Abstract
Three groups (wild—WS, semi-pond—SPS, and pond-cultured—PS) of ide Leuciscus idus (L.) were examined. Each group consisted of 30 females and 10 males, aged 5–8 years. All groups were stimulated with two agents: carp pituitary homogenate (CPH) and a synthetic hormonal preparation Ovopel. The amount of ovulating females decreased in conjunction with females’ domestication level, mainly because of very low efficacy of CPH for pond-reared ide stimulation. The percentage of ovulating females stimulated with Ovopel (97.0 %) was significantly higher comparing to CPH (63.0 %). No significant differences were found in latency time between the groups but spawning synchronization increased in conjunction with ide domestication. The positive correlation between domestication level and latency time, i.e., the higher the domestication level, the higher the latency time, was stated. No significant differences were found in PGSI as well as in total volume of obtained eggs, but groups differed significantly in the number of dry eggs and in number of swollen egg. Significant negative correlation between domestication level and number of swollen eggs, i.e., the higher the domestication level, the lower the number of eggs, was detected. The survival rate at the eyed stage was the highest in WS group (60.4 %) and PS group (57.7 %), and was significantly higher than in SPS group (51.8 %). Eggs obtained after CPH stimulation had higher survival rate at the eyed-egg stage (60.3 %) than eggs obtained from the fish stimulated with Ovopel (53.0 %). The data suggest that some form of domestication appeared in ide cultivated for several generations in carp pond.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rapid advancement in human civilization, particularly in the nineteenth and twentieth centuries, caused serious alterations and damage to the natural environment, especially in fish communities inhabiting river ecosystems. This statement refers not only to large migratory fish of Acipenseridae (Gershanovich and Burtsev 1993; Ivanov and Maznik 1997) or Salmonidae families (Solomon et al. 1999; Wiśniewolski 2002; Thorstad et al. 2008) but also to smaller and ‘non-migrating’ species like reophilic cyprinids (Lelek 1987; Lusk 1995; Fredrich 2003; Winter and Fredrich 2003). Ide Leuciscus idus (L.) is one of the large fluvial cyprinids, widely distributed in Europe, especially in rivers lower reaches. For many years, this species was very common or even dominant and had great economic importance in Central and Easter Europe (Witkowski et al. 1997). Now, its ecological status is recognized as ‘vulnerable to endangered’ (Lelek 1987; Schiemer and Spindler 1989; Lusk et al. 2004) and thus wild populations need preservation and strengthen. Restocking is one of the most important and popular ways of enhancing fish populations for both fisheries and ecological purposes (Cowx 1994; Bolland et al. 2008). Such programs, however, require numerous and genetically diversified broodstock herds (Philippart et al. 1989; Willot et al. 1997; Pelczarski 1998). In case of fluvial cyprinids, three methods are applied for acquiring broodstock. First, ready-to-spawn wild fish is caught on spawning grounds and directly stripped ‘in situ’ (Penaž 1974; Mills 1980) or transported to hatcheries where hormone stimulation is used for egg acquisition (Kaukoranta and Pennanen 1988; Kamerat and Wüstemann 1994; Kupren et al. 2008). Second, wild matured fish are caught out-of-reproduction season and kept in enclosure until the next spawning (Hochman 1959; Żarski et al. 2008). Third, spawning herds are cultivated from larvae in ponds (Cieśla et al. 2000; Żarski et al. 2009) or tanks (Targońska-Dietrich et al. 2004; Kujawa et al. 2006). Originally, carp pituitary homogenate (CPH) was used for artificial ide reproduction (Kucharczyk et al. 1999; Ciesla et al. 2000); however, since late 90s, alternative agents, such as human chorionic gonadotrophin (hCG) (Kucharczyk et al. 1999), synthetic hormonal preparations containing LH–RH-a or GnRH analogues with dopamine antagonist, like Ovopel or Ovaprim, have been implemented in hatchery practice (Targońska-Dietrich et al. 2004; Jamróz et al. 2008; Kayim et al. 2010; Cejko et al. 2012).
Broodstock strains grown and matured in different environmental conditions or stimulated with different hormonal preparations may significantly differ in reproduction effectiveness and reproductive parameters between Brooks et al. (1997); Shimada et al. (2007); Krejszeff et al. (2009); Kujawa et al. (2011); Křišt’an et al. (2012). This is also true even in the case of species that are a long-term object of aquaculture and cultivation such as carp (Brzuska 2000). The aim of this paper is to show differences in reproductive parameters of ide females raised in different environments before artificial stripping and stimulation with carp pituitary homogenate or Ovopel.
Materials and methods
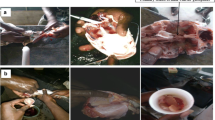
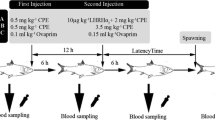
The experiment was carried out at ‘Łąki Jaktorowskie’ Fisheries Research Station (52°6′20.45″N, 20°31′40.9″E), belonging to the Warsaw University of Life Sciences–SGGW, Poland, from 1998 to 2012. Three groups of ide females were studied during the experiment. Each group consisted of 30 females and 10 males, aged 5–8 years. The first group consisted of wild fish (wild strain—WS group, F0 generation), obtained in 1998 from natural spawning grounds by electrofishing in Narew River near the northeastern town of Ostrołęka in Poland. Fish were transported to the hatchery at the Department of Ichthyobiology and Fisheries, Warsaw University of Life Sciences in 1,200-l isothermal plastic tank with oxygenated water. For transportation purposes, fish were sedated with 2-phenoxyethanol (0.02 ml dm−3). Fish were stimulated immediately upon arrival at the hatchery. The second group (semi-pond strain—SPS group) consisted of 1 + to 3 + ide juveniles obtained in 1998 during wild broodstock harvesting. These fish, totaling 200 individuals, were placed in ponds and reared for several years in polyculture with carp. The third group (pond strain—PS group) consisted of ide broodstocks reared from larvae in carp ponds. These fish were the third offspring generation (F3) of wild broodstocks obtained in 1998. Broodstock of males and females were kept separately in RAS unit equipped with six 2.0 × 2.0 × 0.7 m plastic tanks, biofilters, UV lamps, hitting, oxygenating, and aerating systems. Natural daylight regime was maintained and stimulation with water temperature was performed according to Cieśla et al. (2000). Prior to all manipulations, brood fish were anesthetized with 2-phenoxyethanol at the dose of 0.2–0.25 ml dm−3 of water. Measurements of fish were taken at the beginning of the reproduction procedure. Total length was measured to the nearest 1 mm, and the body weight was calculated electronically to nearest 0.1 g. The age of wild fish was calculated by way of studying the annual growth rings present in the scales. Each group of females was divided in two subgroups (n = 15) and stimulated with one of the hormonal agents. Subgroup 1 was injected with carp pituitary homogenate (CPH) (Argent, USA) intraperitoneally, under the left pectoral fin, in two injections. The first injection was 0.2 mg kg BW−1 and the second was 0.6 mg kg BW−1 (total 0.8 mg kg BW−1) at 12-h intervals (Cieśla et al. 2000). Subgroup 2 was stimulated with Ovopel (Unic-trade, Hungary) containing mammalian GnRH analogue (D-ala6, Pro9Net-mGnRH) and metoclopramide as a dopamine antagonist. This hormonal preparation was administered in one dose, 2 pellets kg BW−1 intraperitoneally under the left pectoral fin. Prior to administration, pituitary and Ovopel were homogenized in a mortar and dissolved in 0.9 % NaCl solution. Signs of ovulation were first checked at 16 h after hormone injection and subsequently at 2-h intervals.
Spawn from each female was collected separately into calibrated plastic barrels. The volume of eggs was determined to the nearest 2 ml. Mass of eggs was calculated electronically to nearest 0.1 g. An insulin syringe was used to withdraw 10 × 0.1 ml samples of unfertilized eggs from each female’s spawn to calculate the number of eggs in a 1 ml of ‘dry eggs’ volume. Percentage of ovulating females, latency time, pseudogonadosomatic index (PGSI) (Krejszeff et al. 2010), and post-management broodstock survival were recorded. Males were stimulated with half dose of the same hormonal preparations as used to stimulate the females. Sperm was collected using plastic syringes. Spermatozoa motility was not estimated.
Spawn from all females from each group was collected in plastic bowls and mixed with pooled sperm obtained from a minimum of six males of the same fish strain as females and fertilized using ‘dry method.’ Voynarovich solution was used for eggs rinsing. Ten samples, 100 of rinsed eggs each, were randomly taken to evaluate egg survival at the eyed-egg stage for each strain and group of females. Samples were placed in 250-ml net-made baskets and incubated at 15 ± 0.5 °C (Florez 1972; Cieśla et al. 2000) in RAS unit designed for large-scale ide spawn incubation in jars. To calculate the number of rinsed eggs in 1 ml, ten samples of rinsed eggs—1 ml each—were counted for each strain and subgroup of females. Samples were taken from Weiss jars at the end of egg-hardening process approximately 2 h after eggs rinsing and beginning of the incubation.
Statistical analysis was conducted with the use of two-way ANOVA, and Tukey’s method of multiple comparisons of means was used to calculate differences in hormonal preparation efficiency in each female strain and to calculate the influence of the female’s origin on reproductive parameters. Relationships between examined traits were evaluated with Pearson’s correlation coefficient. The analyses for percentage rates, i.e., embryos survival to eyed-egg stage, were conducted on arcsine transformed data. The correlations between strains (WS = 0, SPS = 1, and PS = 2) and reproductive parameters were calculated to detect the level of ide domestication. The analyses were performed in Statistical 7 (StatSoft). For all analyses, the probability level was set at P = 0.05.
Results
Ide females used for the experiments did not differ statistically in total length and body weight (Table 1). The shortest (365.5 ± 32.4 mm) and the longest (386.5 ± 16.8 mm) females were recorded in the PS group stimulated with Ovopel and with CPH, respectively. The smallest (639.8 ± 162.2 g ind−1) were fish from the PS group stimulated with CPH, and the largest (780.0 ± 106.3 g ind−1) were females from WS group stimulated with Ovopel.
The highest percentage of ovulating females was recorded in WS group (98 %), while in PS group it amounted to 62 % only, mostly because of decreasing efficacy of CPH in conjunction with ide domestication (Table 1). Natural hormonal preparation, CPH, induced ovulation in 95 % of the WS group, but only 33 % in the PS group. Stimulation with Ovopel induced ovulation in 90–100 % of all groups of ide females (Table 1). As a result, average percentage of ovulating females was 63.0 % for CPH and 97.0 % for Ovopel.
Slight differences in latency time were observed between female groups but these differences were not statistically significant (Table 1, ANOVA, P > 0.05). However, calculated correlation showed significant positive correlation between domestication level and latency time, i.e., the higher the domestication level, the higher the latency time (Table 3, r = 0.37, P < 0.05). Generally, there were no significant differences in latency time between stimulating agents, except from WS, where stimulation with CPH resulted in statistically significantly higher latency time comparing to Ovopel (Table 1, ANOVA, P < 0.05). No differences were found in PGSI between ide female strains (Table 1, ANOVA, P > 0.05). But fish from WS and SPS groups stimulated with CPH delivered approximately 2–3 % lower amount of spawn, compared to Ovopel-stimulated fish. In the PS group, a reversed tendency was observed, PGSI of females stimulated with CPH was 2.9 % higher compared to Ovopel-stimulated fish. Ovopel caused serious looses in broodstock by the end of the season. Unfortunately, this parameter was not recorded at the beginning of the experiment for wild stock of females. Average survival rate for females from SPS and PS groups stimulated with CPH was 95 %, while for fish injected with Ovopel, the survival rate was only 42.5 % (Table 1).
There were no statistically significant differences in the volume of obtained eggs, although the differences between mean values for the groups were quite large, especially when considering stimulating agent (Table 2, ANOVA, P > 0.05). For example, in WS group, almost 120 ml of spawn was obtained from one female after stimulation with CPH compared to 160 ml recovered from Ovopel-stimulated fish.
Significant differences were recorded in the number of dry eggs in 1 ml of spawn between the strains of ide’s females, all groups differed statistically significantly (Table 2, ANOVA, P < 0.05). The type of hormonal preparation significantly influenced also the number of dry eggs in 1 ml of unswollen spawn (Table 2). Differences were higher in case of WS and SPS groups of fish. Surprisingly, the highest and the lowest number of eggs were obtained in the same SPS group of females (Table 2). The difference was as high as 130 eggs per ml−1 of spawn. In the PS group of ide females, this difference was tenfold smaller, 14 eggs per ml−1 of spawn, but still statistically significant.
All groups differed statistically significantly in number of swollen eggs in volume unit (Table 2, ANOVA, P < 0.05). The highest amount of swollen eggs, 109 pcs in 1 ml of rinsed spawn, was recorded in the WS group stimulated with CPH, while the lowest, 85 pcs in 1 ml, was recorded in the SPS group stimulated with Ovopel. Significant negative correlation between domestication level and the number of swollen eggs in a volume unit, i.e., the higher the domestication level, the lower the amount of swollen eggs, was detected (Table 3, r = −0.45, P < 0.05). Stimulating agent had also statistically significant influence on the number of swollen eggs (Table 2, ANOVA, P < 0.05); yet, this difference was very small and insignificant between PS group subgroups (Table 2).
Significant differences were observed between strains of the ide females in spawn survival to the eyed-egg stage (Table 2). The highest survival rates were recorded in WS and PS groups, 60.4 and 57.7 % of embryos, respectively. Differences were not significant between both groups but statistically significant comparing to SPS group (Table 2, ANOVA, P < 0.05). Survival of eggs obtained from CPH-stimulated fish (60.3 %) was statistically significantly higher comparing to Ovopel-stimulated ones (53.0 %) (Table 2, ANOVA, P < 0.05). Between subgroups, the highest differences in the influence of hormonal preparations on egg survival at eyed-egg stage were observed in WS and PS group, and in PS group, this difference was even statistically significant (Table 2, ANOVA, P < 0.05). In SPS group, embryos survival until eyed-egg stage was approximately 50 %, difference between stimulating agents did not exceed 2 %, and difference was not statistically significant (Table 2, ANOVA, P > 0.05).
Discussion
Results presented in this paper suggest that long-term rearing in carp ponds may lead to ide domestication and cause significant differences in females’ reproductive parameters and their response to hormonal preparations. Average spawning success in WS group of ide was 98 %, in SPS group it was 80 %, but in PS group 62 % only. Krejszeff et al. 2008, 2010 stated that wild batches of chub L. cephalus (L.) spawn better than cultured stocks, although ovulation rates for CPH, Ovopel, and hCG preparations were relatively low (0–36 % only). Kujawa et al. (2011) also noted higher ovulation success in wild tench Tinca tinca L. than in cultured ones, both after Ovopel or CPH stimulation. In contrast, Targońska et al. (2011) studying barbel Barbus barbus L. found higher ovulation rate (80–90 %) in cultured strain, while in wild strain of females ovulation was observed in 0–25 % only. Differences in reproductive parameters between different fish populations were recorded not only in cyprinids. Křišt’an et al. (2012) analyzed reproduction characteristic for farmed and wild perch Perca fluviatilis L. and reported 92.3 % ovulation rate in farmed fish and 76.9 % in wild females. Although there were no statistically significant differences in latency time between ide groups, significant positive correlation between domestication level and latency time was detected. This result is opposite to other results obtained for fluvial cyprinids, where negative correlation was usually observed (Krejszeff et al. 2008, 2010; Targońska et al. 2011). Domesticated strains of ide not only delivered statistically significantly smaller eggs but also smaller amount of spawn and thus were less fecund than wild ones. Křišt’an et al. (2012) report the same phenomenon for perch. Spawn obtained from wild broodstocks is usually characterized by higher survival rate (Krejszeff et al. 2008, 2010; Kujawa et al. 2011; Targońska et al. 2011; Křišt’an et al. 2012). In our experiment, the highest differences in reproductive parameters (both in ‘+’ and in ‘−’) were usually observed in SPS group, i.e., fish which were rapidly moved from the river into carp ponds. Results for wild and pond-cultivated ide, fish ongrowing in different but stable environments, were usually more close to each other. For example, the difference in spawn survival till eyed-egg stage was the highest in the wild strain of females; however, comparing to pond-reared ide females, this difference was not statistically significant (Table 2).
According to many authors, artificial propagation of cyprinid fish requires stimulation with natural or synthetic hormone preparations (Horvath et al. 1997; Szabo et al. 2002; Linhart et al. 2006; Yaron et al. 2009). For ide, representative of the Cyprinidae family, such stimulation for artificial reproduction also seems to be indispensable (Ciesla et al. 2000; Kucharczyk et al. 2008). Only Targońska-Dietrich et al. (2004) and Krejszeff et al. (2009) report successful ide spawning without hormonal treatment. In our experiment, CPH was very effective when used for wild fish stimulation, with almost 100 % ovulating success. But when used to stimulate ide females cultivated for several years in ponds (PS), efficacy of this preparation was two or three times lower compared to wild stocks (Table 1). Kucharczyk et al. (1999) and (Ciesla et al. 2000) also reported very high percentage of ovulation in wild ide females stimulated with CPH. The use of CPH for the stimulation of pond-reared ide females, especially at two doses, resulted in rapid decrease in its efficacy (Cieśla et al. 2012). Lower efficacy of CPH to Ovopel stimulation was observed in the most domesticated cyprinid species, i.e., carp. Brzuska 2000, 2003 reports very low efficacy of CPH to Ovopel stimulations in different Polish and Hungarian lines of carp C. carpio (L.). Kayim et al. (2010 also) stated that CPH, when used for scaly carp stimulation, is less effective than Ovopel. Described lower efficacy of natural stimulator (CPH) to artificial stimulating preparations (e.g., Ovopel) in carp and pond-reared ide may be considered as indirect proof of ide domestication process in conjunction with cultivation in ponds. CPH usually causes decrease in latency time and makes ovulation more asynchronous (Kucharczyk et al. 1999; Brzuska 2000, 2003, 2006; Kujawa et al. 2011). In our experiment, after CPH administration, latency time was usually longer, but higher ovulation asynchrony was still observed, especially in WS group. However, spawning synchronization was increased in conjunction with ide ‘domestication’ (Table 1). In contrast to CPH, Ovopel was very effective in case of inducing ovulation in ide females. Regardless of the ide broodstocks origin, percentage of ovulating females was nearly 100 %. Szabo et al. (2002) described the stimulation of wild nase Chondrostoma nasus (L.) females with mGnRH analogue with domperidone as dopamine antagonist as more effective for ovulation induction than carp pituitary. Kujawa et al. (2011) report 66.7 % of ovulating pond-reared tench females stimulated with Ovopel and 50.0 % only for wild batch.
Data presented in this paper show that significant differences in reproductive parameters and hormonal preparations efficacy in ovulation induction and/or changes in reproductive parameters occurred between ide females in relation to their origin. The question is whether this is an effect of domestication. Several definitions of fish domestications can be found. According to Balon (2004), only common carp C. carpio L. and the goldfish Carrasius auratus (L.) can be recognized now as domesticated species. Both species are cultivated and kept for human-specific purposes and breeding is fully controlled. Morphology and physiology of cultivated lines of these species are different from wild forms to such extend that in some cases, they would not survive in nature. But even in case of ‘domesticated’ common carp, significant differences were observed during spawning, when different lines or strains of carp were stimulated with different hormonal preparations (Brzuska 2000, 2003, 2006). Following Balon’s definition, some similarities between carp and ide reared for several generations in ponds (F3 generation), in the context of domestication, can be found, for example, changes in ide female reproductive parameters, described in this paper. Ide is now reared on large scale for rivers and reservoirs restocking, and a growing demand for restocking material of this species is observed in Poland currently (Cieśla et al. 2000; Kucharczyk et al. 2008). Reproduction is fully controlled and several different stimulating preparations are successfully used for its artificial propagation. What is worth underlining is that pond-reared ide broodstock also spawns easily in a natural way when kept together during the spawning period in one pond. This phenomenon was never observed in case of wild ides kept in carp ponds. Production of restocking material based on larvae obtained from spawners reared in aquaculture is much stable and higher now, than it was several years ago when production was based on wild fish (Witkowski et al. 1997). All this suggests that some form of ide domestication occurs; nevertheless, this process seems to be at its early stage, and talking about ‘domesticated’ ide would be an overstatement now.
According to Bilio (2007), fish could be recognized as domesticated inter alia when kept for at least three generations in aquaculture. The PS group of ide females described in the present article fully fulfill this criterion. Teletchea and Fontaine (2012) suggest that in case of fish, levels of domestication should be rather taken into consideration than discussion whether species is domesticated or still wild. Following their classification, the PS group of ide should be considered as belonging to ‘domestication level 4,’ because whole production cycle is closed in captivity, is fully independent of wild broodstocks, but no breeding programs have been undertaken so far. It seems worthy to underline that the status of ide in Poland, in general, should be recognized at the level 4 of domestication. Almost all fish producers in Poland have own, local ide stock herds, reared for several years in carp ponds. Although precise data are not available, it can be estimated that these are at least F2–4 or even F5 pond-reared offspring generations.
Fish, unlike any other creature, show enormous environmental plasticity. Particularly, this is evidenced in the variability of fertility of various strains of the same species found in different geographical locations (Vøllestad and L’Abée-Lund 1990; Morita et al. 2009). (Tables 1, 2). Thus, further scrutiny is required to determine whether the reproductive changes will have long-lasting effect as a result of domestication or it is just a result of adaptation to the changing environment.
References
Balon EK (2004) About the oldest domestication among the fishes. J Fish Biol 65(s1):1–27. doi:10.1111/j.0022-1122.2004.00563.x
Bilio M (2007) Controlled reproduction and domestication in aquaculture—the current state of the art, Part II. Aquac Eur 32:5–23
Bolland JD, Cowx IG, Lucas MC (2008) Movements and habitat use of wild and stocked juvenile chub, Leuciscus cephalus (L.). J Fish Biol 71:1810–1819
Brooks S, Tyler CR, Sumpter JP (1997) Egg quality in fish what makes a good egg? Rev Fish Biol Fish 7:387–416
Brzuska E (2000) Artificial spawning of carp Cyprinus carpio L.: differences between the effects on reproduction in females of Polish and Hungarian provenance treated with carp pituitary and (D-Ala6)GnRH-ProNHEt (Kobarelin). Aquac Res 31:457–465
Brzuska E (2003) Artificial spawning of female Polish line 3 carp (Cyprinus carpio L.) after treatment with pituitary homogenate and/or Ovopel. Aquac Res 34:1321–1327
Brzuska E (2006) Artificial propagation of females Hungarian strain 7 carp (Cyprinus carpio) after treatment with carp pituitary homogenate, Ovopel or Dagin. Czech J Anim Sci 51:132–141
Cejko BI, Targońska K, Kowalski RK, Żarski D, Sarosiek B, Kucharczyk D, Głogowski J (2012) The effectiveness of hormonal preparations (Ovopel, Ovaprim, LHRHa, hCG and CPE) in stimulating spermiation in dace Leuciscus leuciscus (L.). J Appl Ichthyol 28:873–877. doi:10.1111/jai.12054
Cieśla M, Wojda R, Śliwiński J, Mizieliński M, Ostaszewska T (2000) Possibilities and threats in pond production of chosen reophilic Cyprinidae fish species. Folia Univ. Agric. Stetin. 214. Folia Univ Agric Stetin: Pisc 214(27):45–54
Cieśla M, Jończyk R, Gozdowski D, Wojda R, Śliwiński J (2012) Comparison of the effectiveness of carp pituitary homogenate (CPH), Ovopel and Ovaprim in controlled reproduction of ide Leuciscus idus L. cultivated in carp ponds. Sc An Pol Ang Ass 25:61–72
Cowx IG (1994) Stocking strategies. Fish Manag Ecol 1:15–30
Florez F (1972) The effect of temperature on incubation time, growth and lethality of embryos, larvae and juveniles of the ide Idus idus L. Inst Fresw Res Drottingholm 52:50–64
Fredrich F (2003) Long-term investigations of migratory behavior of asp (Aspius aspius L.) in the middle part of Elbe River. Ger J Appl Ich 19:294–302
Gershanovich AD, Burtsev IA (1993) Budut li zhit osetry v XXIw (Will acipensers exist in XXI century). Rybn Khoz 4:18–20
Hochman L (1959) Experiments with ide cultivation in ponds. Czech Ryb 5:69
Horvath L, Szabo T, Burke J (1997) Hatchery testing of GnRH analogue-containing pellets on ovulation in four cyprinid species. Pol Arch Hydrobiol 44:221–226
Ivanov VP, Maznik AJ (1997) Rybnoje khazjaistvo Kaspijskovo basseina (Belaja kniga) (The White Book of Caspian Sea fisheries). TOO Zhum Ryb Khoz Moskva 40:17–21
Jamróz M, Kucharczyk D, Hakuć-Błażowska A, Krejszeff S, Kujawa R, Kupren K, Kwiatkowski M, Targońska K, Żarski D, Cejko IB, Głogowski J (2008) Comparing the effectiveness of Ovopel, Ovaprim and LH-RH analogue used in the controlled reproduction of ide, Leuciscus idus (L.). Arch Pol Fish 16:363–370
Kamerat B, Wüstemann O (1994) Zür künslichen Vermerhung des Hasels (Leuciscus leuciscus L.). Fisch Teich 2:59–61
Kaukoranta M, Pennanen J (1988) Propagation and management of the asp (Aspius aspius L.) in Finland. Finn Game Fish Res Int Fish Div 6:1–14
Kayim M, Bozkurt Y, Ogretmen F (2010) Comparing the effectiveness of Ovopel and carp pituitary extract (CPE) on artificial spawning of scaly carp (Cyprinus carpio). J Anim Vet Adv 9:2589–2592. doi:10.3923/java.2010.2589.2592
Krejszeff S, Kucharczyk D, Kupren K, Targońska K, Mamcarz A, Kujawa R, Kaczkowski Z, Ratajski S (2008) Reproduction of chub Leuciscus cephalus L., under controlled conditions. Aquac Res 39:907–912
Krejszeff S, Targońska K, Żarski D, Kucharczyk D (2009) Domestication affects spawning of the ide (Leuciscus idus)—preliminary study. Aquaculture 295:145–147. doi:10.1016/j.aquaculture.2009.06.032
Krejszeff S, Targońska K, Żarski D, Kucharczyk D (2010) Artificial reproduction of two different spaw-forms of the chub. Repr Biol 10:67–74
Křišt’an J, Stejskal V, Policar T (2012) Comparison of reproduction characteristics and broodstock mortality in farmed and wild Eurasian perch (Perca fluviatlis L.) females during spawning season under controlled conditions. Turkish J Fish Aquat Sci 12:191–197. doi:10.4194/1303-2712-v12_2_01
Kucharczyk D, Kujawa R, Mamcarz A, Wyszomirska E, Ulikowski D (1999) Artificial spawning of ide (Leuciscus idus) under controlled conditions. EJPAU 2(2):http://www.ejpau.media.pl/volume2.issue2/fisheries/art-05.html
Kucharczyk D, Targońska K, Żarski D, Kujawa R, Mamcarz A (2008) A review of the reproduction biotechnology for fish from the genus Leuciscus. Arch Pol Fish 16:319–340. doi:10.2478/s10086-008-0021-5
Kujawa R, Kucharczyk D, Mamcarz A (2006) Reproduction of dace (Leuciscus leuciscus) under controlled conditions. In: Zakęś Z, Demska-Zakęś K, Wolnicki J (eds) Reproduction, rearing and prophylactics in cyprind fish and other species. Inland Fisheries Institute, Olsztyn, pp 37–43
Kujawa R, Kucharczyk D, Mamcarz A, Żarski D, Targońska K (2011) Artificial spawning of common tench Tinca tinca (Linnaeus, 1758), obtained from wild and domestic stocks. Aquac Int 19:513–521. doi:10.1007/s10499-010-9366-z
Kupren K, Turkowski K, Kucharczyk D, Krejszeff S, Żarski S, Hakuć-Błażowska A, Targońska K, Kwiatkowski M, Jamróz M, Czarkowski T (2008) Economic aspects of rearing larval asp, Aspius aspius (L.) and ide, Leuciscus idus (L.) in closed recirculating systems. Arch Pol Fish 16:413–420
Lelek A (1987) The freshwater fishes of Europe, vol 9. AULA-Verlag, Wiesbaden
Linhart O, Rodina M, Kocour M, Gela D (2006) Insemination, fertilization and gamete management in tench, Tinca tinca (L.). Aquac Int 14:61–73
Lusk S (1995) The status of Chondrostoma nasus in waters of the Czech Republic. Folia Zool 45:1–8
Lusk S, Hanel L, Luskova V (2004) Red list of the ichthyofauna of the Czech Republic: development and present status. Folia Zool 53:215–226
Mills CA (1980) Spawning and rearing eggs of dace (Leuciscus leuciscus L.). Fish Manag 11:67–72
Morita K, Tamate T, Sugimoto Y, Tago Y, Watanabe T, Konaka H, Sato M, Miyauchi Y, Ohkuma K, Nagasawa T (2009) Latitudinal variation in egg size and number in anadromus masu salmon Oncorhynchus masou. J Fish Biol 74:699–705
Pelczarski W (1998) Reintroduction of whitefish, Coregonus lavaretus L., into Puck Bay (southern Baltic). In: Cowx IG (ed) Stocking and introduction of fish. News Books, pp 294–300
Penaž M (1974) Early development of the nase carp, Chondrostoma nasus L. Zool Listy 23:275–288
Philippart J, Melard Ch, Poncin P (1989) Intensive culture of the common barbel Barbus barbus L. for restocking. In: De Pauv N, Jaspers E, Ackefors H, Wilkins N (eds) Aquaculture—a biochemistry in progress, European Aquaculture Society, Bredene, Belgium, pp 483–491
Schiemer F, Spindler T (1989) Endangered fish species of the Danube River in Austria. Regul Rivers: Res Manag 4:397–407
Shimada Y, Shikano T, Murakami N, Tsuzaki T, Seikal T (2007) Maternal and genetic effects on individual variation during early development in Japanese flounder Paralichthys olivaceus. Fish Sci 73:244–249
Solomon DJ, Sambrook HT, Broad KJ (1999) Salmon migration and river flow. Results of tracking radio tagged salmon in six rivers in South West England. Res Dev Publ 4:1–110
Szabo T, Medgyasszagy C, Horváth L (2002) Ovulation induction in nase (Chondrostoma nasus, Cyprinidae) using carp pituitary extract or GnRH analogue combined with domperidone. Aquaculture 203:389–395. doi:10.1016/S0044-8486(01)00635-4
Targońska K, Kucharczyk D, Żarski D, Cejko BI, Krejszeff S, Kuprem K, Król R, Dryl K, Kowalski RK, Głogowski J (2011) Artificial reproduction of wild and cultured barbel (Barbus barbus, Cyprinidae) under controlled conditions. Acta Vet Hung 59:363–372. doi:10.1556/AVet.2011.021
Targońska-Dietrich K, Zielazny T, Kucharczyk D, Mamcarz A, Kujawa R (2004) Out-of-season spawning of cultured ide (Leuciscus idus L.) under controlled conditions. EJPAU 7:http://www.ejpau.media.pl/volume7/issue2/fisheries/art-02.html
Teletchea F, Fontaine P (2012) Levels of domestication in fish: implications for the sustainable future of aquaculture. Fish Fish. doi:101111/faf.12006
Thorstad EB, Økland F, Aarestrup K, Heggberget TG (2008) Factors affecting the within-river spawning migration of Atlantic salmon, with emphasis on human impacts. Rev Fish Biol Fish 18:345–371
Vøllestad LA, L’Abée-Lund JH (1990) Geographic variation in life-history strategy of female roach, Rutilus rutilus (L.). J Fish Biol 37:853–864
Willot P, Rochard E, Castelnaut G, Rouauld T, Brun R, Lepage M, Ellie P (1997) Biological characteristic of European Atlantic sturgeon, Acipenser sturio, as the basis for restoration program in France. Environ Biol Fish 48:359–370
Winter HV, Fredrich F (2003) Migratory behavior of ide: a comparison between lowland rivers Elbe, Germany and Vecht, The Netherlands. J Fish Biol 63:871–880. doi:10.1046/j.1095-8649.2003.00193.x
Wiśniewolski W (2002) Positive and negative factors influencing fish populations in streaming waters. Acta Hydrobiol 3:1–28
Witkowski A, Cieśla M, Napora K (1997) Ide. Inland Fisheries Institute, Olsztyn
Yaron Z, Bogomolnaya A, Drori S, Biton I, Aizen J, Kulikovsky Z, Levavi-Sivan B (2009) Spawning induction in the carp, past experience and future prospects. Isr J Aquac-Bamidgeh 61:5–26
Żarski D, Kucharczyk D, Targońska K, Chwaluwczyk R, Krejszeff S, Kwiatkowski M, Jamróz M (2008) Impact of the hormonal preparation Ovaprim on the effectiveness of asp (Aspius aspius) reproduction under controlled conditions. In: Zakęś Z, Wolnicki J, Demska-Zakęś K, Kamiński R, Ulikowski D (eds) Biotechnology in aquaculture. Inland Fisheries Institute, Olsztyn, pp 153–158
Żarski D, Kucharczyk D, Targońska K, Jamróz M, Krejszeff S, Mamcarz A (2009) Application of Ovopel and Ovaprim and their combinations in controlled reproduction of two reophilic cyprinid fish species. Pol J Natur Sci 24:235–244
Acknowledgments
The authors would like to thank the two anonymous reviewers for their critical review, helpful comments, and positive suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Cieśla, M., Jończyk, R., Gozdowski, D. et al. Changes in ide Leuciscus idus (L.) females’ reproductive parameters after stimulation with carp pituitary homogenate (CPH) and Ovopel: the effect of domestication?. Aquacult Int 22, 77–88 (2014). https://doi.org/10.1007/s10499-013-9668-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-013-9668-z