Abstract
The aim of this study was to determine the influence of the fillet structure on the deboning force required to remove salmon and trout pin bones. Salmon and trout fillets with differing fillet structure were used, in order to study the importance of the fillet structure on the deboning process. In the first test naturally gaping and non-gaping fillets were compared. To confirm the role that the collagen plays within the fillet structure, the fillets underwent series of treatments. Fillets were put into (i) a collagenase solution to remove the collagen in the fillet and (ii) a calcium chloride solution to determine if collagen was the main influential factor. Both treated salmon and trout fillets were again compared to untreated fillets from the same batch. The results indicate that collagenase and calcium chloride have a large interaction on deboning force compared to water or no treatments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fish is a major part of the human diet that contains important nutritive substance that have a positive effect on human health and have been shown to negate certain diseases (Fuentes-Gandara et al. 2018). Salmo salar (Atlantic salmon) and Oncorhynchus mykiss (rainbow trout) contain a relatively similar protein content of 20% and provide all essential amino acids that are required to maintain the human body (Fuentes-Gandara et al. 2018). As with most foodstuff, consistency in the chemical composition differs due to the fish species, sex, age, environment, diet and season of harvest (Hernández-Martínez et al. 2013).
Rigour mortis is an important phase in which the flesh of the fish turns into meat and takes approximately a maximum of 5 days (to conclude) for Atlantic salmon (Skjervold et al. 2001; Kiessling et al. 2006). The fillet size shrinks by 10 to 15% due to muscle contractions during rigour mortis, which leads to a firmer structure of the fillet and a darker red colour (Einen et al. 2002). Texture defects like gaping and muscle softness can occur after the rigour mortis process due to the different pre- and post-mortem treatments (Skjervold et al. 2001; Cheng et al. 2014), in the case of this study, post-mortem treatments.
Collagen is the structure protein in fish and serves as the scaffold of the fish muscle (Tierney et al. 2009). It is found in the skin, bones, tendons, teeth and blood vessels of the fish (Suphatharaprateep et al. 2011). Collagen plays an important role in the texture and structure of the flesh as it is responsible for the stability of the muscles (Cheng et al. 2014), represents 30% of the total protein content of the fish and is the main structural component of the connective tissue (Darmanto et al. 2014). Fish collagen is different from mammalian collagen as it has a lower temperature of denaturation and lower hydroxyproline content (Safandowska and Pietrucha 2013). The important characteristics of fish collagen are its thermal stability and mechanical strengths (Tierney et al. 2009).
Gaping occurs through the collapsing of muscle fibrils and connective tissues, which affects the structure of the fillet (Skjervold et al. 2001; Cheng et al. 2014). The collapsing originates from the disconnection of muscle fibres and can be affected by seasonality, the rapid growth of the fish and maturity of farmed fish, temperature during rigour and the muscle activity of the fish (Skjervold et al. 2001; Roth et al. 2006). Often, these factors often occur in combination; this increases the gaping within the fish muscle and therefore affects the quality of the saleable fish (Roth et al. 2006). Jacobsen et al. (2015) showed that leaving blood and other contaminate in the abdominal area post rigour would leave to higher gaping than any other factor.
Balaban et al. (2015) investigated the removal of pin bones from salmon, kahawai and red snapper and found that a method using a texture analyser to remove pin bones and record the force required and the force required to break pin bones was a good way of providing qualitative data on the removal of pin bones from species. Information on the removal of pin bones is very limited. The aim of this study is to understand if treatments with collagenase, calcium chloride or water treatments and the fillet structure (gaping or not gaping) affect the deboning force of salmon and trout species.
Materials and methods
Fresh fish samples of Salmo salar (Atlantic salmon) and Oncorhynchus mykiss (rainbow trout) were obtained from Dawnfresh Seafoods Ltd. and were classified industrially into the 4–5-kg weight range, all samples were female triploids. Each farmed fish sample were fileted but not deboned and was provided post rigour and analysed before 6 days from harvest. All samples were quality-assessed industrially and analysed in triplicate.
A TA.XT plus texture analyser (Stable Microsystems, Surrey, UK) with a friction rig attachment and 50-kg load cell was used to measure the force required to remove pin bones from the fish fillet. The test mode was tension, with a pre-test speed of 2 mm/s, test speed 1 mm/s and post-test speed at 10 mm/s. The distance travelled stopped measuring at 20 mm.
Gaping analysis
This test measured the force of four gaping and non-gaping fillets of salmon and trout 4–5 kg. The gaping fillets were chosen based on previous experiences and following methodology set out by Espe et al. (2004). The puller was adjusted to the top of the texture analyser and the sample holder was adjusted to the bottom. The fillet was added to the cutting board and pin bones were manually counted and were numbered from the tail to the neck starting at the tail at pin bone—29 for salmon and 31 for trout. The fillet was placed on the sample holder and a tensile grip was used to fasten the fillet to the sample holder. The puller was attached to the first pin bone and then connected to the sample holder so that the puller was at a horizontal force for pulling the pin bone in the fillet, after which the puller was tightened. The test was started and the measurement recorded, until each pin bone was measured and recorded. This was repeated three times per species of fish (trout and salmon) and between gaping and none gaping fillets. The data presented show the mean force (N) per fish bone.
Collagenase and calcium chloride
For the collagen analysis, salmon and trout fillets were as follows: (A) left untreated, (B) placed into deionised water, (C) in a 12-mM calcium chloride solution (CaCl2) or (D) in a collagenase and calcium chloride solution. The collagenase solution was prepared with one litre of deionised water, 12 mM CaCl2, in a 1.2% PBS buffer, into which 0.5% of the enzyme collagenase A was added (each piece of fish was added to 500 ml of solution). The excess side and tail parts of the fillets were removed and the fillet was cut into two halves; it was deemed that pin bone 20 was the optimum area for splitting the fish fillet for all fish samples, due to size of the fillet and surface area to water ratio. Each sectioned piece was placed into the solution until the fillets were completely covered. The samples were placed into an incubator for 2 h at 37 °C, which allowed collagenase to react, and therefore, each solution was tested this way. The fillets were then removed from the solution and the measurement of force was undertaken using the same methodology as the gaping analysis.
Statistical analysis
All error bars shown represent the standard deviation from the mean. The data was analysed by various statistical methods in IBM SPSS version 24; firstly, data between gaping and none gaping fish fillets were analysed using a one-way ANOVA, a T test for species differences and a two-way ANOVA for analysing the treatments of water, calcium chloride and collagenase. There were three null hypotheses of interest—(a) gaping of fillets had an effect of the force required to extract a pin bones, (b) pulling force did not differ between fish species, (c) treatments of water, collagenase and calcium chloride had no effect on force required to extract the pin bone.
Results and discussion
Gaping analysis
Figure 1 shows the results from the gaping analysis completed for gapped and none gapped salmon fillets—(a) none gapping flesh and (b) gaping flesh. None gapping fillets’ highest pulling force was 7.55 N for pin bone 23 and the lowest was 4.12 N for pin bone 3. Pin bones 21 to 24 of the gaping salmon required the highest pulling force for deboning. Gaping salmon fillets require less pulling force for deboning when compared with none gaping salmon fillets. The highest pulling force was 4.95 N for pin bone 21 (gaping fillets) and the lowest force was pin bone 27 at 2.73 N.
Figure 2 shows the results between (a) none gaping and (b) gaping trout fillets showing that the lowest pulling force for the non-gaping trout was measured for pin bone 12 with 2.43 N while it was 1.91 N at pin bone 12 for the gaping trout. The pin bones in the back area of the trout require more pulling force for deboning (pin bones 21 to 29 for both none gaping and gaping fillets). Gaping trout fillets required more pulling force to debone than fillets for non-gaping trout from pin bones 13 to 30, where pin bone 31 of non-gaping trout fillets required slightly higher pulling force to remove the pin bones than the gaping trout.
The results were subjected to a one-way between subjects ANOVA and significant differences were observed (F = 26.25 [p < 0.05]). However, using a post hoc analysis (Tukey) revealed Salmon non-gaping fillets were significantly different to those that were gaped, and both trout samples (gaping and none gaping) p < 0.05. Salmon fillets that were gaping were significantly different to non-gaping salmon fillets and gaping trout fillets (p < 0.05). Trout none gaping fillets were significantly different to trout gaping and salmon none gaping (p < 0.05), but were not significant compared with salmon gaping fillets (p > 0.05). In this study, trout gaping fillets were significantly different to all other samples (p < 0.05).
There is no significant difference between the gaping and non-gaping trout. It is noted that a large variance in the data for gaping fillets maybe due to the gaping area within the fish fillet. As an example, pin bone 9 (trout gaping fillets) had an average force value of 2.86 N, which is calculated using the measured pulling force of 3.72 N, 2.33 N, 4.43 N and 0.96 N. It is clear that pin bone 9 from fillet 4 was affected from the gaping while the others were not. It was observed that none of the analysed gaping fillets were badly gaping in the back pin bone area. The gaping occurred mostly in the neck or belly area of the fillet. The results for trout thus confirm the observation that gaping trout fillets require less force in the deboning process.
The results confirm that gaping has an influence on the deboning force required for salmon and trout. It also depends on the intensity of the gaping as pin bones in highly gaping areas can be removed with nearly no effort while the force required to remove the pin bones is only reduced for other areas. The gaping analysis suggests that the firmness of the fillet may have an important role in the force needed to debone salmon and trout; therefore, collagen may act as the scaffold and structural protein of the fillet, and it could play an important role on the deboning process (Shoulders and Raines 2009). It is however rare that the entire fillet is affected by gaping, as gaping is usually only evident on a few areas of the fillet (Jacobsen et al. 2015). For this reason, salmon and trout fillets were placed into various treatments to reduce the deboning force required to remove pin bones. The enzyme, collagenase A, was used to reduce the collagen concentration within the fillet; a calcium chloride solution and a water solution were also used. The results were compared with the deboning force of an untreated fillet.
Treatments for reducing pin bone pulling force
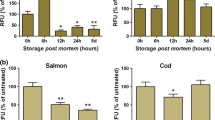
The results from the collagenase and calcium chloride experiments (Table 1) for salmon show that of the 28 pin bones analysed, the treatments showed four significant changes in the fillet, mainly colour loss, size shrinkage, softness of the fillet and gaping. It can be observed that the collagenase-treated salmon requires lower pulling force than the untreated salmon in order to remove pin bones through the fillet. Pin bone 1 measured a force of 2.84 ± 1.45 N for the untreated salmon and 3.11 ± 0.92 N for the collagenase-treated salmon, this could be an effect of bones closer to the neck being more embedded within the fillet than other pin bones.
The untreated salmon shows a peak pulling force at pin bone 25 of 5.17 ± 1.40 N. The pulling force required to remove the following pin bones (26–28) slowly decreases. The lowest force was measured for pin bone 15 (2.74 ± 1.13 N) compared with the highest deboning force for collagenase treated salmon pin bone 1 (3.11 ± 0.92 N) and pin bone 28 being the lowest (0.75 ± 0.00 N). Apart from pin bone 1, the pulling force for collagenase samples was consistently lower than untreated salmon fillets, where they range from 0.97 ± 0.14 to 2.20 ± 0.03 N. The data suggests that the collagenase-treated salmon reduces the peak shown from pin bones 17 (4.00 ± 1.42) through to 27 (4.15 ± 2.51) where pin bone 25 has the highest pulling force required to remove the pin bones on the untreated salmon fillets.
The analysis was repeated with a calcium chloride solution and a loss in colour was observed. The highest force measured for the salmon after the calcium chloride treatment was 2.48 ± 0.09 N at pin bone 10. The lowest force value was measured at pin bone 25 (1.00 ± 0.21 N). When comparing with untreated salmon, the highest force was for pin bone 25 (5.17 ± 1.40 N) and the lowest force was for pin bone 15 (2.74 ± 1.13 N). Salmon placed simply into water for the same period of time showed a bridge between the untreated and treated samples; of note, pin bone 7 had the lowest pulling force (1.34 ± 0.19), whereas pin bone 25 showed the highest pulling force (4.37 ± 0.27).
The results from the collagenase and calcium chloride treatments for trout are shown in Table 2. In general, the untreated trout required a higher deboning force than the collagenase-treated trout. Pin bone 1 was an exception; trout untreated had a lower pulling force than the rest of the bones within the fillet (1.57 ± 1.79), but the variance within the sample was large. The highest values for the trout with the collagenase treatment were however measured for pin bone 28 (2.54 ± 0.36). The highest force for the untreated trout was measured at pin bone 22 (5.31 ± 0.88 N) and pin bone 1 the lowest, a decrease in the pulling force was observed for pin bone 2 (1.90 ± 1.53 N). Not considering pin bones 4 and 5 (0.27 ± 0.38), the lowest value for the force was pin bones 6 (0.58 ± 0.82) and 8 (0.76 ± 0.20 N).
When comparing the addition of calcium chloride to the fillet compared with untreated trout samples, the highest force required to remove a pin bone was at pin bones 3 and 2 (0.58 ± 0.08 and 0.75 ± 0.67, respectively), as the pin bones were not as well connected to the fillet. The highest pulling force for the calcium chloride-treated trout was for pin bone 31 with a value of 2.26 ± 0.00 N. Water treatment, similarly to the salmon fillets showed a bridging effect on the fillets, whereby the highest pulling force was 4.35 ± 0.75 (pin bone 25) and the lowest was 1.68 ± 1.02 (pin bone 1), but also contained a large variance in the data.
Using an independent T test to understand significant differences between the two varieties of fish and the measured forces of different treatments showed a significant difference between the two varieties of fish (p < 0.05). Treated salmon and trout fillets were subjected to a two-way between subjects ANOVA and significant differences were observed (p < 0.05) F = 5.712. A post hoc analysis revealed that trout and salmon fillets showed a significant difference between each other (p < 0.05). Calcium chloride and collagenase were significantly different when compared with the water and none treated salmon and trout fillets (p < 0.05). When comparing salmon calcium chloride and collagenase together, there were no significant differences (p > 0.05), similar to that of salmon and trout non treated and water-treated fillets (p > 0.05). When compared against each other, only one significant difference was observed, this being untreated fillets and species (p < 0.05), all other treatments and species did not show significant differences (p < 0.05).
Both treated salmon fillets show no increase in the force measured in the back area of the fish, unlike the two untreated salmon samples (water solution and no solution). Consequently, both solutions have an impact on the fillet’s texture and the deboning force required. The results show that the removal of collagen by collagenase has a higher impact on the fillet structure and therefore collagen plays an important role in the deboning process. Similarly to salmon, but more pronounced, were the results from the treatments of calcium chloride and collagenase for trout samples, these results showed that pin bones require less force than pin bones in water-immersed or non-treated fillets. Collagen is known to act like scaffold to the bones within the muscle (Song et al. 2006; Ferreira et al. 2012) and mineralise to form pin bones in fish, but some studies have shown the importance of collagen as a mechanism for holding pin bones in fish physiology (Summers and Long 2005).
It is assumed that pin bones towards the neck area were not affected by the collagenase solution, which may be explained by the depth of muscle and exposure to the pin bone area during treatment. Generally, the force does not increase significantly when fish fillets are subjected to collagenase and the back pin bone area and did not have the highest values in the force required to remove the pin bones (a drop in force was observed). The trend of the collagenase-treated salmon when compared to untreated salmon shows different effects (the collagenase samples require less force than untreated samples). The results suggest that the collagenase solution had a substantial influence on the deboning force required to remove the pin bones and that gaping originates from collagen collapse.
Surprisingly, calcium chloride solution showed interesting data which suggest further optimisation to fully understand its role in reducing deboning force in salmon products. Collagen has been shown to prefer to chelate calcium ions in solutions and these subsequently form nucleation sites. Carbonyl groups on the surface of collagen are readily the nucleation sites of calcium phosphate (producing in this case calcium phosphate crystals). Although this data was gathered using calcium phosphate, this could be a reason as to why differences were observed between water and calcium chloride solutions, where the calcium within solution binds with collagen and reduce deboning force (Zhang et al. 2003).
The magnitude of the difference between the means depends on the treatment but not the fish, as the use of a two-way ANOVA between subjects showed interesting results. The results showed a significant difference between the treatments—calcium chloride but not between the species of fish. Water and untreated fish samples were similar to each other, indicating no affect from the pre-soaking of fillets within the testing. Interesting is the impact of calcium chloride and collagenase on the fillet structure, collagenase uses the same solution as calcium chloride, so if the effect it has is an issue of collagenase as well as the calcium chloride (Table 3).
Overall, the results show that the chemical composition of the fish has an impact on the force required for the deboning process. The chemical composition of fish is, however, not constant as it depends on various factors (Hernández-Martínez et al. 2013). Due to the different genetics and life environments of the fish, the chemical composition develops differently and both factors influence the anatomical development and appearance on the fish (Thielemann et al. 2007). This may explain the reason behind the large variances in data noticed as the standard deviation from the mean, and this could prove to be problematic when running similar tests in the future. However, standardising the experiment as well as the treatments can only do so much; one has to understand the differences in the fish products, where they grow to the conditions of feed, environment and many other quality factors. What would be of interest and could have affected this experiment is the date from harvest (quality is known to decrease post rigour), temperature of water, stress within the fish at time of harvest and many other factors. This project was based on the industrial processes within industry; industry seldom have the opportunity to control every variable and when developing a deboning process, it has to be adjusted for all types of fish farming practises.
It is nearly impossible to adjust the deboning process to meet the requirements of every fish. Nevertheless, it would be beneficial to further evaluate and optimise the pre-treatments for deboning fish, as these practices may reduce the likelihood of bones being present in the final product. Collagenase and calcium chloride solutions seem to be a good start to reduce the pulling force required to remove the pin bones from fish fillets; however, further work is required to optimise the effects of these to reduce colour and texture impacts on the final fish fillet.
References
Balaban MO, Jie H, Yin Yee Y, Alçiçek Z (2015) Method to measure the force to pull and to break pin bones of fish. J Food Sci 80(2):E334–E340
Cheng JH, Sun DW, Han Z, Zeng XA (2014) Texture and structure measurements and analyses for evaluation of fish and fillet freshness quality: a review. Compr Rev Food Sci Food Saf 13(1):52–61
Darmanto, Y.S., Agustini, T.W., Swastawati, F. and Al Bulushi, I., 2014. The effect of fish bone collagens in improving food quality. Int Food Res J, 21(3)
Einen O, Guerin T, Fjæra SO, Skjervold PO (2002) Freezing of pre-rigor fillets of Atlantic salmon. Aquaculture 212(1):129–140
Espe M, Ruohonen K, Bjørnevik M, Frøyland L, Nortvedt R, Kiessling A (2004) Interactions between ice storage time, collagen composition, gaping and textural properties in farmed salmon muscle harvested at different times of the year. Aquaculture 240(1):489–504
Ferreira AM, Gentile P, Chiono V, Ciardelli G (2012) Collagen for bone tissue regeneration. Acta Biomater 8(9):3191–3200
Fuentes-Gandara F, Pinedo-Hernández J, Marrugo-Negrete J, Díez S (2018) Human health impacts of exposure to metals through extreme consumption of fish from the Colombian Caribbean Sea. Environ Geochem Health 40(1):229–242
Hernández-Martínez M, Gallardo-Velázquez T, Osorio-Revilla G, Almaraz-Abarca N, Ponce-Mendoza A, Vásquez-Murrieta MS (2013) Prediction of total fat, fatty acid composition and nutritional parameters in fish fillets using MID-FTIR spectroscopy and chemometrics. LWT-Food Science and Technology 52(1):12–20
Jacobsen, Á., Joensen, H. and Eysturskarð, J., 2015. Gaping and loss of fillet firmness in farmed salmon (Salmo salar L.) closely correlated with post-slaughter cleaning of the abdominal cavity. Aquaculture Research
Kiessling A, Stien LH, Torslett Ø, Suontama J, Slinde E (2006) Effect of pre-and post-mortem temperature on rigor in Atlantic salmon muscle as measured by four different techniques. Aquaculture 259(1):390–402
Roth B, Slinde E, Arildsen J (2006) Pre or post mortem muscle activity in Atlantic salmon (Salmo salar). The effect on rigor mortis and the physical properties of flesh. Aquaculture 257(1):504–510
Safandowska M, Pietrucha K (2013) Effect of fish collagen modification on its thermal and rheological properties. Int J Biol Macromol 53:32–37
Shoulders MD, Raines RT (2009) Collagen structure and stability. Annu Rev Biochem 78:929–958
Skjervold PO, Rørå AMB, Fjæra SO, Vegusdal A, Vorre A, Einen O (2001) Effects of pre-, in-, or post-rigor filleting of live chilled Atlantic salmon. Aquaculture 194(3):315–326
Song E, Kim SY, Chun T, Byun HJ, Lee YM (2006) Collagen scaffolds derived from a marine source and their biocompatibility. Biomaterials 27(15):2951–2961
Summers AP, Long JH (2005) Skin and bones, sinew and gristle: the mechanical behavior of fish skeletal tissues. Fish physiology 23:141–177
Suphatharaprateep W, Cheirsilp B, Jongjareonrak A (2011) Production and properties of two collagenases from bacteria and their application for collagen extraction. New Biotechnol 28(6):649–655
Thielemann, J.T., Kirkhus, T., Kavli, T., Schumann-Olsen, H., Haugland, O. and Westavik, H., 2007, August. System for estimation of pin bone positions in pre-rigor salmon. In International Conference on Advanced Concepts for Intelligent Vision Systems (pp. 888–896). Springer Berlin Heidelberg
Tierney CM, Haugh MG, Liedl J, Mulcahy F, Hayes B, O’Brien FJ (2009) The effects of collagen concentration and crosslink density on the biological, structural and mechanical properties of collagen-GAG scaffolds for bone tissue engineering. J Mech Behav Biomed Mater 2(2):202–209
Zhang W, Huang ZL, Liao SS, Cui FZ (2003) Nucleation sites of calcium phosphate crystals during collagen mineralization. J Am Ceram Soc 86(6):1052–1054
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Schroeder, S., Grigor, J.M., Stathopoulos, C.E. et al. The effect of collagenase, water and calcium chloride on the removal of Salmo salar (salmon) and Oncorhynchus mykiss (trout) pin bones. Aquacult Int 26, 1353–1363 (2018). https://doi.org/10.1007/s10499-018-0288-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-018-0288-5