Abstract
Two groups of Anguilla japonica were treated with hormone, one on a weekly basis, the other on a biweekly basis. Intramuscular injection was applied at a dose of 0.75 mg/kg BW carp pituitary extract (CPE) plus 150 IU/kg BW human chorionic gonadotropin (hCG) for female fish, while males received half of this dose. The average total lipid content of the gonads from the weekly treated group, i.e. 20.6 ± 1.2 % for females and 18.0 ± 2.3 % for males, was significantly higher than the biweekly treated group, 16.8 ± 0.7 and 15.5 ± 1.3 %, respectively (p < 0.05). For both muscle and liver, the readings were not significantly different. The gonads from the weekly treated fish had more fatty acids, particularly saturated fatty acids, polyunsaturated fatty acids, highly unsaturated fatty acids, eicosapentaenoic acid (20:5n-3, EPA), docosahexaenoic acid (22:6n-3, DHA) and arachidonic acid (20:4n-6, ARA). Histological examination showed that the ovaries of both the weekly and the biweekly treated fish were mainly at stage IV. However, the weekly treated females had bigger oocyte diameter (722.0 ± 60.9 μm) than the biweekly females (611.6 ± 22.6 μm). These results suggest that CPE and hCG promoted the maturation process for both scheduled induction and that the frequency of hormone injection influenced the biochemical composition of gonads, especially their lipids. Our study describes for the first time the effect of hormone injection frequency on the lipid content and fatty acid composition in the gonads of A. japonica during artificial maturation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anguillid eels have exceptional life history traits. They can spend more than 5 years growing in fresh water, and then travel back, once adult, many kilometres to their oceanic spawning grounds (Tesch 2003). Information about their sexual maturation as they are going to spawn is limited (Tsukamoto 1992), making artificial maturation a genuine task. Over the past years, overfishing, environmental destruction and other unknown factors singly or in combination have led to the decrease in the Japanese glass eel, Anguilla japonica in East Asia. This creates a crucial requirement for artificial breeding of seedlings for aquaculture (Tatsukawa 2003). However, Japanese eels do not mature in captivity based on the fact that eels do not activate ovarian steroidogenesis spontaneously during sexual maturation without being stimulated by exogenous hormones (Kazeto et al. 2011). Thus, scientists have performed many studies on artificially inducing maturation. Yamauchi and Yamamoto (1982) demonstrated the effectiveness of a hormone, 17, 20β-dihydroxy-4-pregnen-3-one (DHP) to induce maturation of oocytes and ovulation of mature eggs. A number of researchers have successfully produced larvae after using salmon pituitary extracts and human chorionic gonadotropin (hCG) for female and male, respectively (Tanaka et al. 2003). Although the production of leptocephali larvae (Tanaka et al. 2001) and glass eels (Tanaka et al. 2003) has been achieved using feed developed for eel larvae, still the production of leptocephali and cultured glass eels is low. Kagawa et al. (2005) developed the method for induction of maturation and ovulation in the Japanese eel by using the maturation-inducing steroid (DHP). However, there were major differences in the extent of egg quality of the female eels induced in individual fish by this treatment and the reasons for inadequate quality of eggs were not elucidated. The egg quality obtained from the female eels induced by hormonal treatment procedures varies among the female eels; thus, further studies are necessary to define the factors associated with egg quality and improve technical procedures (Kagawa et al. 2013). The effect of the injection frequency on the biochemical composition of eggs is less often considered.
The ability to produce good quality fertilised eggs, giving larvae with high survival rate and good growth, is a major bottleneck. According to Kjørsvik et al. (1990), the biochemical composition of fish gonads has a major influence on egg quality, since the gonads contain all the nutrients for the eggs. Other authors suggested earlier that vitamins and essential fatty acids (EFA) are of great importance to egg quality in both freshwater and marine fishes (Takeuchi et al. 1981; Soliman et al. 1986). It is also documented that ARA and DHA are accumulate in ovarian polar lipids during maturation (Støttrup et al. 2013), and optimising egg arachidonic acid (20:4 n-6) (ARA), eicosapentaenoic acid (20:5 n-3) (EPA) and docosahexaenoic acid (22:6 n-3) (DHA) contents and their mutual ratios is important for embryonic development and larval survival (Tomkiewicz et al. 2013). Although different administration techniques of hormones to eels are used, little or nothing is known about the effect of hormone injection frequency on gonadal biochemical composition in them. The aim of this study is to assess the effect of hormone injection frequency, i.e. weekly versus biweekly, on the gonadal biochemical composition with emphasis on lipids in Japanese eel, A. japonica.
Materials and methods
Animals
Downstream migrating silver Japanese eels, A. japonica (males and females), were captured in freshwater rivers by a local supplier from Jiangsu province, China, in November and transported to the aquarium facilities at Shanghai Ocean University. The eels were first acclimatised to sea water in outdoor concrete ponds for 2 month. Female eels were firstly treated in concrete ponds, and then the fish of both sex for lipid experiment were transferred to aquaria before frequency test of hormone treatment. Fish were kept in 30 g/L artificial seawater (Red Sea, China) in 300-L aquaria connected to a recirculation system. Males were kept at a density of 3 per 300-L tank, whereas females were kept at 2 per 300-L tank. Roofing tiles were added to serve as shelter. Fish were not fed during the entire period of the experiment. Injection wounds were treated manually with solutions of potassium dichromate (1 %) and silver nitrate (1 %). All experiments were performed in accordance with China Home Office Regulatory requirements.
Hormonal treatment
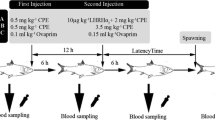
All female fish from experimental group were firstly treated with hormone every 2 weeks in concrete ponds during February 2010, and then kept in aquaria during March and April for a further 4 treatments. All male fish were not treated with hormone in concrete ponds during February 2010, and received 4 treatments in aquaria during March and April. The eels were divided into two experimental groups. Experimental group 1 consisted of the weekly (once every week) hormone-treated fish and experimental group 2 the biweekly (once every two weeks) hormone-treated fish. The total number of administered hormone injections was six for each female group and four for each male group. The females were injected with locally purchased carp pituitary extract (CPE, Hunan, China) and human chorionic gonadotropin (hCG, Ningbo, China) at a dose of 0.75 mg CPE/kg BW plus 150 IU/kg BW hCG. The males received exactly half-dose as compared to the females and the first two injections were not administered to males (Zhang et al. 2007). Before injection, fish were anaesthetised in 100 mg/L tricaine methanesulfonate (MS-222, Shandong, China).
Final measurements
At the end of the experiment, fish were humanely killed using a standard protocol detailed under China Home Office license procedures; the body weight (BW), waist length (WL), eye diameter, fin length (Lf) and total length (TL) were measured; and gonads, liver and muscle were removed and weighed and then stored at −80 °C. All samples were taken during the daytime.
Six morphometric indices were calculated as follows:
-
1.
The ocular index (OI) determines the eye area and takes into consideration the TL of the eel (Pankhurst 1982), this was obtained as follows:
$$ {\text{OI = [}}({{({\text{C}} + {\text{D}}} \mathord{\left/ {\vphantom {{({\text{C}} + {\text{D}}} 4}} \right. \kern-0pt} 4})2 \times \pi /{\text{TL]}} \times 100 $$where C and D stands for horizontal and vertical diameters, respectively, of the eye.
-
2.
The fin index (FI) was calculated as follows
$$ {\text{FI}} = [\text{L}_{\text{f}} /{\text{TL}}] \times 100 $$Lf stands for fin length.
-
3.
The gonadosomatic index (GSI) was calculated as follows:
$$ {\text{GSI}}\,=\,[{\text{GW}}/{\text{BW}}] \times 100 $$GW and BW stand for gonad weight and body weight, respectively.
-
4.
The hepatosomatic index (HSI) was calculated as follows:
$$ {\text{HSI = [LW}}/{\text{BW]}} \times 100 $$LW stands for liver weight.
-
5.
The BW index (BWI) was calculated as follows:
$$ {\text{BWI}} = [{\text{BW}}/{\text{IBW}}] \times 100 $$IBW stands for initial body weight.
-
6.
The BW increase was calculated as follows:
$$ {\text{BW}}\,{\text{increase}} = [{\text{BWt }} - {\text{BWto}}]/{\text{t}} $$BWt, BWto and t stand for finial body weight, initial body weight and time.
Examination of gonad maturity
Preparation of eel gonads sections. The eel gonads were dissected and fixed in 4 % paraformaldehyde in phosphate-buffered saline. Tissues were dehydrated through graded concentrations of ethanol and embedded in paraffin wax. Longitudinal 6-μm-thick sections were cut, mounted on glass slides and incubated at 37 °C overnight.
After dewaxing in three changes in xylene, the sections were rehydrated through a series of ethanols and finally into water. All samples were then stained with haematoxylin and eosin. The samples were observed under the microscope (Zeiss Stereo Discovery V12, Germany) connected to a computer. The mean oocyte diameter (OD ± 1 μm) of the ovary was determined by Image—Pro Plus (1999). For each female, 30 random oocytes with clear nucleus were considered, and both their vertical and horizontal diameters were measured. The stage of maturation for oocytes and spermatogonia was evaluated following Yamamoto and Yamauchi (1974), Guraya et al. (1975) and Miura et al. (1991). Each oocyte was categorised into one of the following stages): stage I, oogonium; stage II, previtellogenic oocyte (very few or no oil droplets); stage III, presence of cortical alveolus (many oil droplets); stage IV, early vitellogenic oocyte (peripheral yolk granules); stage V, midvitellogenic oocyte (peripheral yolk granules as well as central yolk platelets).
Assays of water and protein content
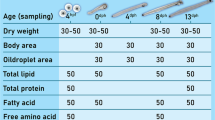
The dorsal muscle, liver and gonads from all hormone-treated groups were analysed in duplicate for moisture and protein according to classic methods (AOAC 1984, 1996): moisture was determined by oven drying at 105 °C to constant weight. Crude protein was determined using the Kjeldahl method and by multiplying the nitrogen content by 6.25. Water content was expressed as a percentage; protein and total lipid were expressed as %DW.
Analyses of lipid content and fatty acid composition
The remaining tissues were freeze-dried at −46 °C. Before the assay was performed, dried tissues were ground to a powder individually. TL of each sample was extracted with chloroform–methanol (2:1, V/V), according to the method of Folch et al. (1957). Fatty acid methyl esters were prepared by transesterification with 0.4 M KOH–methanol, and then detected by gas chromatograph (GC-6890A, USA) following Huang et al. (2010). Fatty acid content was determined using the normalisation method. All measurements were taken in triplicate, and the fatty acids content was expressed as area percentage.
Statistical analysis
Statistical analysis was performed using two-way ANOVA (GraphPad Prism 4) to detect significant differences between means of analytical data on weekly and biweekly hormone treatment groups for both males and females. However, for the oocyte diameter, significant difference was detected by t test. Statistical significance was determined at the 5 % level. All results are presented as mean ± standard deviation.
Results
The frequency experiment was ended after the 4th injection of fish in aquarium tanks (total 6th injection for females and 4th injection for male). During the experiment, a gradual skin colour change was noted, i.e. the dorsal side became blacker and the ventral side more silver. This occurred for both treatments and sexes, but the control fish did not change colour. There were 6 females for both of the weekly (mean body weight 1289.0 ± 306.8 g; mean total length 924 ± 66 mm) and the biweekly (mean body weight 1,186.0 ± 371.5 g; mean total length 885 ± 64 mm) hormone-treated groups of Japanese eels. There were only 4 males for both of the weekly (mean body weight 558.3 ± 78.5 g; mean total length 731 ± 48 mm) and the biweekly (mean body weight 485.3 ± 111.8 g; mean total length 685 ± 60 mm) hormone-treated groups. No significant correlation was noted between the gonad developmental phases and the body weights or body lengths among Japanese eels during the treatment. GSI and HSI are summarised in Table 1. The GSI of the weekly hormone-treated females (4.8 ± 2.2 %) was not significantly different from the biweekly hormone-treated females (3.5 ± 1.9 %) (p > 0.05). However, the weekly treated males (1.4 ± 0.4 %) was higher than the biweekly treated females (0.4 ± 0.3 %) (p < 0.05). For HSI, there was no significant difference obtained between treatments from both females and males (Table 1).
Gonad development
All gonads of six randomly picked female Japanese eels (1,199.0 ± 206.1) were at stage II (GSI 1.9 ± 0.15 %) before the hormone treatment, and the experimental control groups (1,249.0 ± 267.4) were also found to be at stage II (GSI 2.04 ± 0.4 %) as well. There was variation in the ovaries of the individual fish, but mainly, the ovaries of both the weekly and the biweekly hormone-treated groups were found to be at stage IV: early vitellogenic oocyte. The ovaries of all females treated with hormones every week were in stage IV except one female that was in stage III: cortical alveolus stage. Ovaries of two fish from the biweekly hormone-treated group were found to be at stage III. However, ovaries from the remaining of four females from the biweekly hormone-treated group were in stage IV. Vitellogenic oocytes at stage V were not observed in any of the ovaries examined (n = 12) (Fig. 1; Table 1). Males from both groups had type B spermatogonia following the terminology of Miura et al. (1991). These spermatogonia were mainly in the late type B stage as a result of their smaller size and greater number. However, there were no fish with more advanced spermatocytes, spermatids or spermatozoa (Fig. 1).
Oocyte stages of cultured Japanese eels that underwent hormone injections. a Female from the biweekly hormone-treated group with yolk granules (arrow) in the edge of cytoplasm and the nucleolus is close to the nuclear membrane (thin arrow). b Female from the weekly hormone-treated group with more yolk granules in the edge of cytoplasm (arrow) and the number of nucleolus increased and squeezing from nucleus (thin arrow). c Female from the biweekly hormone-treated group, the oocytes entered early phase of vitellogenesis stage and yolk granules distributed in the centre of cytoplasm, (arrow). d Female from the weekly hormone-treated group, the large oocyte in vitellogenesis stage packed with lipid droplets and yolk granules in cytoplasm (arrow). e Male from the biweekly hormone-treated group with spermatocyte (arrow). f Male from the weekly hormone-treated group with more spermatocyte (arrow). All scale bars = 100 μm
Water, total protein and lipid content in tissues of the weekly and the biweekly treated fishes
The moisture content of the gonads from females and males of the weekly hormone-treated group were 67.3 ± 1.5 and 69.2 ± 2.5 %, respectively. This was almost similar to the fish from the biweekly hormone-treated group, 67.6 ± 1.3 and 68.9 ± 3.2 % for females and males, respectively. The protein content of the three tissues, gonad, muscle and liver for both treatments and sex, did not show significant differences (Table 2).
Total lipid content of the gonads from females of the weekly hormone-treated group (20.6 ± 1.2 %) was higher than that of the biweekly treated group (16.8 ± 0.7 %). A similar difference was also observed in males of these two groups, which had values of 18.0 ± 2.3 % for weekly and 15.5 ± 1.3 % for the biweekly hormone treatments. In the muscle, however, there was no difference observed; females from the weekly and the biweekly treatments had values of 41.0 ± 2.5 and 40.4 ± 3.1 %, respectively. Males from both treatments had almost similar values; 40.9 ± 2.3 and 41.6 ± 2.9 % for the weekly and the biweekly hormone-treated fish, respectively. In the liver, there was no difference observed between males and females in total lipid content; the weekly hormone-treated fish had a value of 25.8 ± 3.8 % (females) and 26.9 ± 0.8 (males), while in the biweekly hormone-treated group, the total lipid content was 24.3 ± 1.2 and 23.7 ± 2.8 % for females and males, respectively.
Fatty acid profiles in the tissues of the weekly and the biweekly treated fishes
The major fatty acids in the gonads were 16.00, 16.01, 17.01, 18.1(n-9) and DHA for males and females in both hormonal treatment groups (Table 3). The DHA levels were for the weekly hormone-treated females (10.0 ± 0.5 %) and males (9.9 ± 0.4 %) and for the biweekly hormone-treated females (7.2 ± 0.6 %) and males (8.3 ± 0.5 %). The EPA levels were for the weekly hormone-treated females (3.6 ± 0.4 %) and males (6.0 ± 1.4 %) and for the biweekly hormone-treated females (2.6 ± 0.4 %) and males (4.4 ± 0.1 %). The ARA levels were for the weekly hormone-treated females (3.3 ± 0.4 %) and males (4.3 ± 0.3 %) and for the biweekly hormone-treated females (2.3 ± 0.4 %) and males (2.4 ± 0.4 %). Furthermore, males had a significantly higher value of EPA than females (p < 0.05). EPA, DHA and ARA were significantly higher in fish from the weekly treatment than the biweekly treatment group.
The major fatty acids in the muscle were 16.00, 16.01, 18.00, 18.1(n-9) and DHA for males and females in both hormonal treatment groups (Table 4). As it was the case in the gonads, males had a significantly higher value of EPA than females (p < 0.05). EPA, DHA and ARA were significantly higher in fish from the weekly treatment than the biweekly treatment group.
The major fatty acids in the liver were 16.00, 16.01, 18.1(n-7), 18.1(n-9) and DHA for males and females in both hormonal treatment groups (Table 5). There was no significant difference in DHA and ARA for the both treatments. However, EPA was the only HUFA that was significantly higher in the weekly than the biweekly treated fish.
Discussion
This study describes for the first time the effect of hormone injection frequency on the lipid content and fatty acid compositions in gonad, muscle and liver of A. japonica during artificially induced gonad maturation. The present study demonstrates that the average total lipid content of the gonads from the weekly hormone-injected group was significantly higher than the biweekly hormone-injected group (p < 0.05). The gonads from the weekly hormone-injected fish had more fatty acids, particularly saturated fatty acids (SFA), polyunsaturated fatty acids (PUFA), highly unsaturated fatty acids (HUFA), eicosapentaenoic acid (20:5n-3, EPA), docosahexaenoic acid (22:6n-3, DHA) and arachidonic acid (20:4n-6, ARA), than the biweekly treated fish. There was no significant difference in the lipid content of muscle and liver. Furthermore, the weekly hormone-treated females had bigger oocyte diameter than the biweekly females. These results indicate that hormone injection frequency affects efficiency of nutrient transfer from muscle and liver to gonads, especially lipids.
During embryogenesis and larval development, growth and available energy in most fish species are dependent on endogenous yolk reserves transferred by the mother fish. Therefore, the nutrient reserves in gonads of the broodstocks are vital for the normal development of the embryo and larvae. The lipid reserves of fish eggs are used by the developing larvae both as substrates for energy metabolism and as structural components in membrane biogenesis (Sargent 1995).
In fish, artificial hormones have been reported to affect various organs like kidney, liver, muscle, testes and ovary (Ikeuchi et al. 1999). Other experiments revealed that females grow faster than males because spermatogenesis occurs much faster than oogenesis during gonadotropin treatment (Ohta et al. 1997). Therefore, to achieve simultaneous maturation between sexes by the same treatment, it was necessary to start hormone treatment in males later, after females have started. In Japanese eel, the change in skin colour during the maturation process is correlated with gonad development (Han et al. 2003). In this study, we confirmed the colour change for all hormone-treated fish, whereas the control group showed no colour change. Although Han et al. (2003) observed more pronounced silver colour on the ventral side and black on the dorsal side in males than females, this was not confirmed in the present study. The low hormone dosage in males might be the cause since the males received only half-dose of the females.
The hormone injection frequency had no influence on BW increase, TL, BWI, OI, GSI, and WL, since we found no significant difference between the weekly and biweekly hormone-treated fish. Furthermore, FI and HSI did not show differences in both treatments and sexes. This is contrary to the findings from other reports, which found higher values in females than in males (Tzeng et al. 2003). The reason might be either low sample size or that the females were still growing.
In this study, spermiation of Japanese eels could not be observed after four injections for both groups of males, the weekly or the biweekly hormone-treated groups. Pedersen (2003) found spermiation to start after four weekly HCG injections. However, this study showed that both groups of males had no more advanced spermatocytes, spermatids or spermatozoa. Also, Ohta and Unuma (2003) documented at least five injections were needed to give spermiation in Japanese male eels. Despite the absence of spermiation observed in both hormone-treated groups, there was a difference in gonad development t stages. The testes of the males that were injected every week seemed to develop much faster than those injected every 2 weeks. Therefore, this indicates that weekly hormone injections had a better effect on testes development. Japanese female eels mature slowly due to complex mechanisms of steroidogenesis and function of sex steroid hormones during gametogenesis. However, there is a good response of oocyte to exogenous hormones (Kazeto et al. 2011). Therefore, they need several injections before ovulation. The high variability in gonad development stages (Table 3) within the same treatment group might be caused by the gonad status at the start of hormone injections. The females had poor gonad development either at stage I: oogonium or at stage II: previtellogenic oocyte when hormone injections started. These stages do not give good results with a few hormone injections (Ijiri et al. 1998). Japanese eels are normally at stage III (cortical alveolus stage) when they start spawning migration. This stage is desirable to start hormone injections for inducing maturation (Ijiri et al. 1998). However, eels show a lot of variation in gonad development, and this might be due to differences in management. There was no fish in stage V: midvitellogenic oocyte (containing peripheral yolk granules and central yolk platelets), it is clear that more injections were needed to reach this stage. The differences in gonad development stages were observed after histological examination. However, some external features, fin length and eye diameter could not show difference between the two groups. This shows that most of the changes start at cellular level before they can be observed externally.
Greater lipid content in the weekly than the biweekly hormone-treated fish is a good indication that this group was more mature, because, as maturation reaches completion, more nutrients are mobilised (Adachi et al. 2003). However, there was no difference in total lipid content for muscle and liver. There might be a direct influence of feed composition on the muscle and liver, because this depends on broodstock nutrition before the start of hormone injections.
Polyunsaturated fatty acids including long chain (≥20 carbon) HUFA are very important and abundant in the eggs of eel. Sargent (1995) documented the importance of eicosapentaenoic acid (20:5n-3, EPA), docosahexaenoic acid (22:6n-3, DHA) and arachidonic acid (20:4n-6, ARA) in the process of hatching and survival of larvae before exogenous feeding. However, broodstock management including the maturation procedure might have an influence on the HUFA in gonads (Sargent et al. 1989). Kagawa et al. 2013 documented variation in egg quality obtained from the female eels injected with exogenous hormones. Different researchers have shown the influence of n-3 and n-6 HUFA on the development of larvae, growth and survival. Fernández-Palacios et al. (1995) observed poor hatching rates and survival in sea bream, linked to low fatty acid content. Also, Furuita et al. (2002, 2003) observed the same thing in Japanese flounder. In marine fish, EPA and, especially, DHA are regarded as EFAs due to their necessity for good growth. ARA stimulates testicular testosterone production in goldfish testes and ovaries by conversion to prostaglandin (Wade and Van Der Kraak 1993; Mercure and Van Der Kraak 1996). There is strong evidence that HUFAs, particularly EPA, DHA and ARA, are involved in steroidogenesis and oocyte maturation in vertebrates via metabolites formed from the cyclooxygenase and lipoxygenase pathways (Murdoch et al. 1993). In our study, the higher EPA, DHA and ARA in both females and males of the weekly hormone-treated group compared with the biweekly hormone-treated group is an indication of the positive effect of weekly hormone injection in the gonad maturation process. These HUFA normally increase as the gonads mature preparing the eggs for better hatching and survival of the larvae (Sargent 1995).
We also expected to have clear differences in these HUFA between males and females in a given treatment, because there are more HUFA in ovaries than in testes (Sargent 1995). However, this is not the case for our study. In each treatment, EPA was higher in males than in females. The hormone dosage for females was twice that of males in each group. Therefore, higher hormone dosage might interfere with the formation of these HUFA (Ozaki et al. 2008). Moreover, the present study confirms that HUFA levels in the liver and gonads are remarkably higher than in muscle for both the weekly hormone-injected fish and the biweekly hormone-injected fish. Higher levels of HUFA in gonads are due to its importance of eggs hatching and survival of larvae (Sargent 1995); also, there is possibility that HUFA was further synthesised in the liver.
We also noticed more PUFA in the weekly hormone-injected group than the biweekly hormone-injected group for gonads and liver but not for muscles. However, for both groups, this was below 20 %. In contrast, monounsaturated fatty acids were above 50 % of total fatty acids in gonads, while liver and muscle had about 50 %. We believe that these results reflect that muscle lipids including these fatty acids were excessively transported to liver and gonads, and lipids were rarely consumed as an energy source in the liver. Consequently, lipids that had not been depleted with respect to saturated and monounsaturated fatty acids by liver energy requirements were transported to gonads (Ozaki et al. 2008).
Generally, we observed a lower ratio of EPA to ARA in the weekly hormone-injected group compared with the biweekly hormone-treated group. This signifies that the weekly hormone-injected group had matured more than the weekly hormone-injected group. ARA-derived prostaglandins are involved in reproduction maturation. During maturation, both EPA and ARA compete for the same cyclooxygenase enzyme, but the rate oxidation of EPA is only 10 % of ARA (Wada et al. 2007; Phillis et al. 2006; Zhao et al. 2004).
There was higher DHA/EPA ratio in the weekly hormone-injected group than the biweekly hormone-injected group for gonads, and the ratio was higher than suggested. The yolk of many wild marine fish eggs contains a DHA/EPA ratio of about 2:1 (Parrish et al. 1994). The higher ratio observed in the present study might be attributed by broodstock nutrition before the start of hormone injections since the dietary lipids have been recognised to modify the fatty acid composition of fish tissues including gonads (Bell et al. 2002; Støttrup et al. 2013).
In conclusion, the results obtained in the present study showed that the frequency of hormone injection had an effect on the biochemical composition of gonads. The average total lipid content of the gonads from the weekly hormone-injected group was significantly higher than the biweekly hormone-injected group. Furthermore, the gonads from the weekly hormone-injected fish had more fatty acids, particularly SFA, PUFA, HUFA, EPA, DHA and ARA, than others. These results suggest that both weekly and biweekly treatment of hormones, human chorionic gonadotropin and carp pituitary extract, can influence the maturation process in eel. Moreover, hormone injection frequency affects biochemical composition of gonads, especially lipids. This is, to our knowledge, the first report documented the effect of hormone injection frequency on the lipid content and fatty acid compositions in gonad of A. japonica during artificial maturation.
References
Adachi S, Ijiri S, Kazeto Y, Yamauchi K (2003) Oogenesis in the Japanese Eel, Anguilla japonica. In: Aida K, Tsukamoto K, Yamauchi K (eds) Eel Biology. Springer, Tokyo, pp 502–518
AOAC (1984) Official methods of analysis, in: Association of official analytical chemists (ed) 14th ed. Arlington, VA, pp. 152–169
AOAC (1996) Official methods of analysis, in: Windham W. (Ed.) 16th ed. Association of Official Analytical Chemists, Gaithersburg, Maryland, USA, p. 38
Bell JG, Henderson RJ, Tocher DR, McGhee F, Dick JR, Porter A (2002) Substituting fish oil with crude palm oil in the diet of Atlantic salmon (Salmo salar) affects muscle fatty acid composition and hepatic fatty acid metabolism. J Nutr 132:222–230
Fernández-Palacios H, Izquierdo MS, Robaina L, Valencia A, Salhi M, Vergara J (1995) Effect of n-3 HUFA level in broodstock diets on egg quality of gilthead seabrea, Sparus aurata. Aquaculture 132:325–337
Folch J, Lees M, Stanley GHS (1957) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226:497–509
Furuita H, Tanaka H, Yamamoto T, Suzuki N, Takeuchi T (2002) Effects of high levels of n-3 HUFA in broodstock diet on egg quality and egg fatty acid composition of the Japanese flounder, Paralichthys olivaceus. Aquaculture 210:323–333
Furuita H, Yamamoto T, Shima T, Suzuki N, Takeuchi T (2003) Effect of arachidonic acid levels in broodstock diet on larval and egg quality of Japanese flounder, Paralichthys olivaceus. Aquaculture 220:725–735
Guraya SS, Kaur R, Saxena PK (1975) Morphology of ovarian changes during the reproductive cycle of the fish, Mystus tengara. Acta Anat 91:222–260
Han YS, Liao IC, Huang YS, He JT, Chang CW, Tzeng WN (2003) Synchronous changes of morphology and gonadal development of silvering Japanese eel Anguilla japonica. Aquaculture 219:783–796
Huang X, Yin Y, Shi Z, Li W, Zhou H, Lv W (2010) Lipid content and fatty acid composition in wild-caught silver pomfret (Pampus argenteus) broodstocks: effects on gonad development. Aquaculture 310:192–199
Ijiri S, Kayaba T, Takeda N, Tachiki H, Adachi S, Yamauchi K (1998) Pretreatment reproductive stage and oocyte development induced by salmon pituitary homogenate in the Japanese eel, Anguilla japonica. Fish Sci 64:531–537
Ikeuchi T, Todo T, Kobayashi T, Nagahama Y (1999) cDNA cloning of a novel androgen receptor subtype. J Biol Chem 274:25205–25209
Image-Pro Plus (1999) Reference Guide for Windows, Version 6.0. Media Cybernetics
Kagawa H, Tanaka H, Ohta H, Unuma T, Nomura K (2005) The first success of glass eel production in the world: basic biology on fish reproduction advances new applied technology in aquaculture. Fish Physiol Biochem 31:193–199
Kagawa H, Sakurai Y, Horiuchi R, Kazeto Y, Gen K, Imaizumi H, Masuda Y (2013) Mechanism of oocyte maturation and ovulation and its application to seed production in the Japanese eel. Fish Physiol Biochem 39:13–17
Kazeto Y, Tosaka R, Matsubara H, Ijiri S, Adachi S (2011) Ovarian steroidogenesis and the role of sex steroid hormones on ovarian growth and maturation of the Japanese eel. J Steroid Biochem Mol Biol 127:149–154
Kjørsvik E, Mangor-Jensen A, Holmefjord I (1990) Egg quality in fishes. Adv Mar Biol 26:71–113
Mercure F, Van der Kraak G (1996) Mechanisms of action of free arachidonic acid on ovarian steroid production in the goldfish. Gen Comp Endocrinol 102:130–140
Miura T, Yamauchi K, Nagahama Y, Takahashi H (1991) Induction of spermatogenesis in male Japanese eel, Anguilla japonica, by a single injection of human chorionic gonadotropin. Zool Sci 8:63–73
Murdoch WJ, Hansen TR, McPherson LA (1993) A review—role of eicosanoids in vertebrate ovulation. Prostaglandins 46:85–115
Ohta H, Unuma T (2003) Induction of sperm maturation. In: Aida K, Tsukamoto K, Yamauchi K (eds) Eel Biology. Springer, Heidelberg, pp 415–424
Ohta H, Kagawa H, Tanaka H, Okuzawa K, Iinuma N (1997) Artificial induction of maturation and fertilization in the Japanese eel, Anguilla japonica. Fish Physiol Biochem 17:163–169
Ozaki Y, Koga H, Takahashi T, Adachi S, Yamauchi K (2008) Lipid content and fatty acid composition of muscle, liver, ovary and eggs of captive-reared and wild silver Japanese eel, Anguilla japonica during artificial maturation. Fish Sci 74:362–371
Pankhurst NW (1982) Relation of visual changes to the onset of sexual maturation in the European eel, Anguilla anguilla. J Fish Biol 21:127–140
Parrish CC, Castell JD, Brown JA, Boston L, Strickland JS, Somerton DC (1994) Fatty acid composition of Atlantic halibut eggs in relation to fertilization. Bull Aquac Assoc Can 94:36–38
Pedersen BH (2003) Induced sexual maturation of the European eel, Anguilla anguilla and fertilisation of the eggs. Aquaculture 224:323–338
Phillis JW, Horrocks LA, Farooqui AA (2006) Cyclooxygenases, lipoxygenases, epoxygenases in CNS: their role and involvement in neurological disorders. Brain Res Rev 52:201–243
Sargent JR (1995) Origins and functions of egg lipids: nutritional implications. In: Bromage NR, Roberts RJ (eds) Broodstock Management and Egg and Larval Quality. Blackwell Science, Oxford, pp 353–372
Sargent JR, Henderson RJ, Tocher DR (1989) The lipids. In: Halver JE (ed) Fish Nutrition, 2nd edn. Academic Press, San Diego, pp 153–218
Soliman AK, Jauncey K, Robert RJ (1986) The effect of dietary ascorbic acid supplementation on hatchability, survival rate and fry performance in Oreochromis mosambicus. Aquaculture 59:197–208
Støttrup JG, Jacobsen C, Tomkiewicz J, Jarlbæk H (2013) Modification of essential fatty acid composition in broodstock of cultured European eel Anguilla anguilla. Aquac Nutr 19:172–185
Takeuchi M, Ishii S, Ogiso T (1981) Effect of dietary vitamin E on growth, vitamin E distribution, and mortalities of the fertilized eggs and fry in Plecoglossus altivelis. Bull Tohoku Reg Fish Res Lab 104:111–121
Tanaka H, Kagawa H, Ohta H (2001) Production of leptocephali of Japanese eel, Anguilla japonica in captivity. Aquaculture 201:51–60
Tanaka H, Kagawa H, Ohta H, Unuma K, Nomura N (2003) The first production of glass eel in captivity: fish reproductive physiology facilitates great progress in aquaculture. Fish Physiol Biochem 28:493–497
Tatsukawa K (2003) Eel resources in East Asia. In: Aida K, Tsukamoto K, Yamauchi K (eds) Eel biology. Springer, Tokyo, pp 293–298
Tesch FK (2003) The Eel. Blackwell Science Ltd, Oxford, p 408
Tomkiewicz J, Støttrup JG, Corraze G, Kausik S, Holst LK, McEvoy F, Dufour S, Lafont AG, Asturiano JF, Sørensen SR, Tveiten H, De Schryver P, Butts IAE, Munk P, Zambonino-Infante J, Politis SN, Krüger-Johnsen M, Lauesen P (2013) Reproduction of European eel and larval culture. Aquaculture Europe, Trondheim 190
Tsukamoto K (1992) Discovery of the spawning area of the Japanese eel. Nature 356:789–791
Tzeng WN, Han YS, Liao IC, Huang YS, He JT, Chang CW (2003) Synchronous changes of morphology and gonadal development of silvering Japanese eel, Anguilla japonica. Aquaculture 219:783–796
Wada M, DeLong CJ, Hong YH, Rieke CJ, Sidhu RS, Yuan C, Warnock M, Schmaier AH, Yokoyama C, Smyth EM, Wilson SJ, FitzGerald GA, Garavito RM, de Sui X, Regan JW, Smith WL (2007) Enzymes and receptors of prostaglandin pathways with arachidonic acid-derived versus eicosapentaenoic acid-derived substrates and products. J Biol Chem 282:22254–22266
Wade MG, Van der Kraak G (1993) Arachidonic acid and prostaglandin E2 stimulate testosterone production by goldfish testes in vitro. Gen Comp Endocrinol 90:109–118
Yamamoto K, Yamauchi K (1974) Sexual maturation of Japanese eel and production of eel larvae in the aquarium. Nature 251:220–222
Yamauchi K, Yamamoto K (1982) Experiments on artificial maturation and fertilization of the Japanese eel, Anguilla japonica. In: Richter CJ and Th. Goos GJ (eds) Reproductive physiology of fish. Pudoc, Wageningen, pp. 185–189
Zhang J, Liu L, Guo F, Zhang T (2007) Histological study on artificial induction of gonadal development in Japanese eel Anguilla japonica. J Fish Sci China 14(4):593–599
Zhao Y, Joshi-Barve S, Barve S, Chen LH (2004) Eicosapentaenoic acid prevents LPS induced TNF-alpha expression by preventing NF-kappaB activation. J Am Coll Nutr 23:71–78
Acknowledgments
This work was supported by Shanghai Science & Technology Committee (08391910200 and 11PJ1404500) and Ghent University (VLIR-UOS MSc grant to EJR). The study was also supported by the AqASEM project (245020) under FP7, Shanghai Universities First-class Disciplines Project of Fisheries and Shanghai University Knowledge Service Platform, Shanghai Ocean University Aquatic Animal Breeding Center (ZF1206). We also acknowledge the valuable technical assistance from staff and students at the Fishery and Life Science College of Shanghai Ocean University.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Rupia, E.J., Shen, J., Wu, J. et al. Effect of hormone injection frequency on the lipid content and fatty acid compositions in gonad, muscle and liver of Anguilla japonica during artificial maturation. Aquacult Int 22, 1105–1120 (2014). https://doi.org/10.1007/s10499-013-9731-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-013-9731-9