Abstract
Two experiments were conducted to evaluate the efficacy of an oil-based substrate, The Water Cleanser™ (TWC). The first experiment studied the effects two substrates of different oil composition (TWC, TWC +), and a commercial bacterial additive (Bio-Aid) on concentrations of nitrogen and phosphorous in indoor aquaria. The second experiment studied the effects of TWC, a bacterial additive, and a combination (TWC + B) on concentrations of nitrogen and phosphorous, phytoplankton abundance and diversity in outdoor freshwater crayfish (Cherax cainii, Austin and Ryan, Invertebr Syst 16:357–367, 2002) tanks. In the first experiment, the concentration of TAN decreased more rapidly with Bio-Aid, whilst the maximum concentrations of NO2-N and NO3-N were reduced with the substrates. The concentration of orthophosphate was reduced in aquaria with TWC + . In the second experiment, concentrations of TAN, NO2-N, NO3-N and total phosphate were not significantly affected by TWC. After addition of TWC + B, there was a significant decrease in the concentrations of nitrate and total phosphate, and a higher abundance of phytoplankton was maintained than with other treatments. Additionally, a population Bacillus sp. was found on the substrate surface. TWC had no adverse effects on phytoplankton abundance or diversity, or C. cainii physiology, weight gain or survival. TWC and TWC + were effective bioremediators of eutrophic water, whilst a combination of TWC + B was effective in short term bioremediation and in promoting phytoplankton abundance in C. cainii tank culture.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In Australia, freshwater crayfish (Cherax cainii, Austin and Ryan 2002) have been farmed for over 30 years with considerable progress in terms of production technologies and scientific understanding of the animals; however, the industry is still in its developmental stage and annual production remains under 100 t/annum (Wingfield 2008; FAO 2021). If the industry is to expand, intensification may be required, whilst still maintaining good water quality and quality produce. In intensive finfish aquaculture, high stocking results in increases in nutrient loading from uneaten feed and metabolic waste products (David et al. 2015), leading to eutrophic conditions and algal blooms (Seymour 1980), which can also occur in crayfish ponds subjected to high nutrient inputs (Cole et al. 2019). An accumulation of nitrogenous and phosphorous wastes can also have adverse impacts on the ecosystem (Hargreaves 1998; Jørgensen 2008). Concomitantly, an increase in ammonium and nitrite may damage the health of aquatic organisms (Mires 1995). In finfish, nitrite leads to the oxidation of functional haemoglobin to methaemoglobin, which cannot bind oxygen, whilst in crayfish, nitrite competes with chlorine and bromine ions for the same uptake mechanism within the haemolymph (Jensen 2003). Nitrite can also be harmful to crayfish, for example, Jensen (1996) has shown that nitrite levels of 1 mM can interfere with ionic balance in noble crayfish (Astacus astacus). Poor water quality and excessive organic matter can also cause epibionts such as Epistylis and Temnocephala to thrive, which can be problematic for the health and market value of cultured species, including Cherax cainii (Austin and Ryan 2002) (Morrissy et al. 1990; Lawrence & Jones 2002).
Microbial bioremediation is one solution to decrease the build-up of nutrients in the aquaculture environment (Martinez-Porchas et al. 2014). Modification of the bacterial composition of water and sediments can improve various water quality parameters, reduce toxins in the water and inhibit growth of pathogenic bacteria (Hong et al. 2005; Perumal et al. 2015). Bacterial additives of heterotrophic and polyphosphate-accumulating bacteria have been used to remove nitrogen and phosphorous, respectively, in aquatic systems (Zokaeifar et al. 2014; Wang et al. 2015; Wang et al. 2015). Microorganisms can influence the development and decline of algal blooms (Subashchandrabose et al. 2011), regulating algal populations in water bodies so as to avoid algal blooms (Zhou et al. 2009), or alternatively can benefit phytoplankton by increasing nutrient availability through remineralization of organic matter (Amin et al. 2012). In turn, an increased abundance of phytoplankton can increase the rate of phytoremediation (Lananan et al. 2014; Labbé et al. 2017). This may also provide more food sources for freshwater crayfish, which consume phytoplankton, zooplankton and the detritus formed when they decay (Saoud et al. 2012).
Substrates can also be used for bioremediation, or for providing a complementary food source as biofilm (Viau et al. 2012). Suitable substrates provide sites for the attachment of biofilms, which has been shown to increase ammonia oxidation rates to nearly 60 times higher than in water particles (Holl et al. 2011). Substrates also harbour beneficial bacteria and periphyton, both of which may improve water quality (Schveitzer et al. 2013). Various studies have investigated the effects of substrates on water quality in shrimp aquaculture (Khatoon et al. 2007; Schveitzer et al. 2013; Thompson et al. 2002; Viau et al. 2013), whilst relatively few studies have applied substrates to Cherax crayfish culture (Jones et al. 2002; Viau et al. 2012). Artificial substrates are most commonly made up of plastic, polyethylene mesh, mats, netting and other similar materials. The material used may determine the type of organisms that grow thereon, and the amount of surface area available. Furthermore, artificial substrates may create a habitat for nitrifying bacteria (Otoshi et al. 2006). Substrates may also enhance the degradation of organic matter, as this can at times be limited by the amount of substrate available, rather than the microbial biomass (Avnimelech et al. 1995).
The Water Cleanser™ (TWCFootnote 1) is a waxy, oil-based substrate designed primarily for remediation of eutrophic or polluted waters. A key ingredient, crude oil, can be utilised by microbes as a carbon and energy source (Jobson et al. 1972). As well as crude oil, the water cleanser plus (TWC +) contains tea-tree oil. Because of the antibacterial properties of tea-tree oil (Southwell et al. 1993), TWC + is thought to be more selective in terms of the bacterial species that colonise the substrate than TWC (personal communication with marine easy clean).
Past trials by Marine Easy Clean (2015) and Cole et al. (2019) suggest that TWC provides a substrate for bacteria already present in the surrounding water, whilst also providing a carbon source. A carbon source is essential for heterotrophic bacteria processes including denitrification (Hamlin et al. 2008), the process of reducing nitrate to nitrogen gas. Heterotrophic bacteria, including Bacillus spp., are also known to affect the water quality and microbial ecology of aquatic systems, and health of decapod crustaceans (Nimrat et al. 2012; Ambas et al. 2013; Hai et al. 2009).
Freshwater crayfish aquaculture is dependent on maintaining good water quality, and in promoting the pond ecology to provide natural food sources. C. cainii in particular requires near-pristine waters to thrive (Beatty et al. 2011), depends on natural food sources for their nutrition and may also consume microbes growing on substrates. Oil-based substrates may provide a solution for improving water quality and ecology in C. cainii aquaculture systems. Two experiments were conducted to evaluate the effects of TWC and different bacterial additives on nutrient concentrations, bacterial and phytoplankton abundance and C. cainii growth and health. The first experiment was a preliminary trial to investigate the efficacy of the TWC and TWC + substrates in bioremediation, without animals. The second experiment investigated the efficacy of TWC, TWC with Bacillus sp. (TWC + B), and a bacterial additive, E-viro 3 on C. cainii growth and physiology, and microalgae abundance and species richness. The effects of oil-based substrates on nitrogen and phosphorous dynamics were studied in two different aquatic environments.
Materials and methods
First experiment
First experiment design
All laboratory work was conducted at the Curtin Aquatic Research Laboratory, Technology Park, Bentley, Western Australia.
Twelve 15 L capacity glass aquaria (36 cm × 22 cm × 26 cm) were filled with 12 L of sediment filtered freshwater sourced from tap water stored for the purpose of animal husbandry, with aeration and without mechanical filtration. Ammonium chloride (NH4Cl) and potassium phosphate dibasic (K2HPO4) were added to all tanks to attain an initial concentration of 4.11 mg/L TAN (total ammonia nitrogen) and 3.5 mg/L PO4 (total phosphate). Four treatments were used in triplicate in a completely randomised design: (1) control; (2) the water cleanser (TWC) substrate by Marine Easy Clean Pty Ltd., made with crude oil and waxes, at 22 g/tank, as two spherical balls 28 mm each in diameter, with a dorsal hole to provide more surface area; (3) the water cleanser plus (TWC +) substrate, made with tea tree oils and waxes, at 22 g/tank, as two rectangular blocks (35 mm × 34 mm × 20 mm) with ridges to provide more surface area and (4) a Bio-Aid commercial probiotic (Bio-Aid) by Aquasonic Pty Ltd., at 0.1 mL. This was the recommended initial dosage of Bio-Aid, which contains a mix of 27 different strains of heterotrophic probiotic bacteria. TWC and TWC + were compared in terms of composition, not surface area to volume ratio. The experiment ran for 13 weeks.
Water quality
Dissolved oxygen (DO), temperature, pH and conductivity were monitored biweekly using an Oxyguard Handy Polaris DO metre and an Ecoscan pH 5 m, respectively, for both experiments. TAN, NO2-N, NO3-N and total phosphate (PO4) were measured weekly using chemical test kits (Aquarium Pharmaceuticals™, McLean, VA, USA). These readings (except for PO4) were standardised every 20 days using permachem reagents from HACH® and a skalar colorimeter auto analyser (Downs et al. 2008), and orthophosphate was measured every 20 days. Permachem tests were conducted according to the methods described in the Standard Methods for the Examination of Water and Wastewater (APHA 1998). Readings from the Aquarium Pharmaceuticals™ test kits were compared to readings from the permachem method to achieve water quality readings within detection limits of 0.25 mg/L for TAN, 0.1 mg/L for NO2-N, 0.25 for PO4 and 2.5 mg/L for NO3-N. Results by colour from the chemical test kits were matched with the associated permachem readings regularly to maintain accuracy. Where the colour readings were indeterminate, samples were further diluted with distilled water to attain an accurate reading.
Bacteria abundance and diversity
Heterotrophic plate counts (HPC) in colony-forming units (CFU) per mL were determined after 11 weeks, with a dilution of 10−1.3. From each tank, a water sample was taken and a 50 µL subsample was transferred onto an agar plate using aseptic techniques. Samples were spread using a hoop, which was flamed between samples. Samples were cultured on standard plate count agar (Tryptone glucose yeast agar) at 30 °C for 48 h. Viable colonies were counted and identified according to colony morphology and colour to estimate diversity.
Second experiment
Second experiment design
For the second experiment, the standard, crude oil-based TWC was selected over the TWC + due to its greater commercial availability. The experiment was conducted outdoors, subject to natural sunlight, with a photoperiod close to 12:12 and fluctuations in temperature, rainfall and evaporation. The trial period was 18 weeks from the 26th of May (late autumn) until the 25th of September (early spring), 2016, with average maximum and minimum temperatures and rainfall of 18.6 °C, 8.9 °C and 109.4 mm in June; 17.7 °C, 7.5 °C and 139.6 mm in July; 17.7 °C, 7.9 °C and 123.2 mm in August and 18.5 °C, 7.6 °C and 68.8 mm in September, respectively (BOM 2021). Twelve white plastic cylindrical tanks (46 cm h × 58 cm d) were filled to 85 ± 5L with sediment filtered freshwater sourced from tap water stored for the purpose of animal husbandry, and aerated. Tanks were half covered with mesh shade around the aeration line to prevent escapees. Four treatments were used in triplicate in a randomised block design: (1) a control with no additions; (2) TWC at 200 g rectangular floating blocks (155 × 107 × 20 mm), with ridges to provide more surface area; (3) the water cleanser with Bacillus sp. in inactive form (TWC + B) at 200 g rectangular blocks and (4) E-viro 3 commercial probiotic (E-viro 3) by Enviroplus Pty Ltd (containing a range of bacteria for improving water quality) at 40 mL per tank initially. Initially, 1.85 g of Aquasol fertiliser by Selleys Yates Pty Ltd (containing NPK 23:3.95:14, and trace elements S, Mg, Mn, Cu, Fe, Zn and B) was added per tank to increase nutrient availability.
Water quality
Dissolved oxygen, temperature, pH and conductivity were measured twice a week, diurnally. Concentrations of TAN, NO2-N and NO2-N were measured every 20 days, and orthophosphate every 30 days, using the permachem method in experiment 1. Concentrations of TAN, NO2-N, NO3-N and total PO4 were monitored twice a week during the first month with API test kits, and standardised. Samples were kept refrigerated and analysed within 7 days according to standard procedures (AS/NZS 5667.1, 1998).
Phytoplankton
Phytoplankton species were procured from commercial C. cainii ponds in Manjimup, WA and cultured outdoors in an aerated stock tank. Initially, approximately 10 L of stock water was added to each experimental tank in order to cultivate a population of phytoplankton. Phytoplankton abundance and species composition were measured every 2 weeks. Water samples were taken directly from the water surface into 100 mL plastic containers. Lugol’s iodine was added at 3% concentration for staining and preservation, and samples were counted within 3 h. Phytoplankton were counted using a haemocytometer with a heavy cover slip, and viewed using a Motic compound microscope at 400 × magnification. Phytoplankton abundance (cells/L) was calculated by using equations from Tulsankar et al. (2020).
where Total Vol. = total volume of water (L) collected from the pond, Conc. Vol. = volume of water (mL) containing concentrated plankton after sieving, Sub. Vol. = sub-sample of water (mL) from concentrated volume in which plankton is counted and No. = mean number of cells or individuals counted.
Cells were identified to genus level, with reference to Ingram et al. (1997), and the number of genera per sample counted. The species diversity was further calculated using the Shannon diversity index with the following formula: \(H=-\left[\sum {P}_{i}\times \mathrm{Ln}\left({P}_{i}\right)\right]\), where H = diversity index, Pi = the number each species in the sample/total number of samples and Ln(Pi) = the natural logarithm of this proportion.
Bacteria abundance and diversity
Bacterial abundance in the water column was measured by diluting water samples by 10−2, by mixing 1 mL samples in 100 mL of sterile 0.85% saline solution. A total of 100 µL of diluted sample was then transferred onto agar plates using aseptic techniques. Samples were cultured and counted as per experiment 1. Heterotrophic Plate Count (HPC), Bacillus sp. count and estimated diversity were determined on TWC surface by taking a swab of 1 cm3 from the submerged surface, transferring to agar plates and culturing as per the water sampling techniques.
Animals
Freshwater crayfish (C. cainii) were procured from Blue Ridge Marron, Manjimup, and acclimatised in stock tanks for 7 days before trial, with aeration and regular water exchange. Thirty-six animals (51.3 ± 1.3 g; 56.5 ± 0.5 mm OCL) were stocked at 3 animals per tank to simulate an aquaculture environment, with regular nutrient inputs of feed and waste. PVC pipes were provided as shelters. Feed was provided at a rate of 0.5% of wet body weight, 3 times per week. Individual C. cainii weight was measured monthly. Tanks were checked daily for mortalities.
Haemocyte count
Haemolymph samples were taken after 20 days, 55 and 80 days to conduct total haemocyte count (THC) and differential haemocyte count (DHC) as per established procedure (Sang et a. 2009). A 0.3 mL aliquot of haemolymph was withdrawn into a 1 mL syringes containing 0.3 mL of trisodium citrate anticoagulant. THC was counted within 8 h of sampling, using a haemocytometer (Neubauer, Wertheim, Germany) and a compound microscope (Motic, Xiamen, China) at 400 × magnification. For DHC, samples were stained with Giemsa and May-Grunwald and granular, semi-granular and hyaline cells were counted.
Statistical Analysis
All data obtained were expressed as mean ± standard error. All statistical analysis was done using SPSS version 23.0. Data were analysed using a one-way ANOVA and least significant difference (LSD) post-hoc test to determine significant differences between treatments. A Kruskal–Wallis test was used where data lacked normality and/or homogeneity of variance. A Spearman’s rank test was used to correlate parameters. Differences were considered significant at α = 0.05.
Results
First experiment
Water quality
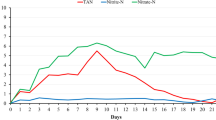
The physico-chemical parameters pH (mean = 7.91 ± 0.02, min = 7.01, max = 8.45) (F (3272) = 0.139, p = 0.937), temperature (mean = 18.45 ± 0.11, min = 15.10 °C, max = 23.10 °C) (F (3296) = 0.156, p = 0.926), DO (mean = 8.80 ± 0.04, min = 7.40 mg/L, max = 10.60 mg/L) (F (3296) = 0.186, p = 0.906) and conductivity (mean = − 26.30 ± 1.35, min = − 72.0, max = 46.0) (F (3272) = 0.053, p = 0.984) were not significantly affected by treatment. The substrates, TWC and TWC + , changed the dynamics of nutrient concentration remediation in the aquaria. Whilst in the control and Bio-Aid treatments, the TAN was broken down more quickly; the nitrites and nitrates rose to significantly higher levels. TAN decreased steadily in all treatments, but was lower in aquaria with Bio-Aid than with substrates after 7, 8, 9 and 11 weeks (p < 0.05), and lower in control than with substrates after 9 and 11 weeks (p < 0.05) (Fig. 1). TAN fell to < 0.02 mg/L by week 13.
Over time, the concentrations of NO2-N and NO3-N were often lower (p < 0.05) and more stable in TWC and TWC + aquaria than in the control and Bio-Aid aquaria, which showed large fluctuations, peaks and high standard errors in both NO2-N and NO3-N (Fig. 2).
The concentration of orthophosphate was significantly lower with TWC + than with Bio-Aid or control in week 4 (0.85 ± 0.02, 1.01 ± 0.03 mg/L and 0.96 ± 0.04, respectively) (F (3, 8) = 4.325, p = 0.043), and lower than with Bio-Aid in week 7 (0.96 ± 0.01, 1.15 ± 0.04 mg/L, respectively) (F (3, 8) = 5.220, p = 0.027). Concentrations of total phosphate (F (3, 8) = 6.524, p = 0.015) and NO3-N (F (3, 8) = 6.308, p = 0.017) were lower with TWC + than with Bio-Aid or control at the end of trial (Fig. 3).
Concentrations of nitrate-nitrogen (NO3-N) and total phosphate after 13 weeks (experiment 1). Values are the mean ± standard error (n = 3). Different letters (a, b) indicate significant differences in total phosphate and numbers (1, 2) indicate significant differences in NO3-N between treatments (α < 0.05)
Bacteria abundance and observations
No significant differences were found amongst heterotrophic plate count (HPC) after 10 weeks (F (3, 8) = 1.44, p = 0.302). Mean HPC was 51.6 ± 35.2, 55.7 ± 11.1, 25.0 ± 7.94 and 7.87 ± 0.29 (CFU/mL × 102) in control, TWC, TWC + and Bio-Aid tanks, respectively.
Biofilm grew at the bottom of aquaria in all tanks. A thick layer of biofilm developed on TWC and TWC + after 4 weeks, varied in colour and texture. Both cocci and rod-shaped bacteria were found within the biofilm, as determined by microscopy. The surface of the substrates also contained a varied community of larger microbes, protozoans and nematodes.
Second experiment
Water quality
The physico-chemical parameters pH (p = 0.067), temperature (p = 0.894), DO (p = 0.707) and conductivity (p = 0.481) were not significantly affected by treatment. Diurnal water temperature ranged from 10.3 to 19.2 °C, with an overall mean of 14.8 ± 0.1 °C. Temperatures remained low throughout the experiment, relative to C. cainii optimal temperature (Morrissy 1990). The pH ranged from 7.78 to 10.22 with an overall mean of 7.91 ± 0.02. Higher phytoplankton abundance was correlated with higher pH levels (R = 0.629, p = 0.028). Dissolved oxygen remained high in all tanks, with an overall mean of 10.98 ± 0.11. A thin layer of biofilm was visible growing on the surface of TWC after 79 days.
After 5 days, NO3-N (F (3, 8) = 9.94, p = 0.004) and total phosphate (F (3, 8) = 9.03, p = 0.006) were significantly lower in TWC + B treatment than all other treatments (Fig. 4). The concentration of orthophosphate was also significantly lower in TWC + B tanks (0.100 ± 0.100) than control (0.953 ± 0.119), TWC (0.973 ± 0.079) and Eviro3 (1.083 ± 0.108) tanks in the first week (F (3, 8) = 19.76, p < 0.001). There were no other significant differences in terms of total phosphate (p = 0.962) (Fig. 4) or orthophosphate (p = 0.599). The concentrations of orthophosphate were not significantly different between control, TWC, TWC + B and Eviro 3 tanks after 5 weeks (1.04 ± 0.136, 0.856 ± 0.069, 0.627 ± 0.077 and 1.35 ± 0.260, respectively) and decreased in all treatments after 10 weeks (0.097 ± 0.024, 0.027 ± 0.027, 0.007 ± 0.003 and 0.110 ± 0.038, respectively). Mean TAN, NO2-N, NO3-N and PO4 remained below 0.10 mg/L, 0.01 mg/L, 3.00 mg/L and 0.11 mg/L, respectively after week 3 (Fig. 4). NO2-N rose in week 3 in control tanks only, though the difference was not significant (F (3, 20) = 1.00, p = 0.413). NO3-N was negatively correlated with phytoplankton abundance (R = − 0.740, p < 0.001), as was TAN (R = − 0.675, p < 0.001).
Phytoplankton
The phytoplankton abundance was highest in TWC + B tanks in week 5 (F (3, 8) = 10.18, p = 0.004) and week 7 (F (3, 8) = 10.94, p = 0.003), and showed weekly fluctuations (Fig. 5). Phytoplankton abundance grew exponentially and peaked after 3 weeks, and then fell again after 5 weeks. The diversity of genera (F (3, 80) = 0.201, p = 0.895) and the Shannon–Wiener Diversity Index (F (3, 80) = 1.607, p = 0.194) were not significantly different between treatments. Within TWC tanks, the Shannon–Wiener Index was significantly higher in week 5 than in weeks 3, 11 and 18, at 1.21 ± 0.04, 0.98 ± 0.03, 0.93 ± 0.08 and 0.90 ± 0.10, respectively (F (6, 14) = 5.57, p = 0.004). In all tanks, the number of genera increased from the start to end of experiment, from 3.08 ± 0.23 to 6.33 ± 0.41 (F (6, 77), p < 0.001). The stock water was dominated by Scenedesmus sp., Chlorella sp. and Chlamydomonas sp., whilst Scenedesmus sp., Chlorella sp., Chlamydomonas sp., Euglena sp., Monoraphidium sp. and Tetraedron sp. were found throughout the experiment.
Bacteria abundance
The Heterotrophic Plate Count (HPC) was not significantly different between treatments at any time (F (3, 20) = 0.789, p = 0.514) (Table 1). Mean bacterial abundance was comparably higher in experiment 2 than experiment 1, probably due to the increase in nutrient inputs from feed. During experiment 2, where Bacillus sp. was added (TWC + B), they remained present in the water of all 3 tanks after 7 weeks and of 2 tanks after 10 weeks. Bacillus sp. was also present in one of the E-viro 3 tanks after 7 weeks. On TWC surface, Bacillus sp. was found in TWC + B tanks but not in TWC tanks. HPC on TWC surface was not significantly different between TWC and TWC + B, at 859 ± 133 and 392 ± 189 CFU/cm3 respectively (t (4) = 2.01, p = 0.395).
Growth, survival and haemocyte count
Survival (F (3, 8) = 0.89, p = 0.487) and SGR (F (3, 19) = 0.44, p = 0.728) of C. cainii were not significantly affected by treatment. One mortality was found each in the control and TWC + B treatments, and 2 in TWC treatment. Cannibalism was the most frequent cause of mortality, often occurring post-moult.
The THC (F (3, 32) = 0.46, p = 0.711) and DHC (F (3, 8) = 0.17, p = 0.916) were not significantly different between treatments at any time. Within control tanks, THC was significantly higher after 12 weeks, at 4.96 ± 0.97 × 106 haemocytes/mL, than after 3 weeks and 8 weeks, at 1.13 ± 0.17 × 106 and 1.69 ± 0.43 × 106 haemocytes/mL, respectively (F (2, 6) = 11.11, p = 0.01). THC was negatively correlated with TAN (p = 0.017, R = − 0.670). After 12 weeks, the proportion of granulocytes ranged from 12.0 ± 0.7 to 14.3 ± 3.6%.
Discussion
The Water Cleanser™ developed a biofilm in both experiments, and had a greater effect on water quality in the more eutrophic environment of the first experiment. In experiment one, the peak concentrations of nitrite and nitrate were significantly reduced with TWC and TWC + . Both substrates were similarly effective as bioremediators, accelerating the nitrification of nitrite, and denitrification of nitrate. Addition of an artificial substrate, with attached biofilm, has been shown to maintain lower levels of ammonium and nitrite in Cherax quadricarinatus culture (Viau et al. 2012). Substrates can have varied effects; however, for example, in one study, addition of a substrate with attached biofilm resulted in a reduction of ammonium, but not of nitrite and nitrate (Thompson et al. 2002). In comparison to previously studied substrates, it is possible that TWC may function differently as a bioremediator, though this still requires validation from a comparative study. TWC and TWC + may also help with the start-up of new aquarium or aquaculture systems by exposing animals to lower levels of nitrites and nitrates. Jones et al. (2002) reported a maximum mean nitrite concentration of 1.3 mg/L in a study using a different substrate (Aquamat™). In our study, the nitrite concentrations remained lower, with a maximum mean value of 0.58 mg/L in tanks with TWC in experiment 1. Meanwhile, the acclimation of a conventional biological filter using chemical addition can take up to 36 days before nitrite concentrations are reduced to levels below 1 mg/L, exposing animals to acute toxicity during this time (Manthe & Malone 1987). The use of oil-based substrates may help to reduce exposure of aquatic animals to high levels of nitrite during the acclimation phase of a new system.
Whilst aquaria with TWC and TWC + exhibited greater reduction of nitrite, nitrate and phosphate concentrations, there was a slower rate of ammonia oxidation. The Bio-Aid probiotic effectively oxidised ammonia, but was only added initially, which may explain why the nitrates and phosphates were not removed quickly, and increased over time. The addition of probiotics has previously given mixed results. Some studies have reported only limited effects of probiotics on water quality (Boyd & Gross 1998; Chiayvareesajja & Boyd 1993), whilst others have reported significant reductions in ammonia levels with probiotic treatments (Nimrat et al. 2012). The difference that we observed between the substrates and the probiotic can be partly explained by variation in the numbers of nitrifying and heterotrophic bacteria, which in aquaria and aquaculture systems are often associated with changes in filter conditions (Itoi et al. 2006). In aquaria with TWC and TWC + , increased ammonia could have been sourced from mineralisation of organic matter by bacteria attached to the substrates (Prabu & Santhiya 2016). Also present on TWC surface were protozoans, which can also work as mineralizers by excreting ammonium and returning nitrogen incorporated by the bacteria to the water (Caron 1994). The degree of grazing by ciliates and other protozoans on TWC surface was not studied, though this predation pressure likely affected the bacterial abundance (Thompson et al. 2002). The composition of attached biofilm and periphyton, and effects on water quality, may vary between substrates composed of different materials.
Past studies have found mixed results based on the effects of artificial substrates on water quality, depending largely on substrate type. Substrates made from mineral media have been used to culture microbial mats, which can then be used to remediate nutrient-enriched aquaculture effluent (Bender & Phillips 2004), or used directly as filter media in aquaculture systems (Bender et al. 2004). Culturing biofilm on TWC prior to application could similarly improve the rate of bioremediation, as biofilm can take several weeks to develop on TWC surface. In contrast, a polyethylene mesh substrate was not effective in reducing concentrations of ammonia, nitrite, nitrate and orthophosphate in one study (Schveitzer et al. 2013). Our second experiment also showed no significant effects of the water cleanser on water quality. Schveitzer et al. (2013) attributed the limited effects to a low amount of biological growth on the substrate surface. Biofilm was present on TWC in experiment 2; however, the nutrient concentrations were likely to have been reduced mainly by phytoremediation from microalgae, as similar effects were observed with TWC and without substrate. Whilst microalgae such as diatoms and cyanobacteria can be grown on substrates to reduce nutrient concentrations in culture systems (Khatoon et al. 2007; Thompson et al. 2002), our observations suggest that TWC encourages the growth of a diverse consortium of heterotrophic rather than autotrophic microbes for bioremediation. Substrates are rarely used in combination with probiotics to promote microbial growth. The combination of TWC and Bacillus sp. significantly reduced concentrations of nutrients in the first week of experiment 2. This is mainly attributed to the probiotic, as biofilm on TWC takes several weeks to become established. The commercial probiotic, E-viro 3, did not reduce the concentrations of nitrogen and phosphate, although previous studies have been found to decrease inorganic nitrogen and phosphate concentration in aquaculture water (Wang et al. 2005). TWC may help to maintain the effectiveness of probiotics for a longer time by providing habitat and a carbon source for the bacteria to thrive. In experiment 2, TWC + B maintained a population of Bacillus sp. on TWC surface.
The surface of TWC became abundant in heterotrophic bacteria after several weeks in the aquatic environment. TWC provides a habitat, and contains hydrocarbons, which is likely to provide an accessible carbon source for Bacillus sp. (Hu et al. 2017). Bacillus sp. is commonly used to improve water quality and in nitrate and phosphate removal (Hong et al. 2005; Boyd & Gross 1998; Hu et al. 2017). The first experiment did not determine whether Bacillus sp. was present, though in experiment 2, Bacillus sp. was found on TWC surface where the bacteria were added initially. This suggests that addition of probiotics into a system containing TWC can result in a continued population living on TWC surface. Observations showed that TWC surface also contained microalgae, but was dominated by heterotrophic bacteria and microbes.
There was a high abundance of phytoplankton and epiphytic microalgae present in the tanks during experiment 2, influencing the tank environment. Nitrate and phosphate levels remain low when microalgae biomass is high (Lananan et al. 2014). Nutrient levels are also a limiting factor on microalgae abundance (Zhihong et al. 2010). Nitrate and ammonia both had an inverse relationship with phytoplankton abundance, which can be partly explained by the uptake of nutrients as phytoplankton biomass increases, and the steady release of nutrients from dead algae cells (Seymour 1980). Meanwhile, TWC + B had a positive impact on phytoplankton abundance, which may be attributed to the addition of heterotrophic bacteria, important for nutrient mineralisation in many planktonic ecosystems (Caron 1994). Addition of Bacillus sp. and Lactobacillus sp. has likewise been found to increase Chlorophyta abundance in shrimp recirculating systems (Litopenaeus vannamei) (De Paiva-Maia et al. 2013). Microbial communities play a major role in recycling the autochthonous nutrients accumulating within the system (Avnimelech et al. 1995). In the current study, microalgae including Scenedesmus sp. and Chlorella sp. were abundant, and it has previously been demonstrated that both are useful in the reduction of nutrients (Lananan et al. 2014; Uma Devi et al. 2014). Lananan et al. (2014) found that a combination of Chlorella and a microorganism was more effective in decreasing orthophosphate than either organism alone. A combination of microalgae and the Bacillus sp. added to TWC + B tanks reduced concentrations of orthophosphate effectively for the first 2 weeks.
In the present study, the phytoplankton grew exponentially at the start of experiment, as nutrients were available, until the phytoplankton community crashed to a much lower biomass following the depletion of nutrients, reflecting patterns of an algal bloom. However, in the TWC + B tanks, the phytoplankton density did not collapse to such low levels. If applied to natural systems, this may help to prevent the depletion of oxygen associated with the collapse of algal blooms. The collapse of dense algal blooms often causes mortality due to depletion of oxygen by algal decomposition (Seymour 1980). The use of aerators prevented any depletion of oxygen in the present study. Here, as in natural systems, the ‘bloom’ of excess algae resulted in accumulation of dead algae (Pace & Lovett 2013). Following the initial drop in phytoplankton abundance, the steady release of nutrients from animal waste, excess feed and organic matter decomposition allowed the algae population to recover.
The slow growth of C. cainii was mainly attributed to the low temperatures, as temperatures below 11–13 °C can be limiting to growth (Morrissy 1990). It is unclear whether the senescent microalgae on the bottom provided any significant nutrition to the animals. There was biofilm present on TWC; however, it was physically inaccessible to the benthic C. cainii, providing no complementary food source. Accessible bottom substrates are very important in growth performance of crayfish (Karadal & Türkmen 2017). Substrates increase growth rates in shrimp and crayfish, by promoting the growth of periphyton for use as a complementary food source, or by providing habitat (Jones et al. 2002; Schveitzer et al. 2013). For example, Schveitzer et al. (2013) found an increase in growth and survival of L. vannamei attributed to a substrate providing increased surface area for habitat and food sources as periphyton. Meanwhile, Viau et al. (2012) have attributed improved growth and survival of C. quadricarinatus to maintenance of good water quality using biofilm.
The relationship that was found between TAN and THC is common in aquaculture environments. Exposure to high levels of ammonia can reduce the THCs of C. cainii (Sang et al. 2009). In the current study, no treatment was able to improve the THCs compared to the control, although TAN remained below 1.20 mg/L. In another study, the addition of Bacillus sp. improved the granular cell count in shrimp (Penaeus brasiliensis) (De Souza et al. 2012); however, in our study, no improvement was found. Moreover, E-viro 3 has been shown to improve health and survival of animals in L. vannamei ponds treated with commercial probiotics (Wang et al. 2005), though no difference was found, likely due to the good condition of C. cainii throughout the experiment.
In the current study, substrates were added to aquaria and C. cainii culture tanks, at a rate of approximately 0.18 and 0.24% mass to volume, respectively. This rate is applicable to aquaria and small aquaculture systems, though would not be applicable to large systems, including commercial aquaculture ponds, due to the high volume of product needed. In a separate study on C. cainii aquaculture ponds, a rate of 10 kg to 1,987,500 L or approximately 0.0005% mass to volume was sufficient to improve water quality of ponds and juvenile C. cainii production (Cole et al. 2019).
Conclusions
The substrates used in this study provided a habitat for heterotrophic bacteria, and significantly reduced nutrient concentrations in experiment 1, although were less effective in bioremediation in experiment 2. TWC and TWC + were more effective in decreasing concentrations of nitrates and orthophosphates, and maintaining a lower level of nitrite and nitrate, than adding probiotics initially or without treatment. In experiment 2, the outdoor conditions may have reduced the effect of TWC. The combination with Bacillus sp. was effective in bioremediation of nitrogenous metabolites and phosphates initially, whilst also maintaining the phytoplankton community, and a population of Bacillus sp. in the water column and substrate surface. The oil-based substrates (TWC, TWC +) may provide an alternative to conventional substrates used in aquaculture. TWC provides a habitat and carbon source for heterotrophic bacteria which can maintain lower concentrations of nitrites and nitrates in aquaria and provide habitat for Bacillus sp. with no ill effects for phytoplankton or C. cainii.
Data Availability
Raw data is available upon request.
Code availability
Not applicable.
Notes
TWC = The Water Cleanser.
References
(2015) ‘Enhancing nature to clean and restore nature’, the water cleanser by marine easy clean; booklet. www.thewatercleanser.com.au
Ambas I, Suriawan A, Fotedar R (2013) Immunological responses of customised probiotics-fed marron, Cherax tenuimanus, (Smith 1912) when challenged with Vibrio mimicus. Fish Shellfish Immunol 35(2):262–270. https://doi.org/10.1016/j.fsi.2013.04.026
American Public Health Association (APHA), 1998. Standard methods for the examination of water and wastewater (20th ed.). APHA, Washington, DC
Amin SA, Parker MS, Armbrust EV (2012) Interactions between diatoms and bacteria. Microbiol Mol Biol Rev 76(3):667–684
Austin CM, Ryan SG (2002) Allozyme evidence for a new species of freshwater crayfish of the genus Cherax Erichson (Decapoda : Parastacidae) from the south-west of Western Australia. Invertebr Syst 16:357–367
AS/NZS 5667.1:1998. Water quality – sampling; part 1: guidance on the design of sampling programs, sampling techniques and the preservation and handling of samples. Retrieved from: https://www.saiglobal.com/pdftemp/previews/osh/as/as5000/5600/56671.pdf
Avnimelech Y, Mozes N, Kochba M (1995) Rates of organic carbon and nitrogen degradation in intensive fish ponds. Aquaculture 134:211–216
Beatty S, de Graaf M, Molony B, Nguyen V, Pollock K (2011) Plasticity in population biology of Cherax cainii (Decapoda: Parastacidae) inhabiting lentic and lotic environments in south-western Australia: implications for the sustainable management of the recreational fishery. Fish Res 110(2):312–324. https://doi.org/10.1016/j.fishres.2011.04.021
Bender J, Lee R, Sheppard M, Brinkley K, Phillips P, Yeboah Y, Wah RC (2004) A waste effluent treatment system based on microbial mats for black sea bass Centropristis striata recycled-water mariculture. Aquacult Eng 31(1–2):73–82. https://doi.org/10.1016/j.aquaeng.2004.02.001
Bender J, Phillips P (2004) Microbial mats for multiple applications in aquaculture and bioremediation. Biores Technol 94(3):229–238. https://doi.org/10.1016/j.biortech.2003.12.016
Boyd, C. E., & Gross, A. (1998). Use of probiotics for improving soil and water quality in aquaculture ponds. In Flegel TW (Ed.) Advances in shrimp biotechnology. Bangkok, Thailand: National Center for Genetic Engineering and Biotechnology.
Bureau of Meteorology (2021) ‘Monthly climate summary for Perth.’ Available at http://www.bom.gov.au/climate/current/month/wa/archive/201606.perth.shtml [Verified 28 October 2021]
Caron DA (1994) Inorganic nutrients, bacteria and the microbial loop. Microb Ecol 28:295–298
Chiayvareesajja S, Boyd CE (1993) Effects of zeolite, formalin, bacterial augmentation, and aeration on total ammonia nitro- gen concentrations. Aquaculture 116:33–45
Cole AJ, Tulsankar SS, Saunders BJ, Fotedar R (2019) Effects of pond age and a commercial substrate (The Water Cleanser™) on natural productivity, bacterial abundance, nutrient concentrations, and growth and survival of marron (Cherax cainii Austin, 2002) in semi-intensive pond culture. Aquaculture 502:242–249. https://doi.org/10.1016/j.aquaculture.2018.12.046
Cui H, Bai M, Li C, Liu R, Lin L (2018) Fabrication of chitosan nanofibers containing tea tree oil liposomes against Salmonella spp. in chicken. Lwt - Food Sci Technol 96:671–678. https://doi.org/10.1016/j.lwt.2018.06.026
David GS, Carvalho ED, Lemos D, Silveira AN, Dall’Aglio-Sobrinho M (2015) Ecological carrying capacity for intensive tilapia (Oreochromis niloticus) cage aquaculture in a large hydroelectrical reservoir in Southeastern Brazil. Aquacul Eng 66:30–40. https://doi.org/10.1016/j.aquaeng.2015.02.003
De Paiva-Maia E, Alves-Modesto G, Otavio-Brito L, Oliveira A, Vasconcelos-Gesteira TC (2013) Effect of a commercial probiotic on bacterial and phytoplankton concentration in intensive shrimp farming (Litopenaeus vannamei) recirculation systems. Lat Am J Aquat Res 41(1):126–137
De Souza DM, Suita SM, Leite FPL, Romano LA, Wasielesky W, Ballester ELC (2012) The use of probiotics during the nursery rearing of the pink shrimp Farfantepenaeus brasiliensis (Latreille, 1817) in a zero exchange system. Aquac Res 43(12):1828–1837
Downs TM, Schallenberg M, Burns CW (2008) Responses of lake phytoplankton to micronutrient enrichment: a study in two New Zealand lakes and an analysis of published data. Aquat Sci 70(4):347–360
FAO. 2021. Fishery and aquaculture statistics. Global aquaculture production 1950–2019 (FishstatJ). In: FAO Fisheries division [online]. Rome. Updated 2021. www.fao.org/fishery/statistics/software/fishstatj/en
Hai NV, Buller N, Fotedar R (2009) Effects of probiotics (Pseudomonas synxantha and Pseudomonas aeruginosa) on the growth, survival and immune parameters of juvenile western king prawns (Penaeus latisulcatus kishinouye, 1896). Aquac Res 40(5):590–602. https://doi.org/10.1111/j.1365-2109.2008.02135.x
Hamlina HJ, Michaelsa JT, Beaulatona CM, Grahama WF, Dutta W, Steinbachb P, Losordoc TM, Schraderd KK, Maina KL (2008) Comparing denitrification rates and carbon sources in commercial scale upflow denitrification biological filters in aquaculture. Aquacult Eng 38(2):79–92
Hargreaves JA (1998) Nitrogen biogeochemistry of aquaculture ponds. Aquaculture 166(3–4):181–212
Holl CM, Otoshi C, Unabia CR (2011) Nitrifying biofilms critical for water quality in intensive shrimp RAS. Glob Aquacul Advocate 14:38–39
Hong HA, Le HD, Cutting SM (2005) The use of bacterial spore formers as probiotics. FEMS Microbiol Rev 29(4):813–835
Hu X, Cao Y, Wen G, Zhang X, Xu Y, Xu W, Xu Y, Li Z (2017) Effect of combined use of Bacillus and molasses on microbial communities in shrimp cultural enclosure systems. Aquac Res 48(6):2691–2705
Ingram, B.A., Hawking, J.H., & Shiel, R.J. 1997. Aquatic life in freshwater ponds: a guide to the identification and ecology of life in aquaculture ponds and farm dams in South Eastern Australia. Albury, Australia: The co-operative research centre for freshwater ecology.
Itoi S, Niki A, Sugita H (2006) Changes in microbial communities associated with the conditioning of filter material in recirculating aquaculture systems of the pufferfish Takifugu rubripes. Aquaculture 256(1–4):287–295. https://doi.org/10.1016/j.aquaculture.2006.02.037
Jensen FB (1996) Uptake, elimination and effects of nitrite and nitrate in freshwater crayfish (Astacus astacus). Aquat Toxicol 34:95–104
Jensen FB (2003) Nitrite disrupts multiple physiological functions in aquatic animals. Comp Biochem Physiol - A Mol Integr Physio 135(1):9–24. https://doi.org/10.1016/S1095-6433(02)00323-9
Jobson A, Cook FD, Westlake DWS (1972) Microbial utilization of crude oil. Appl Microbiol 23(6):1082–1089
Jones PL, Thanuthong T, Kerr P (2002) Preliminary study on the use of synthetic substrate for juvenile stage production of the yabby, Cherax destructor (Clark) (Decapoda: Parastacidae). Aquac Res 33(10):811–818
Jørgensen, S. E. (2008). Freshwater lakes. In Jørgensen, S. E., & Faith, B. D. (Eds.) Encyclopedia of ecology (1st ed.). Oxford, England: Elsevier.
Karadal O, Türkmen G (2017) Growth performance and substrate preference of juvenile mexican dwarf orange crayfish (Cambarellus patzcuarensis) in different substrate types. J Limnol Freshw Fisheries Res 3(3):167–173
Khatoon H, Yusoff FM, Banerjee S, Shariff M, Mohamed S (2007) Use of periphytic cyanobacterium and mixed diatoms coated substrate for improving water quality, survival and growth of Penaeus monodon Fabricius postlarvae. Aquaculture 271(1–4):196–205
Labbé JI, Ramos-Suárez JL, Hernández-Pérez A, Baeza A, Hansen F (2017) Microalgae growth in polluted effluents from the dairy industry for biomass production and phytoremediation. J Environ Chem Eng 5(1):635–643. https://doi.org/10.1016/j.jece.2016.12.040
Lananan F, Abdul Hamid SH, Din WNS, Ali N, Khatoon H, Jusoh A, Endut A (2014) Symbiotic bioremediation of aquaculture wastewater in reducing ammonia and phosphorus utilizing effective microorganism (EM-1) and microalgae (Chlorella sp.). Int Biodeterior Biodegradation 95:127–134
Lawrence, C., & Jones, C. (2002). Cherax. In: Holdich DM (Ed.) Biology of freshwater crayfish. Blackwell Science Ltd, 377–438.
Manthe DP, Malone RF (1987) Chemical addition for accelerated biological filter acclimation in closed blue crab shedding systems. Aquacult Eng 6(3):227–236. https://doi.org/10.1016/0144-8609(87)90006-9
Martinez-Porchas, M., Martinez-Cordova, L. R., Lopez-Elias, J. A., & Porchas-Cornejo, M. A. (2014). Bioremediation of aquaculture effluents. In: Microbial biodegradation and bioremediation, pp. 539–553.
Mires D (1995) Aquaculture and the aquatic environment: mutual impact and preventive management. Isr J Aquac-Bamidgeh 47:163–172
Morrissy NM (1990) Optimum and favourable temperatures for growth of Cherax tenuimanus (Smith 1912) (Decapoda: Parastacidae). Aust J Mar Freshw Res 41:735–746
Morrissy NM, Evans L, Huner JV (1990) Australian freshwater crayfish: aquaculture species. World Aquac 21:113–122
Nimrat S, Suksawat S, Boonthai T, Vuthiphandchai V (2012) Potential Bacillus probiotics enhance bacterial numbers, water quality and growth during early development of white shrimp (Litopenaeus vannamei). Vet Microbiol 159(3–4):443–450
Otoshi CA, Montgomery AD, Matsuda EM, Moss SM (2006) Effects of artificial substrate and water source on growth of juvenile pacific white shrimp, Litopenaeus vannamei. J World Aquaculture Soc 37:210–213
Pace, M. L., & Lovett, G. (2013). Primary production: the foundation of ecosystems. Fundamentals of ecosystem science. Elsevier, Amsterdam, 27–51. https://doi.org/10.1016/B978-0-08-091680-4.00002-0
Perumal, S., Thirunavukkarasu, A. R., & Pachiappan, P. (Eds.). 2015. Advances in marine and brackishwater aquaculture. Chennai, India: Springer (India) Pvt. Ltd.
Prabu E, Santhiya AAV (2016) An overview of bioremediation towards aquaculture. J Aquac Tropics 31(3–4):155–164
Romano N, Zeng C (2007) Acute toxicity of ammonia and its effects on the haemolymph osmolality, ammonia-N, pH and ionic composition of early juvenile mud crabs, Scylla serrata (Forskål). Comp Biochem Physiol 148(Part A):278–285. https://doi.org/10.1016/j.cbpa.2007.04.018
Sang HM, Ky LT, Fotedar R (2009) Dietary supplementation of mannan oligosaccharide improves the immune responses and survival of marron, Cherax tenuimanus (Smith, 1912) when challenged with different stressors. Fish Shellfish Immunol 27(2):341–348
Saoud IP, Garza de Yta A, Ghanawi J (2012) A review of nutritional biology and dietary requirements of redclaw crayfish Cherax quadricarinatus (von Martens 1868). Aquac Nutr 18(4):349–368
Schveitzer R, Arantes R, Baloi MF, Costódio PFS, Arana LV, Seiffert WQ, Andreatta ER (2013) Use of artificial substrates in the culture of Litopenaeus vannamei (biofloc system) at different stocking densities: effects on microbial activity, water quality and production rates. Aquacult Eng 54:93–103
Seymour EA (1980) The effects and control of algal blooms in fish ponds. Aquaculture 19:55–74
Southwell IA, Hayes AJ, Markham J, Leach DN (1993) The search for optimally bioactive Australian tea tree oil. Int Symp Med Aromat Plants 344:256–265
Subashchandrabose SR, Ramakrishnan B, Megharaj M, Venkateswarlu K, Naidu R (2011) Consortia of cyanobacteria/microalgae and bacteria: biotechnological potential. Biotechnol Adv 29(6):896–907. https://doi.org/10.1016/j.biotechadv.2011.07.009
Thompson FL, Abreu PC, Wasielesky W (2002) Importance of biofilm for water quality and nourishment in intensive shrimp culture. Aquaculture 203(3–4):263–278
Tulsankar SS, Cole AJ, Gagnon MM, Fotedar R (2020) Temporal variations and pond age effect on plankton communities in semi-intensive freshwater marron (Cherax cainii, Austin and Ryan, 2002) earthen aquaculture ponds in Western Australia. Saudi J Bio Sci 28(2):1392–1400
Uma Devi, K., Swapna, G., & Suneetha, S. (2014). Microalgae in bioremediation: sequestration of greenhouse gases, clearout of fugitive nutrient minerals, and subtraction of toxic elements from waters. In Das, S. (Ed.) Microbial biodegradation and bioremediation, Elsevier Inc (pp. 433–454).
Viau VE, Ostera JM, Tolivia A, Ballester ELC, Abreu PC, Rodríguez EM (2012) Contribution of biofilm to water quality, survival and growth of juveniles of the freshwater crayfish Cherax quadricarinatus (Decapoda, Parastacidae). Aquaculture 324–325:70–78
Viau VE, De Souza DM, Rodríguez EM, Wasielesky W, Abreu PC, Ballester ELC (2013) Biofilm feeding by postlarvae of the pink shrimp Farfantepenaeus brasiliensis (Decapoda, Penaidae). Aquac Res 44(5):783–794
Wang YB, Xu ZR, Xia MS (2005) The effectiveness of commercial probiotics in northern white shrimp Penaeus vannamei ponds. Fish Sci 71(5):1036–1041
Wang Z, Meng Y, Fan T, Du Y, Tang J, Fan S (2015) Phosphorus removal and N2O production in anaerobic/anoxic denitrifying phosphorus removal process: long-term impact of influent phosphorus concentration. Biores Technol 179:585–594
Wingfield M (2008) An updated overview of the Australian freshwater crayfish farming industry. Freshwater Crayfish 16:15–17
Zhihong, W., Shiguang, C., & Xin, C. (2010). Micro-nutrients effects on algae colony: growth rate and biomass response to various micro-nutrients and competitive inhibitions among multi-microelements. 2010 4th international conference on bioinformatics and biomedical engineering, ICBBE 2010, (9151009001000048), 1–8. https://doi.org/10.1109/ICBBE.2010.5516174
Zhou Q, Li K, Jun X, Bo L (2009) Role and functions of beneficial microorganisms in sustainable aquaculture. Biores Technol 100(16):3780–3786
Zokaeifar H, Babaei N, Saad CR, Kamarudin MS, Sijam K, Balcázar JL (2014) Administration of Bacillus subtilis strains in the rearing water enhances the water quality, growth performance, immune response, and resistance against Vibrio harveyi infection in juvenile white shrimp Litopenaeus Vannamei. Fish Shellfish Immunol 36(1):68–74
Acknowledgements
The described work was part of a Masters course of the first author, with no conflicts of interest related to this article.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. This work was supported by Marine Easy Clean Pty Ltd through an industry scholarship.
Author information
Authors and Affiliations
Contributions
The first (A.C.) and final (R.F.) authors contributed to the study conception and design. The first (A.C.) and second (S.T.) author contributed to the data collection, analysis, writing and revising of the manuscript. The third (B.S.) and fourth (R.F.) authors assisted in writing and revising the work. The first draft was written by the first author (A.C.) and reviewed by all authors. The final manuscript was read and approved by all authors for publishing.
Corresponding author
Ethics declarations
Ethics approval
Animal ethics approval was not required for working with freshwater crayfish, as they are crustaceans.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare that the research was conducted in the presence of a financial scholarship which could be construed as a potential conflict of interest. However, it should be noted that the funders had no role in the design of the study, the collection, analyses or interpretation of data, in the writing of the manuscript or in the decision to publish the results in a peer reviewed journal.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Handling Editor: Dr. Elena Mente
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cole, A.J., Tulsankar, S.S., Saunders, B.J. et al. Effects of an oil-based substrate (The Water Cleanser™) and bacterial additives on nitrogen and phosphorous dynamics in freshwater crayfish (Cherax cainii, Austin and Ryan 2002) aquaculture. Aquacult Int 30, 937–954 (2022). https://doi.org/10.1007/s10499-022-00842-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-022-00842-6