Abstract
The study shows how water temperature, water hardness, and color of tank affect the swim bladder inflation effectiveness (SBIE) and survival in Eurasian perch larvae in controlled conditions. Three separate experimental rearings (in 50-L tanks) were conducted, where (1) water temperature (12 days, 3000 ind./tank; 15, 20, and 25 °C), (2) water hardness (12 days, 4600 ind./tank; 15 °C; and 5, 10, and 15°n), and (3) tank wall color (20 days, 25,000 ind./tank; tanks with black and white walls; 15 °C) constituted tested variables. During all of the experiments, the photoperiod was 24 h (24L:0D), and the intensity of light was 1500 lx at the water surface. In experiments 1 and 2, perch larvae were not fed and in experiment 3, from 5-day post-hatch (DPH), they were fed ad libitum with Artemia sp. nauplii. In experiment 1, the highest SBIE was observed for larvae reared at 15 °C (20.6 ± 1.1%) for 20 °C and 25 °C; the maximum SBIE was significantly lower (8.4 ± 2.9% and 13.0 ± 5.8%, respectively; p < 0.05). The mortality trend was similar for all of the tested temperatures, although at higher temperatures, the larvae were seen to die sooner. In experiment 2, there were no statistical differences (p > 0.05) in mortality of larvae and in final SBIE. The results of experiment 3 clearly suggests that the white tanks caused a significant (p < 0.05) reduction in survival rate (4.9 ± 3.2% and 24.6 ± 6.4%, for white and black tanks, respectively) as well as SBIE (7.7 ± 2.7% and 32.9 ± 3.6%, for white and black tanks, respectively; p < 0.05). The outcomes indicate that Eurasian perch larval rearing at 15 °C in black tanks is preferred.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Eurasian perch, Perca fluviatilis L., is one of the most promising candidates for diversification of European intensive freshwater aquaculture. Despite the huge progress in the field of Eurasian perch larviculture, there is still very limited data available on the factors determining the effectiveness of the initial larvae rearing—during the first days and weeks of life—under controlled conditions and that is when, most of the crucial changes in fish morphology and physiology occur (Urho 2002), as for example the commencement of exogenous feeding and swim bladder inflation (Battaglene and Talbot 1993; Czesny et al. 2005).
Papers aimed at larviculture of perch have been focused mainly on the final outcome of the rearing. The data available in the literature provide some general principles of larval rearing where, e.g., optimal thermal regimes (around 18–23 °C), first feeding protocols (small Artemia between 2 and 4 DPH), and weaning strategies (co-feeding between 21 and 24 DPH) could be found (see Kestemont et al. 2008). However, the data published so far has mostly been obtained from different, not standardized initial rearing protocols, which makes a comparison of the published data very difficult (see Table 1). The published results are sometimes even contradictory, where the same variable (i.e., black tank wall color) was reported to have both a positive (Jentoft et al. 2006) and a negative (Tamazouzt et al. 2000) effect on the rearing effectiveness. In effect, according to the published data, aquaculturists have accessible protocols for rearing juveniles and adult fish, but almost no protocols for efficient initial rearing phase of the larvae.
Swim bladder inflation is an extremely important event during the early larval stage in most fish species. Fish with a non-inflated swim bladder (if they are able to survive) spend more energy to keep the body in horizontal position, have problems with finding food, and more often exhibit skeletal deformities (Ostaszewska 2005; Ostaszewska et al. 2005; Kestemont et al. 2015). It has been reported that the effectiveness of swim bladder inflation is highly dependent on abiotic factors, including photoperiod, turbidity of water, salinity, and temperature (Hadley et al. 1987; Battaglene and Talbot 1993; Martín-Robichaud and Peterson 1998). Although Eurasian perch is a species which can survive without an inflated swim bladder (Egloff 1996), swim bladder inflation effectiveness (SBIE) is one of the most limiting factors in larviculture of freshwater percids (e.g., Bein and Ribi 1994; Kestemont and Melard 2000). Perch is a physoclistous fish species in which swim bladder is formed as a dorsal part of a gut. Connection of swim bladder with the gut is lost during the larval metamorphosis. Around 4 DPH perch larvae starts to fill their swim bladder by swimming to the surface and swallowing the air (this moment also coincided with the onset of exogenous feeding). Air gulped from the surface is forced through the pneumatic duct into the swim bladder (e.g., Żarski et al. 2011). This makes the inflation of swim bladder highly crucial process in this species, especially that in both—commercial and lab conditions—highly variable and very often low (sometimes did not exceed 10%) inflation of swim bladder is observed (own data and personal communication of P. Fontaine). But to date, information about factors that determine swim bladder inflation in Eurasian perch larvae is still very scarce and contradictory. It also includes the temperature which was found to be important factors affecting fish rearing effectiveness (e.g., Nwosu and Holzlohnev 2000; Trottera et al. 2003; Silva et al. 2005).
The aim of the study was to investigate how water hardness, water temperature, and tank wall color affect the swim bladder inflation effectiveness and survival of Eurasian perch larvae reared under controlled conditions.
Materials and methods
Larvae source for experimental studies
Eurasian perch larvae, used in experiments, were obtained after induced spawning of wild spawners. Twenty females (with an average weight of 240 ± 55 g) and 15 males (with an average weight of 130 ± 80 g) were kept in the hatchery of the Aquaculture and Ecological Engineering Center (University of Warmia and Mazury, Olsztyn, Poland) where they were reproduced with the method described by Żarski et al. (2017). Fertilized egg ribbons were incubated in a recirculating aquaculture system (RAS) in square-shaped 50-L tanks at 14 ± 0.1 °C at constant light conditions (~ 100 lx). The temperature was raised to 15 °C when the first hatched larvae were observed. Twelve hours after the first larvae hatching, the unhatched eggs were removed. This moment was considered as 0 DPH (0 days post-hatched). The average wet body weight (WBW) and total length (TL) of 0 DPH larvae were 1.01 ± 0.06 mg and 5.83 ± 0.18 mm, respectively.
Rearing conditions and measurements
Three different experiments on Eurasian perch larvae were conducted: (1) “water temperature” rearing, (2) “water hardness” rearing, and (3) “tank wall color” rearing. During all of the experiments, larvae were reared in the same RAS systems, consisting of head and sump tanks with rearing tanks in between. Moreover, systems were equipped with biological filtration (moving-bed filter), aeration, UV sterilizers, automatic control of temperature (± 0.2 °C), and light control system. The photoperiod was 24 h (24L:0D), and the intensity of light was 1500 lx at the water surface. The concentration of ammonia was measured every day (with the photometer DR5000 Hach Lange, Berlin, Germany) and it was below 0.01 mg L−1 throughout all of the rearing periods. The determination of oxygen saturation was made twice a day, with a use of an oxygen probe (with a Handy Polaris 2.0 OxyGuard, Farum, Denmark), and all the time was greater than 80%. All tanks were supplied with water from the top-inlet (full water exchange in the rearing tanks occurred after 1 h), but no surface skimmer was applied and the water level in the tanks was set at 10 cm. In experiments 1 and 2, TL (± 0.01 mm, with the use of stereo microscope; Zeiss steREO Discovery V.20, Jena, Germany), WBW (± 0.1 mg, with the use of electronic precise balance, Kern ABJ 120-4M, Germany), and swim bladder inflation effectiveness (SBIE, %) were determined daily. The evaluation of WBW, TL, and SBIE in experiment 3 was made only on the last day of the experiment.
Determination of SBIE was made with the use of stereo microscope, by triple counting (on Petri dishes) the randomly caught larvae with and without swim bladder filled (in total, around 400 larvae were counted each time). Moreover, measurements of the WBW were performed by a harmless method. This was done by means of a platform of nylon net with a 200 μm mesh. Larvae (n = 60 for each experimental treatment) were placed on the net and excess water was drained off with the use of filter paper. This method helped to avoid any physical damage to the body of the larvae (as described by Krejszeff et al. 2013). Before manipulations related with measurements, larvae were anesthetized in MS-222 solution (at a dose of 150 mg L−1). After the measurements, larvae were recovered in a separate tank and returned to the rearing tanks from which they were collected.
“Water temperature” rearing
During these experiments, the effect of different thermal regimes (15, 20, and 25 °C) on the SBIE and survival of the larvae was investigated. Freshly hatched larvae (0 DPH) were stocked into rearing tanks (3000 ind. in each tank) with black tank wall color, working in RAS. For each temperature, separate RAS systems (with the same construction) were used. At the beginning, the temperature in all of the tanks was set at 15 °C. In the tanks where temperatures of 20 °C and 25 °C were required, the initial temperature was raised steadily (1° every 1 h) just after stocking the larvae. Eurasian perch larvae were not fed throughout the entire experiment. Every day, TL and WBW of 20 randomly chosen larvae from each tank (n = 60 for each water temperature) were determined. At the end of day 0, dead larvae were removed and counted in order to estimate the initial mortality of the larvae. Also, each morning, dead larvae were carefully removed and counted in order to determine the daily mortality rate. Starting from 4 DPH (when the first larvae were found to inflate the swim bladder), the SBIE of the larvae were determined every day until the end of the rearing period, which was manifested by the excessive mortality of the larvae (for larvae kept at 15 °C, it was 12 DPH, and for larvae at 20 °C and 25 °C, it was 10 and 7 DPH, respectively). The experiment was performed in three replications for each treatment group.
“Water hardness” rearing
During the experiment, the effect of different water hardness (WH) (5, 10, and 15°n, where 1°n = 10 mg CaO/dm3) on the SBIE and survival of the larvae was investigated. Freshly hatched larvae (0 DPH) were stocked into rearing tanks (around 4600 ind. in each tank) with black tank wall color at 15 °C, working in RAS (the same as in experiment 1). For each WH, separate RAS systems (with the same construction) were used. To keep the proper WH in each tank, the total WH, magnesium (Mg 2+), and calcium (Ca 2+) were determined every day by the wersenian method (according to Siepak 1992; Hermanowicz et al. 1999). Perch larvae were not fed during all of the rearing period. Every day, TL and WBW of 30 randomly chosen larvae from each tank (n = 60 for each WH) were calculated. Moreover, every day, mortality of the larvae from each tank was counted, and starting from 4 DPH, also the SBIE of the larvae were determined until the end of the rearing. The experiment was performed in three replications for each treatment group.
“Tank wall color” rearing
Freshly hatched larvae (0 DPH) were stocked into a six rearing 50-L tanks (25,000 ind. in each tank)—three with white tank wall color (wTWC) and three with black tank wall color (bTWC). In this experiment, a much higher stocking density was used to mimic the common high density commercial conditions (up to 500 ind. per L). For this experiment, three different RAS were used. Each RAS was composed of two tanks—one with wTWC and one with bTWC. The water flow and light conditions were the same as in experiments 1 and 2. In each tank, the temperature was set to 15 °C. In this experiment, larvae were fed ad libitum two times a day with Artemia sp. (SF origin, Inve Aquaculture, Belgium) from 5 DPH, and starting from this day, tanks were also cleaned once a day (every morning before feeding). On day 20 of the experiment (the last day of rearing), SBIE, TL, and WBW were recorded (on the basis of n = 60 of Eurasian perch larvae from each tank). Survival rate (S, %) was verified only at the end of the experiment on the basis of the number of live larvae at the end of the experiment in each rearing tank. The number of dead larvae was not counted during the rearing period (as in experiments 1 and 2) since uneaten food and feces made the counting of the dead larvae unreliable (the counting of dead larvae was hindered by the uneaten food and feces). Additionally, on the last day of the experiment, the presence of food in the digestive system was recorded (by observing larvae under a stereoscopic microscope) during the evaluation of the SBIE, which allowed the foraging rate of the larvae between the treatments to be compared (Table 2).
Data analysis and statistics
The statistical differences for “water temperature” and “water hardness” rearing were analyzed with one-way analysis of variance (ANOVA) and Tukey’s post hoc test at the significance level below 5% (p < 0.05). For “tank wall colour” rearing, the data were analyzed with the t test at a significance level of 5% (p < 0.05). Before the analysis, the data expressed in the percentage values (S and SBIE) were arcsine transformed. The statistical analysis was performed with Microsoft Excel and STATISTICA (data analysis software system) version 10 (StatSoft Inc., Tulsa, USA).
Results
“Water temperature” rearing
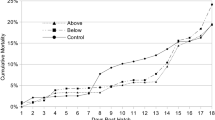
For 0 DPH, there were no significant differences between the temperature treatments (15, 20, and 25 °C) for TL, WBW (p > 0.05), and SBIE (p > 0.05) (see Figs. 1, 2, and 4). At the first DPH, larvae reared at 25 °C had the highest TL (p < 0.05). The TL of larvae reared at 15 °C and 20 °C was similar until 5 DPH. Significant differences between temperature treatments occurred on 6 DPH and 7 DPH for TL, WBW, and SBIE (see Figs. 1, 2, and 4). The significantly different (p < 0.05) order of preference was 15 > 20 > 25 °C for TL, WBW, and SBIE. Although in the general trend, larvae reared at lower-tested temperatures reached maximum TL later, in effect, they were able to reach higher TL during the experimental period (Fig. 1). For WBW, regardless of the temperature tested, from the moment that the fish reached the maximum WBW, this parameter continuously decreased until the end of the experiment (Fig. 2). The cumulative mortality increased over time in all of the treatments, and the larvae at higher temperatures were found to die sooner (Fig. 3). For 15 °C and 25 °C treatments, SBIE increased initially but later decreased over time (Fig. 4). For 20 °C treatment, SBIE did not have a distinct trend. The highest SBIE for larvae reared at 15 °C and 20 °C was observed on 8 DPH (20.6 ± 1.1% and 13.0 ± 5.8% for 15 and 20 °C, respectively; p < 0.05).
Changes in Eurasian perch larvae total length (TL, mm) reared till 12 DPH (days post-hatch) in three temperatures 15, 20, and 25 °C. Data (mean ± SD) between the groups among the same DPH marked with different letters were statistically different (p < 0.05). For clarity, neighboring data points without statistical differences were encircled
Changes in Eurasian perch larvae wet body weight (WBW, mg) reared till 12 DPH (days post-hatch) in three temperatures 15, 20, and 25 °C. Data (mean ± SD) between the groups among the same DPH marked with different letters were statistically different (p < 0.05). For clarity, neighboring data points without statistical differences were encircled
Cumulative mortality (CM) of Eurasian perch larvae reared till 12 DPH (days post-hatch) in three temperatures 15, 20, and 25 °C. Data (mean ± SD) between the groups among the same DPH marked with different letters were statistically different (p < 0.05). For clarity, neighboring data points without statistical differences were encircled
“Water hardness” rearing
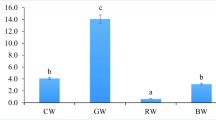
There were no differences (p > 0.05) in TL and WBW of the larvae from the tanks with different water hardness (WH) at the end of the experiment (Table 2). There were also no statistical differences (p > 0.05) in CM of larvae during all of the experiment period (Fig. 5). The final SBIE was 17.7 ± 4.8%, 17.5 ± 8.2%, and 16.9 ± 6.4% for 5, 10, and 15°n of WH, respectively) but there were also no statistical differences observed (p > 0.05) (Table 2).
Cumulative mortality (CM) of Eurasian perch larvae reared till 12 DPH (days post-hatch) in three different water hardness 5, 10, and 15°n. Data (mean ± SD) between the groups among the same DPH marked with the same letter were statistically the same (p < 0.05). For clarity, neighboring data points without statistical differences were encircled
“Tank wall color” rearing
On the last day of rearing (20 DPH), Eurasian perch larvae from wTWC had significantly lower TL and WBW (p < 0.05) than those from bTWC (Table 2). The survival rate and SBIE of larvae from wTWC were also lower (p < 0.05) than those from bTWC. There were no differences in terms of the foraging rate between the two treatments (Table 2).
Discussion
It has to be pointed out that freshwater larviculture suffers from a very little information available on the initial rearing protocols. That is why, it is very important that obtained result showed—for the first time—how water temperature, water hardness, and tank wall color affect the swim bladder inflation effectiveness and survival in perch larvae, during first weeks of rearing in laboratory conditions. Obtained data enable also to create standardized protocols for early rearing of Eurasian perch larvae, what could have important meaning both for aquaculturists and scientist.
In the case of fish that are ectothermic vertebrates, water temperature is one of the main factors that has important influence for eggs development, hatching and survival of the larvae, behavior, utilization of yolk sac, and what goes with it for growth rate (e.g., Szumiec 1988; Nwosu and Holzlohnev 2000). The percids are classified as temperature mesotherms, for which the optimum temperature for adult fish is 25 °C (Hokanson 1977). However, lack of the standardize protocols for rearing perch larvae caused that they were reared in the entire temperature range from 10 °C (Bein and Ribi 1994) to 23 °C (Kestemont et al. 2003; Mandiki et al. 2004, 2007) (see also Table 1). Meanwhile, as Hokanson and Kleiner (1974) showed in the yellow perch, Perca flavescens, temperature has a huge influence on the life expectancy of larvae with yolk sac feeding. This knowledge is important for aquaculturists to the extent that it may have an impact on success or failure of whole rearing. Data obtained in this study showed clearly that in 25 °C, the perch larvae quickly gained weight and become the longest, but it was connected also with the fastest mortality if the proper time of food application would be overlook. Meanwhile, the larvae from the tanks with 15 °C died at the slowest, which resulted in the higher SBIE (20.6 ± 1.1%), while larvae reared in 25 °C reached SBIE only 8.4 ± 2.9%. Obtained results cannot be compared unambiguously with other data, because similar experiment, to this date, was never conducted on perch larvae, only on the larvae of different fish species, as for example on striped trumpeter, Latris lineata, which requires totally different rearing protocols (Trottera et al. 2003).
Water hardness as a concentration of calcium (Ca2+) and magnesium (Mg2+) can affect many stages of fish rearing. It has been shown, for example that eggs swelling is faster and more efficient in water of low hardness (Spade and Bristow 1999), while hatching and survival of the larvae according to water hardness are species-specific (e.g., Ketola et al. 1988; Silva et al. 2005; Kalenda et al. 2007), and Kalenda et al. (2007) observed also SBIE and survival in pikeperch, Sander lucioperca, larvae according to water hardness. Still, there were no data about potential effect of different water hardness on SBIE in perch larvae. Data obtained during experiment 2 shows that water with different degrees of hardness (5, 10, and 15°n) had no significant effect, neither on TL and WBW of the perch larvae nor on their SBIE and survival. The lack of correlation between water hardness and SBIE was observed also by Kalenda et al. (2007) in pikeperch larvae. But in contrast to perch larvae, Kalenda et al. (2007) observed the highest survival, TL, and weight of pikeperch larvae in the group with the highest, from the applied, water hardness (174.85 mg/L CaCO3). Observed differences resulted most likely from the other needs of pikeperch and perch larvae (as a different species) and because Kalenda et al. (2007) conducted their experimental rearing longer and fed the pikeperch larvae ad libitum with Artemia sp. nauplii.
Artificial environments, wherein most of the fish rearing took place, are mostly totally different than natural habitats. Tank wall color (as one of the artificial environment elements) was found to be modulatory factor affecting the behavior of particular fish species and thus having a direct effect on the rearing effectiveness. This concerns, among others, foraging behavior (by having influence on the visibility and contrast of the food in the tank) as well as SBIE (Martín-Robichaud and Peterson 1998; Cobcroft et al. 2012; Sebesta et al. 2018). In Eurasian perch, it was reported that tanks with black walls had a positive effect on the growth rate during the first 5 weeks of rearing without a negative effect on survival rate (Jentoft et al. 2006). On the other hand, Tamazouzt et al. (2000) reported that the black tanks significantly affected lower survival (3%) compared to the white tanks (17% survival rate) within the first 2 weeks of rearing the perch. However, none of those studies investigated the effect of tank wall color on the SBIE. In the current study, the lower SBIE for the larvae reared in wTWC could be associated with the “walling behavior,” reported to be a significant issue in marine species (Cobcroft and Battaglene 2009; Cobcroft et al. 2012). In white tanks, photopositive larvae (such as percids; Manci et al. 1983) exhibited a tendency to gather close to the tank walls being attracted by the bright wall rather than the less “attracting” surface. The current study clearly indicates a positive effect of bTWC, which affected not only significantly higher growth and survival rate but also SBIE compared to fish reared in wTWC. There was also no effect on the foraging rate, indicating that larvae in both cases could easily find the food offered (see Table 2). A clear comparison of the factors responsible for the differences observed is very difficult, as all studies used different approaches. The main common feature of the work of Jentoft et al. (2006) and the present study is the constant light condition provided, which, regardless of the intensity (maximum 150 lx applied by Jentoft et al. (2006) and 1500 lx in our study), could be recommended to be applied during the initial larvae rearing of Eurasian perch.
Obtained data for the first time showed how water temperature, water hardness, and the tank wall color affect the SBIE and survival in perch larvae. It was observed that during the first 12 DPH, the slowest cumulative mortality and what is associated with it, the significantly higher SBIE was in perch larvae kept at temperature 15 °C. It was also proven that in the early period of perch larvae life, water hardness did not have any significant effect neither on survival nor on SBIE. Eventually, proven that larvae kept in the bTWC have much higher survival rate than larvae kept in wTWC, also the SBIE was significantly higher in the case of larvae from bTWC.
The process of filling the swim bladder involves breaking the water surface by larvae and gulping the air which then passes from the digestive tract and through the pneumatic duct into the swim bladder, and it often coincides with the beginning of exogenous feeding. There are two events conditioning SBIE. First, consider pneumatic duct—connecting the digestive tract to the swim bladder—which undergoes atrophy over some time being limiting factor strictly related to developmental advancement of the fish and, consequently, time period when this process is practically possible to occur. Second event, consider the size of the air bubbles swallowed by the larvae. If they will be too big, they will not pass through pneumatic duct and may stay in the gut creating potential threat to the larvae (Rieger 1995; Suchocki 2017). It has to be pointed out, however, that in our study, the larvae were not fed throughout the experiment. It cannot be ruled out that the SBIE could be even more improved when the larvae would have food offered, but in order to verify this hypothesis, a very detailed study involving separate rearing of a single individual should be proceeded.
All of these results are very important from the point of view of the introduction of standardized rearing protocols for perch larvae and serve as a basis for further improvement of perch production effectiveness and for its cost-effectiveness. However, it would be interesting to examine in greater detail in the future, whether feeding the larvae will affect in any way the efficiency of filling the swim bladder in perch. Nevertheless, the outcomes of this study allow to state that in order to achieve the highest SBIE and the highest survival rate in perch, the larvae should be reared for at least 12 DPH at 15 °C in black tanks.
References
Baras E, Kestemont P, Melard C (2003) Effect of stocking density on the dynamics of cannibalism in sibling larvae of Perca fluviatilis under controlled conditions. Aquaculture 219:241–255
Battaglene SC, Talbot RB (1993) Effects of salinity and aeration on survival of and initial swim bladder inflation in larval Australian bass. Progres Fish-Cult 55:35–39
Bein R, Ribi G (1994) Effect of larval density on the development of perch larvae (Perca fluviatilis L.). Aquat Sci 56:97–105
Cobcroft JM, Battaglene SC (2009) Jaw malformation in striped trumpeter Latris lineata larvae linked to walling behavior and tank colour. Aquaculture 289:274–282
Cobcroft JM, Shu-Chien AC, Kuah M-K, Jaya-Ram A, Battaglene SC (2012) The effects of tank colour, live food enrichment and greenwater on the early onset of jaw malformation in striped trumpeter larvae. Aquaculture 356–357:61–72
Cuvier-Peres A, Kestemont P (2002) Development of some digestive enzymes in Eurasian perch larvae Perca fluviatilis. Fish Physiol Biochem 24:279–285
Czesny SJ, Brian D, Graeb S, Dettmers JM (2005) Ecological consequences of swim bladder non inflation for larval yellow perch. Trans Am Fish Soc 134:1011–1020
Egloff M (1996) Failure of swim bladder inflation of perch, Perca fluviatilis L. found in natural populations. Aquat Sci 58:15–23
Hadley CG, Rust MB, Van Eenennaam JP, Doroshov SI (1987) Factors influencing initial swim bladder inflation by striped bass. Am Fish Soc Symph 2:164–169
Hermanowicz W, Dozańska W, Dojlido J, Koziorowski B (1999) Fizyczno-chemiczne badania wody i ścieków. 847 pp. Arkady, Warszawa
Hokanson KEF (1977) Temperature requirements of some percids and adaptation to the seasonal temperature cycle. J Fish Res Board Can 34:1524–1550
Hokanson KEF, Kleiner CF (1974) Effects of constant and rising temperatures on survival and developmental rates of embryonic and larval yellow Perch, Perca flavescens. In: Blaxter JHS (ed) The early life history of fish. Springer-Verlag, Berlin and New York, pp 437–448
Jentoft S, Øxnevad S, Aastveit AH, Andersen Ø (2006) Effects of tank wall color and up-welling water flow on growth and survival of Eurasian perch larvae (Perca fluviatilis). J World Aquacult Soc 37:313–317
Kalenda V, Baránek V, Dvořák J, Kopp R, Mareš J, Spurný P (2007) Effect of different water hardness on growth, survival and swim bladder inflation of pikeperch (Sander lucioperca) larvae. VII th International conference of PhD and MSc students “Genetics and Animal Breeding”, Brno pp. 49–66
Kestemont P, Melard C (2000) Aquaculture. In: Craig JF (ed) Percid fishes: systematics, ecology and exploitation. Blackwell Science, Oxford, UK, pp 191–224
Kestemont P, Jourdan S, Houbart M, Melard C, Paspatis P, Fontaine P, Cuvier A, Kentouri M, Baras E (2003) Size heterogeneity, cannibalism and competition in cultured predatory fish larvae: biotic and abiotic influences. Aquaculture 227:333–356
Kestemont P, Rougeot C, Musil J, Toner D (2008) Chapter 5. In: Farming of Eurasian Perch, larval and juvenile production. BIM, Dublin, pp 30–41 24
Kestemont P, Mélard C, Held JA, Dabrowski K (2015) Culture methods of Eurasian perch and yellow perch early life stages. In: Kestemont P, Dąbrowski K, Summerfelt RC (eds) Biology and culture of percid fishes. Springer Netherlands, Dordrecht, pp 265–293
Ketola HG, Longacre D, Greulich A (1988) High calcium concentration in water increases mortality of salmon and trout eggs. Prog Fish-Cult 50:129–135
Krejszeff S, Żarski D, Palińiska-Żarska K, Trabska I, Kupren K, Targońska K, Bowszys M, Kucharczyk D (2013) Procedure for harmless estimation of fish larvae weight. It J Anim Sci 12:270–274
Manci WE, Malison JA, Kayes TB, Kuczynski TE (1983) Harvesting photopositive juvenile fish from a pond using a lift net and light. Aquaculture 34:157–164
Mandiki SNM, Blanchard G, Mélard C, Koskela J, Kucharczyk D, Fontaine P, Kestemont P (2004) Effects of geographic origin on growth and food intake in Eurasian perch (Perca fluviatilis L.) juveniles under intensive culture conditions. Aquaculture 229:117–128
Mandiki SNM, Babiak I, Krol J, Rasolo JFR, Kestemont P (2007) How initial predator-prey ratio affects intra-cohort cannibalism and growth in Eurasian perch Perca fluviatilis L larvae and juveniles under controlled conditions. Aquaculture 268:149–155
Martín-Robichaud D, Peterson RH (1998) Effects of light intensity, tank colour and photoperiod on swimbladder inflation success in larval striped bass, Morone saxatilis (Walbaum). Aquac Res 29:539–547
Mélard C, Baras E, Mary L, Kestemont P (1996) Relationships between stocking density, growth, cannibalism and survival rate in intensively cultured larvae and juveniles of perch (Perca fluviatilis). Ann Zool Fenn 33:643–651
Nwosu FM, Holzlohnev S (2000) Influence of temperature on eggs hatching, growth and survival of larvae of Heterobranchus longifilis. (Teleostei, Clariidae). J Appl Ichth 16:20–23
Ostaszewska T (2005) Developmental changes of digestive system structures in pike-perch (Sander lucioperca L.). Electr J Ichth 1:65–78
Ostaszewska T, Dabrowski K, Czuminska K, Olech W, Olejniczak M (2005) Rearing of pike-perch larvae using formulated diets - first success with starter feeds. Aquac Res 36:1167–1176
Ribi G (1992) Perch larvae (Perca fluviatilis L.) survive better in dilute sea water. Aquat Sci 54:85–90
Rieger PW (1995) Direct evidence of physotomous gas bladder inflation in physoclistous fish larvae. In: Report for fisheries and aquatic sciences. Mead & Hunt, Inc., Madison, WI
Sebesta R, Stejskal V, Matousek J, Lundova K (2018) The effect of light intensity and tank wall colour on survival and growth of peled Coregonus peled Gmelin 1788 larvae. TrJFAS 19: (in press)
Siepak J (1992) Fizyczno-chemiczna analiza wód i gruntów. 193 pp. UAM, Poznań
Silva LVF, Golombieski JI, Baldisserotto B (2005) Growth and survival of silver catfish larvae, Rhamdia quelen (Heptapteridae), at different calcium and magnesium concentrations. Neotrop Ichth 3:299–304
Spade S, Bristow B (1999) Effects of increasing water hardness on egg diameter and hatch rates of striped bass eggs. N Am J Aquacult 61:263–265
Suchocki CR (2017) The role of Phototaxis in the initial swim bladder inflation of larval yellow perch (Perca Flavescens), Theses and Dissertations 1706, 62 pp
Szumiec MA (1988) A management model of carp growth in ponds. Arch Pol Fish 6(1):83–95
Tamazouzt L, Chatain B, Fontaine P (2000) Tank wall colour and light level affect growth and survival of Eurasian perch larvae (Perca fluviatilis L.). Aquaculture 182:85–90
Trottera AJ, Pankhurst PM, Moreheadb DT, Battaglene SC (2003) Effects of temperature on initial swim bladder inflation and related development in cultured striped trumpeter (Latris lineata) larvae. Aquaculture 221:141–156
Urho L (2002) Characters of larvae – what are they? Folia Zool 51:161–186
Vlavonou RS, Masson G, Moreteau J (1999) Growth of Perca fluviatilis larvae fed with Artemia spp. nauplii and the effects of initial starvation. J Appl Ichth 15:29–33
Żarski D, Palińska K, Targońska K, Bokor Z, Kotnik L, Kreszeff S, Kupren K, Horvath A, Urbanyi B, Kucharczyk D (2011) Oocyte quality indicators in Eurasian perch, Perca fluviatilis L., during reproduction under controlled conditions. Aquaculture 311:84–91
Żarski D, Horváth A, Bernáth G, Krejszeff S, Radóczi J, Palińska-Żarska K, Bokor Z, Kupren K, Urbanyi B (2017) Controlled reproduction of wild Eurasian perch: a hatchery manual. Springer Briefs in Environmental Science, Netherlands, p 102
Acknowledgments
Special thanks to Dariusz Kucharczyk for his support during the experimental period as well as to Joanna Nowosad and Kacper Kwasek for their valuable technical assistance during the rearing procedure.
Funding
This study was financed by the project “Innovations in finfish aquaculture with special reference to reproduction” (acronym: InnovaFish), Operational Programme “Sustainable Development of the Fisheries Sector and Coastal Fishing Areas 2007-2013” (OR14-61724-OR1400003/09/10/11).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed by the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Palińska-Żarska, K., Krejszeff, S., Łopata, M. et al. Effect of water hardness, temperature, and tank wall color, on the effectiveness of swim bladder inflation and survival of Eurasian perch (Perca fluviatilis, L.) larvae reared under controlled conditions. Aquacult Int 27, 931–943 (2019). https://doi.org/10.1007/s10499-018-0333-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-018-0333-4