Abstract
This study compared changes in the biochemical composition of muscles and ovaries of maturing female European eels during hormonal stimulation with carp pituitary homogenate under controlled conditions and in the development of oocytes. It has been found that differentiation of oocyte size in eel is visible from the beginning of the gamete maturation process. This differentiation increases as gonads grow. Furthermore, oocytes are at different development stages and are still highly differentiated in terms of size during ovulation. Moreover, the biochemical composition of the body and gonads was found to change. During the process of maturation, the relative fat content in the eel body decreases both in the muscles (from 21.99 ± 4.3 to 18.48 ± 3.3 %) and in gonads (from 25.76 ± 2.71 to 15.21 ± 4.7 %), with the changes in ovaries being more rapid. Protein content in muscles also decreased (from 15.98 ± 3.13 to 12.35 ± 1.6 %) during the process of female maturation. Different trends were observed for polyunsaturated fatty acids (EPA and DHA). The total amount of these acids decreased in muscles (P < 0.05) but increased in ovaries (P > 0.05).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The population of the European eel (Anguilla anguilla) has decreased dramatically over the past three decades. It is estimated that it has dropped to a mere 1 % of its 1970 levels (Dekker 2003; Bevacqua et al. 2011). Currently, the species is outside the limits of biological safety and, consequently, it has been placed on the Red List of endangered species in Europe (Belpaire et al. 2009; Prigge et al. 2012). Such radical changes in the eel population have been attributed, inter alia, to excessive exploitation of the species, deterioration of the environment, climatic changes and infestation of the swimming bladder with the Anguillicoloides crassus nematode (e.g., Friedland et al. 2007; Belpaire et al. 2009; Clevestam et al. 2011). Intensive research to develop an artificial reproduction technique for this species (Palstra et al. 2005), as well as stocking actions aimed at increasing its population in the waters of Europe (Vogel 2010) is among the measures taken to save the European eel from total extinction.
The eel is a species with an interesting life story—it spends most of its life in European fresh water, but it swims to spawn in the Sargasso Sea area, covering thousands of kilometers during its spawning migration. Many intensive physiological and morphological changes take place in the eels during the maturation process (Müller et al. 2004a; b; van Ginneken et al. 2005a; Clevestam et al. 2011; Nowosad et al. 2014). Although the European eel is regarded as a trophic opportunist (Prigge et al. 2012), it does not consume any food during its migration journey and it takes the energy it needs to travel the thousands of kilometers and undergo the maturation process from the fat accumulated in its body (Clevestam et al. 2011). Proper sexual maturation—vitellogenesis and ovary development brought about by environmental factors—takes place during the migration journey (Duriff et al. 2006). The decreased deposits of fatty tissue (as observed recently in juvenile fish in inland waters) can have consequences for older individuals, by preventing silver state females from reaching spawning grounds and ovaries from maturing properly. In some cases, the accumulated energy may even be insufficient for reaching the spawning ground (Clevestam et al. 2011), which makes the situation even more difficult for the species.
Despite researchers’ considerable interest in the species, there is still only scarce information on the basic biological processes of changes in the bodies of European eel brooders which take place during its spawning migration. It could be said that information about climate fluctuations is more easily available than on the European eel, to be precise—on the changes which occur during its long journey to the spawning ground, maturation and fasting. What changes take place in their muscles? What about gonads? These questions have inspired researchers to study this species in more detail.
The aim of this study was to compare changes in the biochemical composition of muscles and gonads of maturing female European eels and the development of ovaries with and without hormonal stimulation with carp pituitary homogenate (CPH) under controlled conditions.
Materials and methods
Fish
Eel brooders (42 female fish) were caught during commercial fishing activities in freshwater in the Mazury region (the northeast of Poland) as fish which start their spawn migration (at the end of May). The collection of fish was made during few following days. After being caught, the fish were transported to the laboratory of the Department of Lake and River fisheries, University of Warmia and Mazury, Olsztyn, where they were put into a 1-m3 tank, operating in closed water circulation (Kujawa et al. 1999). The initial water temperature was 12 °C (±0.2 °C). The photoperiod applied was natural and photocell-controlled. The oxygen level in the water was at least 6 ppm. The fish stayed about 1 month in the freshwater tank until the start of the experiment.
Handling the fish
All the fish handling was conducted in the state of anesthesia (MS-222 (300 ppt), Finquel, USA). After being anesthetized, the fish were measured (TL, eye diameter), weighed and labelled with individual PIT labels (Biomark, USA). The fish handling was conducted in a slightly dimmed room. The fish (with a mean body weight (BW) of 1,174.9 ± 250.0 g and the mean length (LT) of 86.2 ± 5.6 cm) were divided into two groups: C, control group, no hormonal stimulation (n = 14) and the experimental group T, n = 28. Samples (minimum 5 fish each) were taken from group T in week 2–4—group TI, and in week 13–15 of the experiment—group TII. Subsequently, fish from groups C and T were transferred to 1-m3 tanks operating in closed water circulation. The circulation had controllable physico-chemical conditions (temperature, photoperiod), and the system was also equipped with a polygeser filter and UV lamps (2 × 36 W each). The initial water temperature was set at 12 °C, and the photoperiod was 0 L: 24 D. Subsequently, the water was salted (Aqua Nova, Australia) over 7 days to the level of 33–35 ‰. After 20 days, the water was heated to the level of 15°C (±0.1°C). The fish were not fed throughout the experiment.
Hormonal stimulation
The hormonal stimulation was started after the desired water salinity level was achieved using the method described by Nowosad et al. (2014). After being taken out of the tanks, the fish were put in a container with an anesthetic. After being anesthetized, the fish were weighed and had their eye diameter measured. Subsequently, fish from experimental groups were interperitoneally injected at 18 mg kg−1 of carp pituitary homogenate (CPH, Agrent, USA). The hormones were not given to the fish in the control group. The fish handling was conducted at 5-day intervals, for 15 weeks. Using obtained measurements, the body weight index (BWI) was calculated as follows: BWI = actual body weight × 100 %/initial body weight.
Sampling
Before the samples were taken, fish were anesthetized until their life functions stopped. Ovaries and a fragment of a muscle from the middle (the same) section of a female body were taken for biochemical analyses. Fish were taken for analyses [gonadosomatic index (GSI), chemical composition of muscles and gonads, oocytes development] randomly at the start of the experiment, during its initial period (day 15–30)—group TI and during its final period (day 90–115)—group TII. Samples for analyses were taken from the control group at the beginning and at the end of the experiment. The gonadosomatic index was calculated from the formula: GSI = (GW BW−1) 100 %, where GW: gonad weight (g), BW: body weight (g).
Histological analysis
The gonads were weighed with an accuracy of ±0.01 mg, and subsequently approximately 1-cm3 fragments were removed for histological examinations, placed into glass containers and fixed in Bouin’s fluid. Depending on the size and amount of the material taken for the analysis, the fixing time ranged from 2 to 3 days. The fixed material was put into biopsy cartridges and then into a tissue processor (LEICA TD 1020) for 21 h, where it was washed in ethanol at increasing concentrations (75, 80, 90, 95 %), acetone, xylene and liquid paraffin at 54 °C. The obtained study material was then sealed in paraffin blocks and sliced in a rotating microtome (LEICA RM 2155) into 0.5–0.7-µm thick sequences. The paraffin sections were put onto protein-covered slides. The preparations were made with Mayer’s hemotoxin and eosin (H + E method) (Bagiński 1965). Subsequently, the stained preparations were sealed with cover slips and histokitt (Glaswarenfabrik Karl Hecht GmbH & Co KG, Germany). After drying, the histological preparations were analyzed under an optical microscope (Axio Scope A1, Zeiss, Germany) with AxioVs40 v 4.8. 2.0 software (Carl Zeiss MicroImaging GmbH, Germany).
Analysis of chemical composition of muscles and gonads
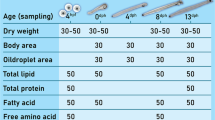
The chemical composition of gonads and muscles was analyzed. The analyzed features included the content of dry matter, protein, ash, fat and fatty acid profiles.
Dry weight: Approximately 1-g samples (±0.0001 g) in duplicate were initially dried at 65–70 °C in quartz tests, then dried to a constant weight at 105 °C for 1 h.
Protein: The protein content was determined following the method of Kjeldahl according to PN-75 A-04018.
Ash: The samples (about 1 ± 0.0001 g) were dry-digested at 680 °C for 6 h in quartz tests.
Fat: The fat content was determined using the Schmidt-Bondzynski-Ratzlaff C method (Berg and Nilsson 1997). To this end, approximately 2-g (±0.0001 g) samples were hydrolyzed in 8 M HCl and transferred to a 100-ml cylinder. 10 ml of 95 % ethanol with an addition of 0.0001 g of BHA (2-tert-Butyl-4-hydroxyanisole) and 25 mL of ethylene ether and 25 mL of petroleum ether were then added in succession to all samples. The ether layer was collected to a 250-mL glass flask. Subsequently, 15 ml of ethylene ether and 15 mL of petroleum ether were added twice. Each time, the samples were shaken and were left overnight. The ether layer was distilled by means of aggregate for distillation of solvents. Odd fat was dried at 105 °C for 6–7 h to a constant weight and was then weighed.
The content of fat (%) was calculated according to the pattern: X = [(b − a) × 100]/c; where: a—weight of flask (g), b—weight of flask with extracted fat (g), c—weight of samples (g).
Fatty acids: In the case of fatty acid analysis, lipids were extracted with the use of Folch’s procedure (Christie 1973). To this end, the studied material was broken up and mixed. 2 g of sample was homogenized for 1 min with 20 mL of methanol. Next, 40 mL of chloroform was added, and the course was continued for 2 min. The prepared mixture was filtered to a 250-mL glass cylinder. The solid residue was re-suspended in 60 mL of chloroform methanol (2:1 v/v) and homogenized again for 3 min. After filtering, the solid was washed once more with 40 mL of chloroform and once with 20 mL of methanol. The combined filtrates were transferred to the same cylinder. 0.88 % sodium chloride in water (determining 1/4 volume of filtrate) was added to the total filtrate and was then shaken and left overnight. The upper layer was removed and a water–methanol mixture (1:1 v/v) was added to the lower layer, and the washing procedure was repeated. The remaining layer was filtered through anhydrous sodium sulfate and was distilled by means of aggregate for distillation of solvents. The fatty acid methyl esters were prepared from total lipid with the Peisker method with chloroform–methanol–sulfuric acid (100:100:1 v/v) (Żegarska et al. 1991).
Methyl esters of fatty acids of each sample were analyzed with capillary gas chromatography with a flame-ionization detector (FID) under the following conditions:
-
capillary column (dimension 30 m × 0.25 μm with a 0.32 mm internal diameter, liquid phase Supelcowax 10).
-
temperature: flame-ionization detector—250 °C, injector—225 °C, column—170 °C
-
carrier gas—helium, flow rate 0.6 mL/min
Individual fatty acids were identified by comparing the relative retention time peaks to known standards of Supelco.
Data analysis and statistics
The data were analyzed by means of a one-way analysis of variance (ANOVA) and subsequently with Duncan’s post hoc test (α = 0.05). The statistical analysis was conducted with Microsoft Excel and Statistica v.10 (StatSoft Inc.2011, USA).
Results
The largest body weight increase (BWI = 115.4 ± 10.7 %) was observed in the European eel females after the hormonal stimulation, in group TII, kept in saltwater for approximately 15 weeks (Table 1). The body weight in fish in the control group was found to have decreased, and the BWI value at the end of the experiment was 93.3 ± 0.7 % (P < 0.05). The percentage of biochemical components of muscles and gonads was found to have changed during the period of maturation of female European eel under controlled conditions (Fig. 1). The content of fat (Fig. 1a), protein (Fig. 1b) and ash (Fig. 1d) was found to have decreased during the experiment. There was a significant difference in ash content (P > 0.05) between muscles in females in the initial weeks (8.04 ± 1.0 %, Group T I) and at the end (5.84 ± 4.2 %, Group T II) of the experiment (Table 1). The gonads were found to contain significantly (P < 0.05) less fat (decrease from 25.76 ± 2.71 to 15.91 ± 4.7 %) in Group TI and Group T II, respectively (Fig. 1b; Table 1). The protein content in gonads was not statistically different at the beginning than at the end of the experiment (P > 0.05).
An analysis of the fatty acid content in gonads and muscles showed a statistically significant (P < 0.05) increase in MUFA content in both organs (Fig. 2b). A statistically significant decrease (P < 0.05) in EPA + DHA (from 8.04 ± 1.0 to 5.84 ± 4.2 %) (Fig. 2c) and polyene acids n-3/n-6 (from 2.02 ± 0.28 to 1.52 ± 0.42 %) (Fig. 2d) was observed in muscles alone. The percentage of EPA +DHA in gonads increased (P > 0.05) from 10.57 ± 1.52 to 11.40 ± 0.8 % (Fig. 2c). The content of saturated fatty acids in gonads or muscles (Fig. 2a) and that of polyene acids in gonads (Fig. 2d) (P > 0.05) did not change.
The percentage of the content of fundamental biochemical components in gonads and muscle to the whole body shows a decrease in the level of all components under study in the whole fish body (P < 0.05), with a simultaneous increase in their content in gonads (Fig. 3).
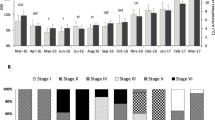
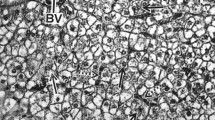
The GSI in the female eel was 1.66 ± 0.25 % during the initial weeks (2–6) of stimulating the maturation process, whereas it grew considerably between weeks 12 and 16 (P < 0.05) and reached 18.0 ± 10.6 % (5.0–31.6 %) on average (Fig. 4; Table 1). Size differentiation of oocytes in eel is visible from the beginning of gamete maturation (Fig. 5a). This size differentiation increases as gonads grow (Fig. 5b, c). At the same time, oocytes are at different stages of development (primary growth, pre-vitellogenic, vitellogenic), and the size differentiation increases during ovulation (Figs. 5d, 6). In group TII, the percentage of oocytes with a diameter under 0.3 mm decreased significantly, and the percentage of oocytes with a diameter above 0.3 mm increased significantly (P < 0.05) compared to the control group (at the experiment end) (Table 2). The average diameter of the largest oocytes in some individuals in the group (TII) was 0.83 ± 0.08 mm (Figs. 5d, 6). Ovaries of the untreated females and the beginning of maturation contained a large amount of fat cells could be found among the oocytes. Fat cells disappeared among the oocytes in the ovary advantage phase. According to histological preparations (Fig. 5), there are large amount of fat tissues among the gametes in picture A and B. In these pictures, the oocytes were seen in the cortical alveolus stage (OD = 80–130 µm). In picture C, the oocytes are between the early vitellogenic oocytes and mid-vitellogenic phase, characterizing large and small peripheral yolk granules in the cytoplasm. The number of fat cells is smaller than in previous stages.
Changes in the size and the stage of oocyte development in female eel at the beginning (a), in week 3 (b), in week 13 (c) and at the end of the experiment, immediately before ovulation (d). The images were taken at the same magnification (Scale bar 100 µm). The arrows indicate fat tissues in the ovaries
Discussion
In order to improve the artificial reproduction of the eel, a detailed analysis of the entire ovary and gamete maturation process is required. The changes in the biochemical composition of the body tissues, mainly muscles and ovaries are an indicator of the changes in the whole process. The knowledge of those changes and their dynamics will help to elucidate the process of vitellogenesis and final oocyte maturation. It will also help to understand what chemical substances are necessary for the process. This study of maturing female eels has shown a statistically significant (P < 0.05) decrease in fat content in gonads, but the percentage of MUFA acids increased (P < 0.05) and that of EDP + DHA increased slightly (P > 0.05). The content of fat, protein (P > 0.05), ash and EPA + DHA and polyene acids n-3/n-6 (P < 0.05) in muscles decreased. As in gonads, the content of MUFA in muscles increased significantly (P < 0.05). Comparing the percentages of fundamental biochemical components in gonads and muscles to the whole body reveals a surprising relationship: a decrease in the level of all the components under study (fat, protein, water and ash) in the whole fish body (P < 0.05) with a simultaneous increase in their content in gonads (P < 0.05).
Hormonal stimulation of female eel in the last phase resulted in a significant increase in the weight of the body and the gonads. In consequence, gonad weight in fish in the experimental group, compared to the control group at the start of the experiment, increased from 15 to 45. six times. The GSI in one of the females was about 32 %, the same as achieved by Pedersen (2003). The mean GSI (18.0 ± 10.6) achieved in this study in female eel after 13–16 weeks of hormonal stimulation is close to the value of GSI immediately before ovulation (20.0 ± 11.3 %) as found by other authors (e.g., van Ginneken et al. 2005a). The fish in this study had a mean BWI of 115.4 ± 10.7 % (106.1–128.5 %), which is a satisfactory result compared to that found by Pedersen (2004): 110.7 ± 9.8 %.
Freshwater eel, which swim long distances to the spawning ground in saltwater, must accumulate sufficient amounts of nutrients to help them make it through the journey and develop high quality gonads. Proteins and fats are regarded as the most important nutrients responsible for successful spawning. However, according to Jędryczkowski and Fischer (1973) and Clevestam et al. (2011), the fats accumulated in an eel’s body are mainly responsible for successful migration. The level of fat in tissues varies from one eel species to another. For example, in Japanese eel, it ranges from 13 to 20 % (Ozaki et al. 2008; Oku et al. 2009). In European eel in the Baltic region, it ranges from 20 to 38 % (Clevestam et al. 2011). However, for a journey to be successful, the starting fat reserves in European eel, according to Clevestam et al. (2011), should exceed 32 %, otherwise the fish stand little chance of reaching the spawning ground. Such an assumption would be correct if, during its journey, an eel used fat only for swimming metabolism and the basic metabolism. However, van Ginneken et al. (2005b) and Palstra and van den Thillart (2010) estimated that about 20 % of the energy consumed for swimming is produced from proteins. Considering the fact that eel are known to be much more effective swimmers than thought before (van Ginneken et al. 2005b; Palstra and van den Thillart 2010; Clevestam et al. 2011), and they save considerable amounts of energy when swimming to the spawning ground and that they swim (at least initially) at water temperatures considerably lower than those at which metabolic studies have been conducted (Aarestrup et al. 2009), it may be accepted that energy saving for swimming can be even more effective than originally assumed. It is all the more so that, as these studies have shown, eel usually make use of sea currents rather than swimming upstream. However, there is still the issue of gonads, whose weight at the end of the gamete development process is considerably larger and whose growth consumes proteins and fats. The changes in the structure of the body (muscles) and gonads (ovaries) determined in this study indicate several tendencies. As far as percentage is concerned, the protein content in hormonally stimulated females was found to have decreased from 16.0 to 12.4 % and that of fat from 22.0 to 18.5 % (Figs. 1, 3; Table 1). Conversely, the content of proteins in gonads of hormonally stimulated female eel was not found to have decreased, although the fat content decrease was substantial (from 25.8 to 15.9 %). The content of proteins in muscles in fish in the control group at the end of the study was found to have decreased significantly compared to the females in group TII (Table 1). Palstra and van den Thillart (2010) did not find any differences in the percentage biochemical composition (protein, fat, ash) in the bodies of female eel during the 6-month period of forced rest or swimming compared to the initial values. However, the fish in that study were not in the process of sexual maturation, i.e., a period of gonad growth. Therefore, there was no transfer of biochemical components from the fish bodies to their gonads. On the other hand, it may indicate that proteins and fats are consumed for the metabolic processes evenly.
Proteins and fats are important, both because of gonad growth and because they must be used as a source of energy for the basic metabolism and for fish to reach the spawning grounds (van Ginneken et al. 2005b; Clevestam et al. 2011). Reduction of the percentage of fat in ovaries, as found in this study, may be attributed to the increasing growth of GSI, from about 1.5 to over 30 % in some individuals (to 18.0 % on average) and the increase in the water content in the tissues under study. Such changes were not observed in male eel, which, however, were not stimulated hormonally (Palstra and van den Thillart 2010). The considerable decrease in the protein content in the muscles of fish in the control group at the end of this study may suggest that this component plays a very important role in providing energy for metabolism when a fish swims to the spawning ground. However, calculation of the total amounts of proteins, fats, water and ash in gonads of the European eel during the process of maturation (Fig. 3) has shown that their content decreases in the bodies of females and increases in gonads. This means that fats and proteins are transferred actively from muscles and incorporated in gonads. These findings show that the content of proteins in the whole fish body decreased more (nearly 50 %) than that of fat (a little more than 30 %), with a similar increase in both classes of substances in gonads. This may indicate that female eel use more protein than fat for swimming-related metabolism during the spawning journey and the growth of gonads. This was confirmed in the control group at the end of this experiment (Table 1). The decrease in the fat content calculated per gram in fillet and gonad tissues were measurable but, on the other hand, for the gonads, the gonadosomatic index increased from about 15 to 45. six times. The ovaries of the untreated females contained predominantly pre-vitellogenic oocytes to the cortical alveolus stages, and ovary samples of matured eels contained oocytes from the late vitellogenic phase to overripe oocytes without fat cells among the oocytes (an effect of hormonal treatment).
The content of different groups of fatty acids during the process of female fish maturation changed. The content of saturated fatty acids remained unchanged both in gonads and in muscles (Fig. 2a). Similar findings, indicating no changes in the content of different groups of fatty acids in muscles and semen of male eels, were reported by Mazzeo et al. (2010). A slight decrease in MUFA and an increase in PUFA were reported only for the liver. It was found, in the current study, that a considerable increase in the content of MUFA (monounsaturated fatty acids) took place in both muscles and gonads during the period of female maturation (Fig. 2b). On the other hand, the combined content of EPA and DHA was found to have decreased in muscles and increased in gonads (Fig. 2c). The changes in allocation of EPA and DHA indicate considerable movement of those acids from the rest of the body to ovaries. Considering the significant growth of gonads, large amounts of EPA and DHA must have been moved selectively to the ovaries. This indicates the great significance of these acids to the process of gonad maturation in eel. However, there have been no reports on the role or precise percentage changes in fatty acid contents in female European eel during the maturation process (Mazzeo et al. 2010). Furuita et al. (2006) examined the relationship of egg quality to the content of individual substances in the Japanese eel and found high quality eggs to contain slightly less fat (40 % in high quality eggs vs. 44 % in low quality eggs) and that the DHA content in eggs was positively correlated with spawn quality. On the other hand, a negative correlation was found for arachidonic acid (AA). No such studies have been conducted with European eel eggs. However, it cannot be ruled out that an increase in the total content of EPA + DHA in ovaries during the female eel maturation process is associated with oocyte maturation.
An increase in the gonad size during oocyte maturation is a normal, extensively described process. The eel has been described as a monocyclic species with one spawn during a lifetime. However, all oocytes develop simultaneously in such genera as Oncorhynchus (e.g., sockeye salmon, Pavlov et al. 2010). On the other hand, oocytes in the European eel differ in size by the initial development stage (Fig. 5a). The development phase (Fig. 5b, c) is when oocytes were found to grow and differentiate in terms of size and development level. Oocytes in different classes of size and maturity have also been found in ovaries immediately before ovulation (Fig. 5d). Such differentiation in the size and maturity of oocytes is typical of batch-spawning fish (e.g., Krejszeff et al. 2010; Targońska et al. 2011, 2012). This has been confirmed by Unuma et al. (2011) with the Japanese eel. As was shown by Unuma et al. (2011), the diameter of oocytes immediately before ovulation in Japanese eels which spawn in batches range from 0.8 to 1.0 mm, although in earlier study and the current study have shown that in female European eel immediately before ovulation there are oocytes with a wide range of diameters, from about 0.01 do over 0.7 mm. This study has shown that several (4–5) hormonal injections (group TI) bring the same results in terms of the oocyte size in female European eel as keeping female eel for 16 weeks in salt water with no hormonal stimulation (control) under the same conditions. No statistical differences (P > 0.05) were found in these study groups by the end of the experiment in terms of the percentage of oocytes in various size classes (Table 2). The oocyte diameters observed in this study after 5 weeks of stimulating the female fish were larger (0.176 ± 0.018 mm) than those observed by other authors after 6 weeks of the experiment (0.109 ± 0.041 mm) in a study examining the effect of swimming on the size of oocytes (Palstra et al. 2007). It may be concluded that hormonal treatment considerably accelerates the process of gamete maturation, which was also confirmed by a study conducted by Nowosad et al. (2014). The oocytes obtained at the end of the experiment had diameters of up to 0.853 mm, while eggs of a similar size (0.763–0.930 mm) obtained by Pedersen (2003) were regarded as pre-ovulation eggs.
Conclusions
Maturation of gonads in eel is a difficult and lengthy process, which requires large amounts of energy, protein and—to a lesser extent—fats accumulated in muscles. This means that fats and proteins are transferred actively from muscles and abdominal fats and incorporated in gonads. EPA and DHA fatty acids are most probably considerably important for the maturation of female eel gonads. This is shown by changes in their allocation, which indicates the considerable movement of those acids from the rest of the body to the ovaries. More attention should probably be paid to the diet of breeding fish in which spawn is to be induced under controlled conditions, and they should be provided with the optimum amounts of EPA and DHA. When the maturation process is induced in European eel under controlled conditions, oocytes with a wide range of diameters and different stages of development can be observed throughout the whole process of maturation, which shows that this eel species spawns in batches. Hormonal treatment of female eel considerably reduces the duration of oocyte maturation.
References
Aarestrup K, Økland F, Hansen MM, Righton D, Gargan P, Castonguay M, Bernatchez L, Howey P, Sparholt H, Pedersen MI, McKinley RS (2009) Oceanic spawning migration of the European eel (Anguilla anguilla). Science 325(5948):1660
Bagiński S (1965) Technika mikroskopowa. PWN, Warszawa
Belpaire CGJ, Goemans G, Geeraerts C, Quataert P, Parmentier K, Hagel P, De Boer J (2009) Decreasing eel stocks: survival of the fattest? Ecol Freshw Fish 18:197–214
Berg H, Nilsson S (1997) Determination of fat content in meat and meat products with NMR or SFE. Proc Euro-Food Chem IX Interlaken Switz 26:59–64
Bevacqua D, Melia P, De Leo GA, Gatto M (2011) Intra-specific scaling of natural mortality in fish: the paradigmatic case of the European eel. Oecologia 165:333–339
Christie WW (1973) The isolation of lipids from tissues. Recommended procedures. chloroform-methanol (2:1, v/v) extraction and “Folch” wash. W: lipid analysis. Isolation, separation, identification and structural analysis of lipids. Pergamon Press Oxford, New York, pp 39–40
Clevestam PD, Ogonowski M, Sjoberg NB, Wickstrom H (2011) Too short to spawn? Implications of small body size and swimming distance on successful migration and maturation of the European eel Anguilla anguilla. J Fish Biol 78:1073–1089
Dekker W (2003) On the distribution of the European eel (Anguilla anguilla) and its Wsheries. Can J Fish Aquat Sci 60:787–799
Duriff C, Dufour S, Elie P (2006) Impact of silvering stage, age, body size and condition on reproductive potential of the European eel. Mar Ecol Prog Ser 327:171–181
Friedland KD, Miller MI, Knights B (2007) Oceanic changes in the Sargasso Sea and declines in recruitment of the European eel. ICES J Mar Scien 64:519–530
Furuita H, Unuma T, Nomura K, Tanaka H, Okuzawa K, Sugita T, Yamamoto T (2006) Lipid and fatty acid composition of eggs producing larvae with high survival rate in the Japanese eel. J Fish Biol 69:1178–1189
Jędryczkowski W, Fischer Z (1973) Preliminary report on the metabolism of the silver eel (Anguilla anguilla L.). Pol Arch Hydrob 20:507–516
Krejszeff S, Targońska K, Żarski D, Kucharczyk D (2010) Artificial reproduction of two different spawn-forms of the chub. Reprod Biol 10:67–74
Kujawa R, Kucharczyk D, Mamcarz A (1999) A model system for keeping spawners of wild and domestic fish before artificial spawning. Aquacult Eng 20:85–89
Mazzeo I, Gallego V, Pérez L, Peñaranda DS, Jover M, Asturiano JF (2010) Variations in fatty acids composition in different tissues of the European eel (Anguilla anguilla L.) males during induced sexual maturation. J Appl Ichthyol 26:763–774
Müller T, Molnár T, Szabó A, Romvári R, Hancz C, Bercsényi M, Horn P (2004a) Tracking the hormonally-induced female eel maturation by means of computer tomography. Act Vet Hung 52:235–243
Müller T, Romvári R, Bercsényi M, Hancz C, Molnár T, Szabó A, Horn P (2004b) Following the artificially induced eel maturation process by means of in vivo CT scanning. J World Aquacult Soc 35:217–224
Nowosad J, Kucharczyk D, Czarkowski TK, Kwasek K (2014) Changes in body weight and eye size in female European eel kept in fresh and salt water. Ital J Anim Sci 13:382–386
Oku T, Sugawara A, Choudhury M, Komatsu M, Yamada S, Ando S (2009) Lipid and fatty acid compositions differentiate between wild and cultured Japanese eel (Anguilla japonica). Food Chem 115:436–440
Ozaki Y, Koga H, Takahashi T, Adachi S, Yamauchi K (2008) Lipid content and fatty acid composition of muscle, liver, ovary and eggs of captive-reared and wild silver Japanese eel Anguilla japonica during artificial maturation. Fish Sci 74:362–371
Palstra AP, van den Thillart GEEJM (2010) Swimming physiology of European silver eels (Anguilla anguilla L.): energetic costs and effects on sexual maturation and reproduction. Fish Physiol Biochem 36:297–322
Palstra AP, van Ginneken VJT, Murk AJ, van den Thillart GEEJM (2005) Are dioxin-like contaminants responsible for the eel (Anguilla anguilla) drama? Naturwissenschaften 93:145–148
Palstra AP, Curiel D, Fekkes M, de Bakker M, Székely C, van Ginneken V, van den Thillart G (2007) Swimming stimulates oocyte development in European eel. Aquaculture 270:321–332
Pavlov ED, Mikodina EV, Sedova MA, Emel’yanova NG, Markevich GN (2010) The state of gonads of resident sockeye salmon Oncorynchus nerka from the Tolmachev Reservoir. J Ichthyol 50:321–329
Pedersen BH (2003) Induced sexual maturation of the European eel Anguilla anguilla and fertilisation of the eggs. Aquaculture 224:323–338
Pedersen BH (2004) Fertilisation of eggs, rate of embryonic development and hatching following induced maturation of the European eel Anguilla anguilla. Aquaculture 237:461–473
Prigge E, Malzahn AM, Zumholz K, Hanel R (2012) Dietary effects on fatty acid composition in muscle tissue of juvenile European eel, Anguilla anguilla (L.). Helgol Mar Res 66:51–61
Targońska K, Kucharczyk D, Żarski D, Cejko B, Krejszeff S, Kupren K, Król R, Dryl K, Kowalski R, Glogowski J (2011) Artificial reproduction of wild and cultured barbel (Barbus barbus, Cyprinidae) under controlled conditions. Act Vet Hung 59:363–372
Targońska K, Perkowski T, Żarski D, Krejszeff S, Mamcarz A, Kujawa R, Kucharczyk D (2012) Method of evaluation of wild common tench, Tinca tinca (L.), female suitability for artificial reproduction during the spawning season. Ital J Anim Sci 11:164–168
Unuma T, Hasegawa N, Sayumi Sawaguchi S, Tanaka T, Matsubara T, Nomura K, Tanaka H (2011) Fusion of lipid droplets in Japanese eel oocytes: stage classification and its use as a biomarker for induction of final oocyte maturation and ovulation. Aquaculture 322–323:142–148
van Ginneken V, Vianen G, Muusze B, Palstra A, Verschoor L, Lugten O, Onderwater M, van Schie S, Niemantsverdriet P, van Heeswijk R, Eding E, van den Thillart G (2005a) Gonad development and spawning behaviour of artificially-matured European eel (Anguilla anguilla L.). Anim Biol 55:203–218
van Ginneken V, Antonissen E, Muller UK, Booms R, Eding E, Verreth J, van den Thillart G (2005b) Eel migration to the Sargasso: remarkably high swimming efficiency and low energy costs. J Exp Biol 208:1329–1335
Vogel G (2010) Europe tries to save its eels. Science 329:505–507
Żegarska Z, Jaworski J, Borejszo Z (1991) Ocena zmodyfikowanej metody Peiskera otrzymywania estrów metylowych kwasów tłuszczowych. Act Acad Agricult Techn Olst 24:25–33
Acknowledgments
The study was financed “Innovations in finfish aquaculture with special references to reproduction,” Operational Program Sustainable Development of the Fisheries Sector and Coastal Fishing Areas 2007–2013” (OR14-61724-OR1400003/09/10/11).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Nowosad, J., Kucharczyk, D., Łuczyńska, J. et al. Changes in European eel ovary development and body and ovary chemistry during stimulated maturation under controlled conditions: preliminary data. Aquacult Int 23, 13–27 (2015). https://doi.org/10.1007/s10499-014-9794-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-014-9794-2