Abstract
Growth rate is one of the most important factors in the farming of Haliotis midae, and somatic growth rates decline after abalone reach sexual maturity. Artificial diets are suspected to accelerate maturation, in particular when soya meal is used as a protein source, because of this plant’s high concentration of phytoestrogens. The effect of diet on the gonad development of 27-month-old farmed Haliotis midae, raised at two stocking densities, was analysed. We fed two artificial diets and a natural diet, kelp, to cultured abalone. The first artificial diet had fishmeal as its main source of protein, while in the other, Abfeed® S34, 32% of the fish meal was replaced with soya meal. For each gonad sample, the development phase was determined based on both histological criteria and the gonad bulk index (GBIn). The hypothesised link between dietary protein source and gonad development could not be established by either morphological criteria or GBIn. Similarly, stocking density did not have a significant effect on GBIn-values. GBIn values were significantly correlated to the results from the histological analyses in males and females. The results from this study suggest that artificial diets including those using soya meal are a valid alternative in the feeding of abalone. The research provides a database for future research on factors influencing gonad development in this species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to the United Nation’s Food and Agriculture Organization, South Africa has been the largest abalone producer outside Asia since 2004. Concurrently, the economic value of abalone mariculture for coastal communities has increased (Dlaza 2006; Troell et al. 2006; Makhande 2008). Abalone farming requires a high capital investment and relies on formulated diets to improve productivity by improving abalone growth (López-Acuna 1999; Dlaza 2006; Makhande 2008; Cho and Kim 2012) and food conversion rate (Bautista-Teruel et al. 2001; Troell et al. 2006; Makhande 2008). These diets are good alternatives to the natural diet of abalone, i.e., kelp and seaweeds, and they have been used in combination with macroalgal diets (Kemp et al. 2015). One of the advantages of these pelletized diets is that they can be adapted to match the life stages and dietary needs of the farmed abalone (Sales and Britz 2001). They are also more practical than a natural kelp or seaweed diet as they are available throughout the year (Sales and Britz 2001; Makhande 2008). Some farms combine artificial diets and kelp in the production of the South African abalone, Haliotis midae (Makhande 2008), and other haliotids (Bansemer et al. 2016), but the most common application of artificial diets remains during the grow-out period of H. midae (Wu et al. 2019). The most common ingredients in artificial diets are fish meal, defatted soybean meal and casein as protein sources (Fleming et al. 1996; Sales and Britz 2001; Shipton and Britz 2001). A combination of fish meal and soybean meal can result in faster abalone growth than diets with fishmeal as the only main protein source (Riddin 2013; Wu et al. 2019). For example, Abfeed™ S34® (Marifeed Pty Ltd), the diet used in the present study, is a commercial feed for post-weaning of abalone and contains fishmeal and soybean meal as the main sources of protein.
Animals fed Abfeed™ grow faster than animals fed kelp up to an age of two and a half years, after which growth is reduced (Troell et al. 2006). The onset of growth reduction corresponds to the age at which H. midae can reach sexual maturity under farming conditions (Visser-Roux 2011), which may cause the channelling of metabolic energy into gonad development (Wu 2020). This hypothesis is supported by differences in sexual maturation between wild and farmed H. midae (Newman 1968; Tarr 1995; Wood and Buxton 1996; Visser-Roux 2011). In captivity, 50% of male and female abalone reached sexual maturity at 18 and 24 months, respectively (Visser-Roux 2011). Wild abalone, however, attain sexual maturity at an age of approximately 7 years (Tarr 1995). Younger sexual maturity has an impact on the farming of H. midae, as this species takes approximately 4 years from hatching to a marketable weight of 80 g abalone−1 (Troell et al. 2006). Therefore, factors that lead to premature sexual maturation have a detrimental effect on growth and farm productivity in a species in which males and females have equally high gonadosomatic indices of up to 75 mm3 g−1 soft tissue (Wu et al. 2019) and uncontrolled sexual development and spawning that may complicate growth forecasting, which is required for successful farm management, product quality and marketing of the animals.
The ingredients in artificial diets may play an additional role. H. midae raised on formulated feeds with fishmeal and soya meal as the only protein source devoted more energy to gonad development than abalone fed diets that included only fishmeal (Wu et al. 2019). Soybean meal is rich in phytoestrogens, which are suspected to act as endocrine disruptors in H. midae (Wu et al. 2019) and other animal species (Adams 1995; Weber et al. 2001; Le Francois et al. 2010). However, Wu et al. (2019) tested soybean meal in H. midae diets and could not confirm that the flavonoids in soybean meal are responsible for the hypothesised effect on gonad development. In other species, phytoestrogens have been linked to precocious genital development, decreased testosterone levels, altered activity levels, decreased prostate weight, decreased sperm quality, temporary or permanent infertility, abnormal lactation in mammals, an increased incidence of dystocia and feminization (Weber et al. 2001; Cong et al. 2006; Glover and Assinder 2006; Gupta 2012). In fish, soybean-based diets have been associated with shifting of the sex-ratio towards females, low testosterone levels, reduced fertility affecting both sperm quality and the number of eggs spawned, inadequate gametogenesis and a reduction of fertilisation success and hatch rate (Pollack et al. 2003; Tzchori et al. 2004; El-Sayed et al. 2012; Bagheri et al. 2013).
There is a paucity of published information on the effect of soybean meal on abalone gonad development in both males and females when compared to natural diets. For example, in feeding studies that were designed to analyse gonad development of H. midae, kelp was not included as a test diet (Ayres 2013; Riddin 2013; Wu et al. 2019). Capinpin and Corre (1996) suggested that growth was fastest in H. asinina fed artificial diets as compared to red algae, Gracilariopsis heteroclada, until the abalone reached a size at which energy was channelled into gonad development. The authors hypothesised that this was due to the relatively high protein and energy content of these diets. However, gonad mass index (GBI) was not determined, and no histological evidence was provided to support this hypothesis.
An additional factor that may influence gonad development in H. midae is stocking density and water flow rate to the culture tanks. Abalone are broadcast spawners and can be triggered to release eggs and sperm by the presence of gametes in the water column. Spontaneous spawning events have been recorded in H. asinina (Fermin et al. 2000). Counihan et al. (2001) suggested that high stocking densities in tanks may influence the frequency of spawning events in H. asinina due to the fact that abalone are closer together, which may result in males releasing gametes and associated sex pheromones. Thus, the number of abalone in a tank may plausibly influence gonad development or the frequency of spawning events. Furthermore, during spawning season and under favourable conditions on the farm, H. midae broodstock can reproduce approximately every 6 weeks (Visser-Roux 2011), which suggests a fast recruitment of oocytes. Broodstock abalone are kept in a controlled environment at low population densities, which is different from the conditions in the grow-out tanks, with variable and generally high numbers of abalone and consequently fast water flow rates. The effect of stocking density on abalone reproductive development in a grow-out system remains to be tested.
The aim of this study was to use histological examination to compare the effect of a partial substitution of fishmeal in the diet with soybean meal on gonad development and histology of both male and female H. midae. A treatment, in which abalone were fed kelp, their natural diet, was included. Abalone stocking intensity and water flow rate to the culture tanks were used as additional independent variables.
Methods
Animals and culture conditions
Twenty-seven-month old H. midae (30–40 g abalone−1, 55–60 mm length) were randomly collected at the grading station of an on-shore abalone farm in Hermanus, South Africa, in early December 2010 where they had been fed a natural diet of kelp. Based on information available from farms in the Western Cape region of South Africa, it was estimated that the animals would be immature and that only 60% of animals within this size range had started to develop gonadal tissue. As some animals were too immature to be sexed, the ratio of male to female abalone could not be confirmed with histological evidence at the time of collection.
The abalone were transferred to eighteen 300-L tanks with oyster mesh baskets and a horizontal floating top feeding plate on which the artificial food was placed. Abalone are nocturnal feeders and come to the feeding plate at night. Each tank had its own water inflow and outflow with filtered seawater (100 µm) from the farm’s header tank.
The effect of stocking intensity on growth and gonad development was tested by farming the animals at two densities, which were expressed as the percentage of available basket surface area that could be covered by the abalone. To estimate the required biomass of abalone, regression models developed by farm management to predict foot surface area as a function of biomass were used. In the low stocking density (LI), there was a biomass of 8 kg basket−1 with animals covering 17.5% of the surface area of the basket and flow indices of 9 L h−1 kg−1 to 20 L h−1 kg−1. In the high stocking density (HI) treatment, the density of animals was 11 kg basket−1, so that 24% of the available basket surface area was covered by the animals. In this treatment, the flow index ranged from 13 to 29 L h−1 kg−1.
The abalone were fed either a commercial diet with fishmeal (FM) as the main protein source or Abfeed® S34 diet (Marifeed; Pty Ltd) with a combination of fishmeal and soybean meal. The diets were isonitrogenous (33.2% protein) and isocaloric (15.6 MJ kg−1) with an average lipid level of 3.6%. The daily feeding rate was approximately 0.5% of body mass, which is an established feeding rate for the average body mass of abalone used in this experiment (Sales and Britz 2001). Daily rations were calculated at the beginning of the trial, based on the weight of the animals in a basket, and adjusted every 2 weeks according to the growth rate of the animals.
Both LI and HI treatments were randomly assigned to baskets in eight tanks; four tanks in each group received the FM diet, and the other four received Abfeed® S34. Experimental units were randomly allocated to the combinations of artificial diets and stocking density.
Two baskets received locally harvested kelp (Ecklonia maxima), which was fed in excess. These animals were stocked at the low population density with a flow index of 20 L kg−1 h−1.
Sampling and processing of gonadal tissue
Because some immature abalone could not be confidently sexed, three individuals, a presumed female, a presumed male and an immature individual that was not yet displaying clear externally visible sexual characteristics, were sampled at the start of the feeding experiment. In all but one case, histological examination correctly identified the sex of the individuals. The information from the histological preparations was used for data analysis. After the first month, abalone were sampled at the end of each of the following 2 months. The sex of all abalone was now identifiable by their external characteristics. Preparations from 115 animals (n = 115) could be used confidently to determine GBln (%)-values based on the quality of the histological preparations, i.e., 48 animals in December, 35 in January and 32 in February. In one male, GBln (%) could not be determined, but the developmental phase was identifiable.
Following collection, the animals were left overnight for purging in separate bags labelled with the tank number. The gonads were removed the following day after the animals had been shucked according to farm protocol. The entire conical organ was placed in Davidson’s solution for fixation. The gonad tissue was cut and stained at the Division of Anatomy and Histology of Stellenbosch University (Tygerberg, South Africa). Dehydration of the fixed samples was achieved in serial dilutions of ethanol (70% to 100% v/v) for 180 min each. The specimens were cleared with xylene for 150 min and embedded in paraffin wax for 120 min at 60 °C. Specimen blocks were processed in sections of 5-µm thickness and incubated at 60 °C for 60 min in an autoclave. Sections were rehydrated in a serial dilution of ethanol (100% to 70% v/v), stained with haematoxylin–eosin and cleared with xylene. Sections were mounted onto microscope slides with di-n-butyl phthalate in xylene mounting medium (Fluka).
For every specimen, three cross-sections, all originating from the mid-point between the base and the tip of the conical organ, were stained and mounted.
An image capturing device, ScanScope® CS (Aperio), was used to take micrographs of the slides. These digital micrographs were analysed using the viewing software ImageScope Version 11.1 (Aperio). Measurements were taken using this software, and the histology of the samples was interpreted.
Slide analysis and histological examination
Determination of abalone sex and developmental phase
Histology was performed to evaluate the developmental phase of the animals. The development of the ovary occurs at the same pace throughout the length of the organ (Gurney and Mundy 2004). Therefore, for each individual conical organ, a single cross-section, from midway between the base and the tip, was analysed to evaluate the developmental phase of each gonad.
Classification of the germ cells of female and male gonads and the description of the gamete cell developmental stage were based on published criteria for haliotids (Wood and Buxton 1996; Singhakaew et al. 2003; Najmudeen 2008; Visser-Roux 2011). Stages of oogenesis were determined following the criteria proposed by Visser-Roux (Visser-Roux 2011). However, the size of the nucleus and nucleolus did not always fall within the reference range or could not be measured. Therefore, nucleus and nucleolus size were not used as criteria for gamete classification. Each gonad was assigned a developmental phase (immature, proliferative, premature, mature, spawning, spent) according to this classification.
The testicular developmental phases were primarily assigned according to cell stages and their relative abundance. The gonad surface area covered by spermatids and spermatozoa was given values of “ < 25%”, “25–75%” or “ > 75%”.
The ovaries were evaluated using cell counts. Numbers of oogenetic stages along a horizontal and a vertical midline as well as in randomly selected high power fields (HPF) were counted. Frequencies were determined and the presence of yolk throughout the gonad was staged into high, moderate and low levels.
The percentages of each stage present along a vertical and horizontal midline and ten randomly selected HPFs were established. Cell stages were differentiated by measuring cell size and by comparing their morphology with the descriptions given by Visser-Roux (Visser-Roux 2011). While transverse sections along the length of the ovaries were similar to each other (Gurney and Mundy 2004), the various cell cohorts within these sections often reached maturity at different times. In these samples, the phase of each inter-trabecular compartment was determined. The developmental phase dominating the gonad surface area was then established, and the sample was classified based on this developmental phase.
Evaluation of the gonad index
Newman’s gonad bulk index (Newman 1967) was calculated for each sample. The surface area of the conical organ (total area of section) and the hepatopancreas (GI area) were measured using the ImageScope software, Version 11.1. For each individual, the gonad surface area (area of gonad section) was calculated by subtracting the GI area from the total area of the section. The GBIn was then calculated using the following equation: GBIn = (area of gonad section / total area of section) × 100. The percentage GBIn values were then compared to the developmental phase based on the morphological criteria.
Statistical analysis
Statistical analyses were performed using Microsoft EXCEL 2021, IBM SPSS v20 and Statistica v14.0.0.15 (TIBCO). The log-transformed values of GBIn were normally distributed (Kolmogorov–Smirnov test). The effect of the independent variables, month, dietary protein source and stocking density on the continuous dependent variables was analysed by comparing the GBIn of the animals that had received either of the diets using a general linear model (GLM, ANOVA). Using data from the animals sampled in January and February, average values for GBln were compared between (a) the three gonadal developmental phases in both males and females, (b) months and (c) diets. This was done using ANOVA followed by Tukey’s HSD post hoc test when comparing three treatments and Student’s t test for unequal variances for two treatments. GBIn values were compared to reproductive stages in both females and males using Spearman rank R correlation.
Results
The analyses excluded two animals belonging to the stage “spawning”. The animals in this group appeared to have released gametes when handled during the process involved in purging. The two individuals in the “spawning” group made up 1.6% of the total number of animals.
Ovarian histology
In the ovarian sections, a staggered development of cohorts was apparent in most samples. Premature compartments (Fig. 1A, C, and D) were frequently positioned next to mature or proliferative compartments. Areas of the gonad sporadically showed signs of necrosis as described by Najmudeen (Najmudeen 2008). The developmental phase that covered the largest fraction of the gonad area was assigned to that individual based on the criteria described in materials and methods (Fig. 1B and G).
Histological views of the gonads of Haliotis midae at various stages of maturation. A Immature gonad where a = hepatopancreas; b = oogonia; c = inner layer of capsule composed of collagenous fibres and smooth muscle cells and d = outer layer of the capsule composed of a single layer of epithelial cells. B Female gonads in the proliferative stage where a = hepatopancreas; arrow = oogonia and early-stage oocytes; arrowhead = trabeculae. C Low magnification view of a female gonad in the premature stage. D Detailed view of a female gonad in the premature stage where a = hepatopancreas; b = oocytes in the stage 6 of development; arrow = oogonia; arrowhead = trabeculae. E Low magnification view of a male gonad in the premature stage. F Detailed view of a male gonad in the premature stage where arrow = spermatogonia maturing into primary spermatocytes maturing as they get further from the trabeculae; arrowhead = spermatids and spermatozoa. G Low magnification view of a mature female gonad. H Low magnification view of a mature male gonad
In the testes, primary spermatocytes were counted as a single stage, while secondary spermatocytes occurred in negligible numbers (Fig. 1E). This observation is in agreement with that of Singhakaew et al. (2003). Numbers of late stages, i.e., spermatids and spermatozoa, could not be counted, since they were too numerous (Fig. 1F and H), too small and often overlapped. They were therefore described as the mean percentage of total gonad area as estimated in ten randomly selected high power fields (HPF) per gonad.
GBIn-values
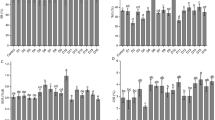
There was a significant difference in mean GBIn-values between all means of the three developmental phases in females (p < 0.001, Fig. 2A). In males, mean GBIn-values did not differ between the two means for the categories “25–75%” and “ > 75%”, respectively (t test, p > 0.05, Fig. 2B).
Gonad bulk index (%) statistics for female and male abalone. The data are grouped based on the developmental phase in females (A) and relative abundance of spermatids and spermatozoa in males (B), the months of the experiment (C, D) and the abalone diets (E, F). Sample sizes are given in each box. Means with different letters shown above the X-axis are significantly different from each other. As samples in December were collected before the start of the feeding study, the data were not used for the analysis (C, D) but box and whisker-plots are shown to compare variability in the dataset
There was no significant difference in both females and males in GBln means between January and February, i.e., during the 2 months when the abalone were fed the three experimental diets (p > 0.26, t test, Fig. 2C and D, respectively). There was no significant interaction (two-way ANOVA, p > 0.05) between month and diet on mean GBIn for females and males with similar average values for the three diets within each sex (ANOVA, p > 0.5 and p > 0.96, Fig. 2E and F, respectively). There was no significant effect stocking density on the developmental phase of females (p = 0.52) and males (p = 0.14).
In neither females nor males was there a significant effect of flow index and farming intensity on the gonad index (p > 0.05). Using pooled data, mean GBIn-values did not differ between females and males during the experimental period (t test; p = 0.16).
Using data from all samples, GBIn values were significantly correlated to the histologically defined maturity stages in females (R = 0.84, p < 0.05) and males (R = 0.38, p < 0.05).
Developmental synchronicity
Figure 3 compares the numbers and percentages of mature females and males with relative abundance values of spermatids and spermatozoa of > 75% for each of the three experimental diets. In abalone fed Abfeed S34, the ratio of mature males to females was 0.9:1, which was closer to the ratio of 1:1 as compared to the other two diets. The percentage of sexually mature individuals was not significantly different between males and females for any given sampling time.
Numbers and percentages of mature females and males with relative abundance of spermatids > 75% in males and mature ovaries in females. The data are from animals sampled in January and February. The frequencies of the numbers of males to females are shown on top of each bar and the percentage ratios are part of the X-axis legend
Discussion
Methodology
The method of quantifying reproductive development in female and male South African abalone, H. midae, made it possible to distinguish developmental stages in both sexes during the months of known reproductive activity. This method differed from other studies on the same species as we estimated gonad development by comparing numbers and percentages of mature females and males between diets. As GBIn was significantly correlated to maturity stage, this method can be used in future research to report or compare GBIn-data with developmental stages.
For comparisons of gonad maturity phases, we used the gonad index GBIn developed by Newman (1967). Gurney and Mundy (2004) have reported that GBIn correlated well with gonad histology and therefore constituted an adequate measure of fluctuations in the reproductive cycle. According to Sobhon et al. (1999), gonad indices relate gonad area to constant parameters, such as the size of the conical organ, and they do not take variation of hepatopancreas size into account. Thus, the reliability with which the area of the conical organ and the hepatopancreas can be measured is a potential limitation for GBIn analyses. For example, eleven samples for which these measurements could not be made were excluded, while the analysis of the remaining samples allowed for an assessment of developmental stages. Therefore, to obtain a good estimate of the development of the gonads, GBIn was used in the present study in combination with histological assessments. Differences in mean GBIn-values between developmental phases within the spawning season could be detected. Thus, this approach contributes new information to our knowledge on reproductive development in farmed South African abalone during the spawning season.
This study also highlights the difficult that can be associated with determining the sex of the abalone. During the initial size-grading procedure, 40% of the animals did not have macroscopically visible sexual characteristics. Microscopic examination allowed for the identification of nascent gonadal tissue in several of the immature abalone, and all but one animal could be assigned to a sex. An even more sensitive method for sex determination may increase the probability of detecting individuals that have not reached maturity at the time of sampling. For example, it was proposed to assess sexual differentiation based on the measurement of the expression levels of the gene coding for the zona pellucida domain 4 (zp4) using real-time quantitative PCR in the disk abalone, Haliotis discus hannai (Choi et al. 2021). Because male abalone are known to reach sexual maturity at a younger age in average, and because this is linked to decreased growth rates, it might be beneficial to test this approach in future studies, especially at the earliest stages of reproductive development.
Husbandry
Stocking density and flow rate
Stocking density and water flow rate to the tanks did not affect mean GBIn-values in either sex. H. midae is a broadcast spawner, in which spatial closeness of abalone to each other and the release of pheromones may trigger spawning events. This species can spawn repeatedly within the reproductive season and spontaneous spawning events have been reported to occur once or twice per month (Henry 1996). These events can lead to a loss of biomass, which can affect feeding intensity. It was therefore assumed that differences in development due to husbandry conditions could be detected within the period chosen for the present study. Only two abalone had spent gonads, but this was likely caused by stress due to handling during purging. Therefore, spontaneous spawning events in H. midae could not be confirmed under the stocking densities and flow rates chosen for this experiment.
Influence of diet of gonadal development
Diet did not affect GBIn-values. Other authors have investigated the effect of diet on the growth of H. midae (Britz 1996a, b; Lopez et al. 1998; Sales and Britz 2001; Shipton and Britz 2001; Dlaza 2006; Naidoo et al. 2006) by comparing diets with soya meal inclusion with diets that only contained fishmeal as the protein source (Ayres 2013; Riddin 2013; Wu et al. 2019; Wu 2020). In these studies, the natural macroalgal diet, kelp, was not included and so the current research contributes further to our understanding of the effect of both natural and artificial diets on abalone reproduction. GBI-values were expressed as the estimated effective gonad volume (mm3) per g of shucked abalone mass. Furthermore, some research investigating abalone kept under different conditions only focused on female development (Ayres 2013; Riddin 2013) while the present study reports on the combined effect of dietary composition and abalone sex on gonad development. Wu et al. (2019) compared frequency distributions of oocyte stages over a 180-day growth period (Wu et al. 2019). Three abalone of each sex were collected every 45 days. In the present study, findings were based on a larger sample size, a combination of different methods and the analysis of gonad development in both sexes. The fact that the soya diet was not associated with an altered gonad development in either sex could suggest that it has no effect as a source of phytoestrogens in abalone.
Synchronicity
The combination of methods used in this study adds information to the study of abalone maturity. For example, the analysis of frequencies of mature females and males for each of the three diets provided a first preliminary assessment of potential developmental synchronicity. The present results suggested that synchronicity was higher in abalone fed a fishmeal soya combination because an approximately equal number of abalone of both sexes that were fed Abfeed S34 had reached maturity, while this ratio of males to females differed from 1:1 in the other two diets. Future studies should quantify whether abalone fed a diet with soya meal have a larger number of spawning events.
Conclusions
In the short term, the gonad development of abalone was not influenced by farm conditions or the inclusion of soya meal as a source of protein. Farmers may be able to feed any of the diets used in the present study for at least 2 months during the natural spawning season without negative effects on gonad development or a loss of biomass due to spawning.
It is hypothesised that the onset of sexual maturation in farmed abalone is determined primarily by abalone size rather than diet. Using the proposed methods, future experiments may benefit from a longer duration as it may take more time for nutritional or environmental factors to have effects on reproductive development.
Due to the likely economic impact of precocious sexual development, more studies addressing this subject will be beneficial to the abalone farming industry.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ANOVA :
-
Analysis of variance
- FM :
-
Fishmeal diet
- GBI :
-
Gonad mass index
- GBIn :
-
Gonad bulk index
- GI :
-
Gastrointestinal
- GLM :
-
General linear model
- HI :
-
High stocking density
- HPF :
-
High power fields
- LI :
-
Low stocking density
- PCR :
-
Polymerase chain reaction
- SPSS :
-
Statistical Package for the Social Sciences
- zp4 :
-
Zone pellucida domain 4
References
Adams NR (1995) Detection of the effects of phytoestrogens on sheep and cattle. J Anim Sci 73:1509–1515. https://doi.org/10.2527/1995.7351509x
Ayres DWP (2013) Effect of diet and sex-sorting on growth and gonad development in farmed South African abalone, Haliotis midae. MSc. thesis. Rhodes University
Bagheri T, Imanpoor MR, Jafari V, Bennetau-Pelissero C (2013) Reproductive impairment and endocrine disruption in goldfish by feeding diets containing soybean meal. Anim Reprod Sci 139:136–144. https://doi.org/10.1016/j.anireprosci.2013.02.003
Bansemer MS, Qin JG, Harris JO et al (2016) Nutritional requirements and use of macroalgae as ingredients in abalone feed. Rev Aquac 8:121–135. https://doi.org/10.1111/raq.12085
Bautista-Teruel MN, Millamena OM, Fermin AC (2001) Reproductive performance of hatchery-bred donkey’s ear abalone, Haliotis asinina, Linne, fed natural and artificial diets. Aquac Res 32:249–254. https://doi.org/10.1046/j.1355-557x.2001.00022.x
Britz PJ (1996a) The suitability of selected protein sources for inclusion in formulated diets for the South African abalone, Haliotis midae. Aquaculture 140:63–73. https://doi.org/10.1016/0044-8486(95)01197-8
Britz PJ (1996b) Effect of dietary protein level on growth performance of South African abalone, Haliotis midae, fed fishmeal-based semi-purified diets. Aquaculture 140:55–61. https://doi.org/10.1016/0044-8486(95)01198-6
Capinpin ECJ, Corre KG (1996) Growth rate of the Philippine abalone, Haliotis asinina fed an artificial diet and macroalgae. Aquaculture 144:81–89. https://doi.org/10.1016/S0044-8486(96)01332-4
Cho SH, Kim DS (2012) Effects of Feed type and temperature on growth of juvenile abalone, Haliotis discus hannai Ino. J World Aquac Soc 43:114–119. https://doi.org/10.1111/j.1749-7345.2011.00517.x
Choi M-J, Oh YD, Kim YR et al (2021) Use of a gene encoding zona pellucida 4 as a female-specific marker for early stage sexual differentiation and size dimorphism in the pacific abalone Haliotis discus hannai. Anim Reprod Sci 225:106687. https://doi.org/10.1016/j.anireprosci.2021.106687
Cong L, Qin Z-F, Jing X-N et al (2006) Xenopus laevis is a potential alternative model animal species to study reproductive toxicity of phytoestrogens. Aquat Toxicol 77:250–256. https://doi.org/10.1016/j.aquatox.2005.12.014
Counihan R, McNamara D, Souter D et al (2001) Pattern, synchrony and predictability of spawning of the tropical abalone Haliotis asinina from Heron Reef, Australia. Mar Ecol Prog Ser 213:193–202. https://doi.org/10.3354/meps213193
Dlaza TS (2006) Growth of juvenile abalone under aquaculture conditions. University of the Western Cape, South Africa, p 98
El-Sayed A-FM, Abdel-Aziz E-SH, Abdel-Ghani HM (2012) Effects of phytoestrogens on sex reversal of Nile tilapia (Oreochromis niloticus) larvae fed diets treated with 17α-Methyltestosterone. Aquaculture 360–361:58–63. https://doi.org/10.1016/j.aquaculture.2012.07.010
Fermin AC, Gapasin RSJ, Teruel MB (2000) Spontaneous spawning, fecundity and spawning periodicity in the Donkey’s ear abalone Haliotis Asinina Linnaeus. In: Hylleberg A (ed) Proceedings of the 10th International Congress and Workshop of the Tropical Marine Mollusc Programme (TMMP), 20-30 October 1999, Hanoi and Haiphong, Vietnam. Phuket, Thailand. Phuket Marine Biological Center, Phuket, pp 195–201
Fleming AE, Van Barneveld RJ, Hone PW (1996) The development of artificial diets for abalone: a review and future directions. Aquaculture 140:5–53. https://doi.org/10.1016/0044-8486(95)01184-6
Glover A, Assinder SJ (2006) Acute exposure of adult male rats to dietary phytoestrogens reduces fecundity and alters epididymal steroid hormone receptor expression. J Endocrinol 189:565–573. https://doi.org/10.1677/joe.1.06709
Gupta RC (2012) Veterinary toxicology: basic and clinical principles, 2nd edn. Elsevier LTD, Oxford
Gurney LJ, Mundy CN (2004) Reproductive biology: identifying spawning cycles In The Genus Haliotis. Tech Rep Ser Tasmanian Aquac Fish Institute; Hobart, Tasmania 23
Henry NR (1996) Studies related to the artificial spawning and culture of the abalone, Haliotis midae linne, 1785, MSc-thesis, University of Cape Town, South Africa, p 135
Kemp JOG, Britz PJ, Toledo Agüero PH (2015) The effect of macroalgal, formulated and combination diets on growth, survival and feed utilisation in the red abalone Haliotis rufescens. Aquaculture 448:306–314. https://doi.org/10.1016/j.aquaculture.2015.06.016
Le Francois NR, Jobling M, Carter C, Blier PU (2010) Finfish aquaculture diversification. CABI Publishing, Wallingford
Lopez L, Tyler PA, Viana MT (1998) The effect of temperature and artificial diets on growth rates of juvenile Haliotis tuberculata (Linnaeus, 1758). J Shellfish Res 17:657–662
López-Acuna LM (1999) Growth and the energy budget of juveniles of the abalone Haliotis tuberculata (L.). PhD Dissertation. University of Southampton
Makhande ED (2008) Growth of the South African abalone (Haliotis midae) on three diets, under commercial conditions. Nelson Mandela Metropolitan University, South Africa
Naidoo K, Maneveldt G, Ruck K, Bolton JJ (2006) A comparison of various seaweed-based diets and formulated feed on growth rate of abalone in a land-based aquaculture system. J Appl Phycol 18:437–443. https://doi.org/10.1007/s10811-006-9045-7
Najmudeen TM (2008) Ultrastructural studies of oogenesis in the variable abalone Haliotis varia (Vetigastropoda: Haliotidae). Aquat Biol 2:143–151
Newman GG (1967) Reproduction of the South African abalone Haliotis midae. Investl Rep Div Sea Fish S Afr 64:1–24
Newman GG (1968) Growth of the South African abalone Haliotis midae. Investl Rep Div Sea Fish Afr 64:1–24
Pollack SJ, Ottinger MA, Sullivan CV, Woods LC (2003) The effects of the soy isoflavone genistein on the reproductive development of striped bass. N Am J Aquac 65:226–234. https://doi.org/10.1577/c02-041
Riddin NA (2013) Growth and gonad size in cultured South African abalone, Haliotis midae. Rhodes University, South Africa, p 140
Sales J, Britz PJ (2001) Research on abalone (Haliotis midae L.) cultivation in South Africa. Aquac Res 32:863–874. https://doi.org/10.1046/j.1365-2109.2001.00629.x
Shipton TA, Britz PJ (2001) The effect of animal size on the ability of Haliotis midae L. to utilize selected dietary protein sources. Aquac Res 32:393–403. https://doi.org/10.1046/j.1365-2109.2001.00572.x
Singhakaew S, Seehabutr V, Kruatrachue M et al (2003) Ultrastructure of male germ cells in the testes of abalone, Haliotis ovina Gmelin. Molluscan Res 23:109–121
Sobhon P, Apisawetakan S, Chanpoo M et al (1999) Classification of germ cells, reproductive cycle and maturation of gonads in Haliotis asinina Linnaeus. ScienceAsia 25:3–21
Tarr RJQ (1995) Growth and movement of the South African abalone Haliotis midae: a reassessment. Mar Freshw Res 46:583–590
Troell M, Robertson-Andersson D, Anderson RJ et al (2006) Abalone farming in South Africa: an overview with perspectives on kelp resources, abalone feed, potential for on-farm seaweed production and socio-economic importance. Aquaculture 257:266–281. https://doi.org/10.1016/j.aquaculture.2006.02.066
Tzchori I, Degani G, Elisha R et al (2004) The influence of phytoestrogens and oestradiol-17β on growth and sex determination in the European eel (Anguilla anguilla). Aquac Res 35:1213–1219. https://doi.org/10.1111/j.1365-2109.2004.01129.x
Visser-Roux A (2011) Reproduction of the South African abalone, Haliotis midae. Stellenbosch University, South Africa, p 102
Weber K, Setchell K, Stocco D, Lephart E (2001) Dietary soy-phytoestrogens decrease testosterone levels and prostate weight without altering LH, prostate 5alpha-reductase or testicular steroidogenic acute regulatory peptide levels in adult male Sprague-Dawley rats. J Endocrinol 170:591–599. https://doi.org/10.1677/joe.0.1700591
Wood AD, Buxton CD (1996) Aspects of the biology of the abalone Haliotis midae (Linne, 1758) on the east coast of South Africa. 1. Feeding biology. South African J Mar Sci 17:61–68. https://doi.org/10.2989/025776196784158590
Wu Y (2020) A holistic approach in understanding the effects of dietary protein sources on the growth and reproductive development of farmed abalone, Haliotis midae. Rhodes University, South Africa, p 165
Wu Y, Kaiser H, Jones CLW (2019) A first study on the effect of dietary soya levels and crystalline isoflavones on growth, gonad development and gonad histology of farmed abalone, Haliotis midae. Aquac Int 27:167–193. https://doi.org/10.1007/s10499-018-0315-6
Acknowledgements
The authors with to acknowledge the invaluable assistance they received from Dr. Alexander Tichy for the statistical analysis of the data.
Funding
Open access funding provided by University of Veterinary Medicine Vienna. This work was supported by the Joint Study scholarship program of the University of Veterinary Medicine of Vienna and Rhodes University, South Africa. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Author information
Authors and Affiliations
Contributions
Conceptualization, design of the experiment and acquisition of funding: MN, HK and MEM. Conduct of the experiments: EM. Supervision of the experiments: MN and HK. Data analysis: EM and SML. Writing—original draft preparation: EM. Writing—review and editing: HK, MN and SML. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study design was conducted with the ethical approval of all relevant bodies in Austria and South Africa.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Handling Editor: Gavin Burnell
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Simple summary
The objective of this study was to describe gonadal development in female and male South African abalone, H. midae, a commercially valuable species of haliotids. The animals were fed two pelleted diets with and without soya meal, respectively, and kelp, which represents their natural diet. In addition, they were kept at two stocking densities and a range of water flow rates. The use of artificial diets is a common practice in the farming of H. midae, and these diets can include soya meal as a partial substitute for fish meal. Stocking density, flow rate and soya meal inclusion did not have a significant effect on the sexual maturity of both male and female H. midae. A combination of histological assessments and measurements of gonad tissue area made it possible to distinguish reproductive development in both sexes between months of the spawning season.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Meusel, E., Menanteau-Ledouble, S., Naylor, M. et al. Gonad development in farmed male and female South African abalone, Haliotis midae, fed artificial and natural diets under a range of husbandry conditions. Aquacult Int 30, 1279–1293 (2022). https://doi.org/10.1007/s10499-022-00850-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-022-00850-6