administration of multispecies synbiotic has increased the number of intestinal bacteria (total viable bacterial count) (Fig. 9). Daniels et al. (2013) described that the synbiotic with the mannan oligosaccharide and probiotic Bacillus sp. is able to increase the total number of bacteria in the gut of European lobster (Homarus gommarus) and improve growth performance.
Conclusion
The application of synbiotic consortiums was able to improve immune response, gene expression, growth performance, and abundance of gut bacteria as well as increase resistance to IMNV and V parahaemolyticus coinfection with the best result in the administration of Sin 9 treatment.
Data availability
All authors agree to publish in aquaculture international.
Code availability
Not applicable.
References
Aguilera-Rivera D, Prieto-Davó A, Rodríguez-Fuentes G et al (2019) A vibriosis outbreak in the Pacific white shrimp, Litopenaeus vannamei reared in biofloc and clear seawater. J Invertebr Pathol 167:107246. https://doi.org/10.1016/j.jip.2019.107246
Amparyup P, Charoensapsri W, Tassanakajon A (2013) Prophenoloxidase system and its role in shrimp immune responses against major pathogens. Fish Shellfish Immunol 34:990–1001. https://doi.org/10.1016/j.fsi.2012.08.019
Ananda Raja R, Sridhar R, Balachandran C et al (2017) Pathogenicity profile of Vibrio parahaemolyticus in farmed Pacific white shrimp, Penaeus vannamei. Fish Shellfish Immunol 67:368–381. https://doi.org/10.1016/j.fsi.2017.06.020
Blaxhall PC, Daisley KW (1973) Routine haematological methods for use with fish blood. J Fish Biol 5:771–781. https://doi.org/10.1111/j.1095-8649.1973.tb04510.x
Cheng W, Liu CH, Tsai CH, Chen JC (2005) Molecular cloning and characterisation of a pattern recognition molecule, lipopolysaccharide- and β-1,3-glucan binding protein (LGBP) from the white shrimp Litopenaeus vannamei. Fish Shellfish Immunol 18:297–310. https://doi.org/10.1016/j.fsi.2004.08.002
Chiu CH, Guu YK, Liu CH et al (2007) Immune responses and gene expression in white shrimp, Litopenaeus vannamei, induced by Lactobacillus plantarum. Fish Shellfish Immunol 23:364–377. https://doi.org/10.1016/j.fsi.2006.11.010
Dangtip S, Sirikharin R, Sanguanrut P et al (2015) AP4 method for two-tube nested PCR detection of AHPND isolates of Vibrio parahaemolyticus. Aquac Reports 2:158–162. https://doi.org/10.1016/j.aqrep.2015.10.002
Daniels CL, Merrifield DL, Ringø E, Davies SJ (2013) Probiotic, prebiotic and synbiotic applications for the improvement of larval European lobster (Homarus gammarus) culture. Aquaculture 416–417:396–406. https://doi.org/10.1016/j.aquaculture.2013.08.001
FAO (2021) GLOBEFISH Highlights 3rd issue 2021, with Jan.–Mar. 2021 Statistics – International Markets on Fisheries and Aquaculture Products. Quarterly update. Globefish Highlights No. 3–2021. Rome. https://doi.org/10.4060/cb7153en
Hamsah H, Widanarni W, Alimuddin A et al (2019) Immune response and resistance of Pacific white shrimp larvae administered probiotic, prebiotic, and synbiotic through the bio-encapsulation of Artemia sp. Aquac Int 27:567–580. https://doi.org/10.1007/s10499-019-00346-w
Huynh TG, Cheng AC, Chi CC et al (2018) A synbiotic improves the immunity of white shrimp, Litopenaeus vannamei: metabolomic analysis reveal compelling evidence. Fish Shellfish Immunol 79:284–293. https://doi.org/10.1016/j.fsi.2018.05.031
Liu C, Chen J (2004) Fish & Shellfish Litopenaeus Vannamei and Its Susceptibility to Vibrio Alginolyticus. Fish Shellfish Immunol 16:321–334. https://doi.org/10.1016/S1050-4648(03)00113-X
Madigan MT, Bender KS, Buckley DH, Sattley WM, Stahl DA (2018) Brock Biology of Microorganisms, 15th edn. Pearson Education, United States
Munaeni W, Widanarni YM et al (2020) Effect in white shrimp Litopenaeus vannamei of Eleutherine bulbosa (Mill.) Urb. Powder on immune genes expression and resistance against Vibrio parahaemolyticus infection. Fish Shellfish Immunol 102:218–227. https://doi.org/10.1016/j.fsi.2020.03.066
Oktaviana A, Widanarni, Yuhana M (2014) The use of synbiotics to prevent IMNV and Vibrio harveyi co-infection in Litopenaeus vannamei. HAYATI J Biosci 21:127–134. https://doi.org/10.4308/hjb.21.3.127
Pang H, Wang G, Zhou S et al (2019) Survival and immune response of white shrimp Litopenaeus vannamei following single and concurrent infections with WSSV and Vibrio parahaemolyticus. Fish Shellfish Immunol 92:712–718. https://doi.org/10.1016/j.fsi.2019.06.039
Prabawati E, Hu S, Chiu S, Balantyne R (2022) Fish and shellfish immunology a synbiotic containing prebiotic prepared from a by-product of king oyster mushroom, Pleurotus eryngii and probiotic, Lactobacillus plantarum incorporated in diet to improve the growth performance and health status of white. Fish Shellfish Immunol 120:155–165. https://doi.org/10.1016/j.fsi.2021.11.031
Rubio-Castro A, Luna-González A, Álvarez-Ruiz P et al (2016) Survival and immune-related gene expression in Litopenaeus vannamei co-infected with WSSV and Vibrio parahaemolyticus. Aquaculture 464:692–698. https://doi.org/10.1016/j.aquaculture.2016.08.024
Senapin S, Phewsaiya K, Briggs M, Flegel TW (2007) Outbreaks of infectious myonecrosis virus (IMNV) in Indonesia confirmed by genome sequencing and use of an alternative RT-PCR detection method. Aquaculture 266:32–38. https://doi.org/10.1016/j.aquaculture.2007.02.026
Song YL, Hsieh YT (1994) Immunostimulation of tiger shrimp (Penaeus monodon) hemocytes for generation of microbicidal substances: analysis of reactive oxygen species. Dev Comp Immunol 18:201–209. https://doi.org/10.1016/0145-305X(94)90012-4
Tang KFJ, Bondad-Reantaso MG, Arthur JR; FAO (2019) Fisheries and aquaculture circular, shrimp infectious myonecrosis strategy manual. Rome
The World Organisation for Animal Health; OIE (2003) Manual of diagnostic tests for aquatic animals, 4th edn. Office International International Des Epizootes, Paris
Thitamadee S, Prachumwat A, Srisala J et al (2016) Review of current disease threats for cultivated penaeid shrimp in Asia. Aquaculture 452:69–87. https://doi.org/10.1016/j.aquaculture.2015.10.028
Tran L, Nunan L, Redman RM et al (2013) Determination of the infectious nature of the agent of acute hepatopancreatic necrosis syndrome affecting penaeid shrimp. Dis Aquat Organ 105:45–55. https://doi.org/10.3354/dao02621
Wang Y, Jiang Z, Kim D et al (2013) Stimulatory effect of the sulfated polysaccharide ascophyllan on the respiratory burst in RAW264.7 macrophages. Int J Biol Macromol 52:164–169. https://doi.org/10.1016/j.ijbiomac.2012.09.008
Wang YC, Hu SY, Chiu CS, Liu CH (2019) Multiple-strain probiotics appear to be more effective in improving the growth performance and health status of white shrimp, Litopenaeus vannamei, than single probiotic strains. Fish Shellfish Immunol 84:1050–1058. https://doi.org/10.1016/j.fsi.2018.11.017
Yao W, Li X, Zhang C et al (2021) Effects of dietary synbiotics supplementation methods on growth, intestinal health, non-specific immunity and disease resistance of Pacific white shrimp, Litopenaeus vannamei. Fish Shellfish Immunol 112:46–55. https://doi.org/10.1016/j.fsi.2021.02.011
Zokaeifar H, Balcázar JL, Saad CR et al (2012) Effects of Bacillus subtilis on the growth performance, digestive enzymes, immune gene expression and disease resistance of white shrimp, Litopenaeus vannamei. Fish Shellfish Immunol 33:683–689. https://doi.org/10.1016/j.fsi.2012.05.027
Funding
This work was partly funded by the Ministry of Education and Culture, Republic of Indonesia, the grant number 079 /SP2H/ LT/ DRPM/ II/2018/Munti Yuhana.
Author information
Authors and Affiliations
Contributions
Agil Setya Utomo performed the experiment, analyzed the data, and wrote the first version of the manuscript. Munti Yuhana designed the study and wrote the first version of the manuscript. Widanarni designed the study, reviewed the first version of the manuscript, and approved it for publication. Usamah Afiff, reviewed the first version of the manuscript and approved it for publication.
Corresponding author
Ethics declarations
Ethics approval
All experiments in this study associated with fish complied with animal welfare and were conducted according to protocol number 183–2020, approved by the Ethics Committee on Animal Use of the IPB University, November 2020.
Consent to participate
All authors consented to participate in all aspects of this study and publication.
Conflict of interest
The authors declare no competing interests.
Additional information
Handling Editor: Brian Austin
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Utomo, A.S., Yuhana, M., Widanarni, W. et al. Immune response, gene expression, and intestinal microbial composition of Pacific white shrimp fed with multispecies synbiotic for the prevention of coinfection disease. Aquacult Int 31, 53–64 (2023). https://doi.org/10.1007/s10499-022-00966-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-022-00966-9