Abstract
Aflatoxicosis, toxicity of aflatoxin, is of great concern in aquaculture. This study was conducted to assess the efficacies of three adsorbents, a hydrated sodium calcium aluminosilicates (HSCAS), Saccharomyces cerevisiae (S.C.) and an esterified glucomannan (EGM), against feed contaminated with contained 200 μg/kg (ppb) aflatoxin B1 (AFB1). A total of 240 Nile tilapia fingerlings, Oreochromis niloticus (15 ± 2 g), were randomly divided into eight experimental groups (30 fish per group) with three replicates. Group T1 represented the negative control fed on a basal diet, and T2 was the positive control group fed on a basal diet supplemented with 200 ppb AFB1. Groups T3, T4 and T5 were fed the AFB1-contaminated diet (200 ppb) supplemented with 0.5 % HSCAS, 0.25 % S.C or 0.25 % EGM, respectively. Groups T6, T7 and T8 were fed a basal diet supplemented with 0.5 % HSCAS, 0.25 % S.C or 0.25 % EGM, respectively. The reduction in AFB1-bioavailability was judged by toxin residues in fish musculature throughout the study beginning at the second week of exposure. AFB1 reduced the survivability, total weight gain, average daily gain and specific growth rate, evident as early as the second week of exposure. The total erythrocyte count, hemoglobin content and total leukocyte count were significantly decreased after AFB1 exposure for 6, 8 and 10 weeks, respectively. Prolonged administration of AFB1 led to significant increases in serum alanine transaminase, aspartate transaminase and creatinine activity, and produced significant decreases in plasma proteins, including serum globulin. The specific immune response was assessed by an agglutinating antibody titer after immunization of the fish with an Aeromonas hydrophila vaccine. The antibody titer and relative level of protection of fish challenged with Aeromonas hydrophila were reduced throughout the period of examination in AFB1-exposed fish. Supplementation with HSCAS, S.C. or EGM significantly improved growth performance, blood parameters and immune status; in addition, these groups showed decreased AFB1 residues in fish musculature when compared with AFB1-treated fish. HSCAS effectively reduced AFB1 toxicity, whereas S.C. and EGM were less efficacious.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aflatoxins are secondary metabolites of the fungus Aspergillus flavus, A. parasiticus and A. nominus. Aflatoxins are produced in low-quality ingredients or upon improper storage of feed (Schoental 1967). Aflatoxin contamination is a major problem related to aquaculture that leads to economic losses and health complications in fish (Shane 1993; Chávez-Sánchez et al. 1994; Santacroce et al. 2008). Aflatoxin B1 (AFB1) is the most frequent, potent and toxic metabolite in humans, animals and aquatic organisms (Kennedy et al. 1998; Hussein and Brasel 2001). In addition, AFB1 is a naturally occurring hepatotoxic carcinogen, mutagen and teratogen (Alpert et al. 1971; JECFA 1998; Han et al. 2008).
Aflatoxin B1 susceptibility widely varies according to the specific aquatic species. Trout are the most sensitive fish to AFB1 (Horn et al. 1989). Other species such as channel catfish, coho salmon and zebrafish are less sensitive (Hendricks and Bailey 1989; Plakas et al. 1991; Tsai 1996). Nile tilapia is also influenced by the deleterious effects of AFB1 as low as 1.5 ppm (Zychowski et al. 2013). Aflatoxin B1 in feed led to a low growth rate, decreased food intake, poor food conversion ratio and increased disease susceptibility (El-Banna et al. 1992; Royes and Yanong 2002). Exposure to higher concentrations of AFB1 led to yellowing of the tilapia surface and an increase in mortality (Cagauan et al. 2004). In aquatic animals, AFB1 can cause abnormalities in serum biochemical and hematological parameters. The RBC (Red Blood Cell) count, hematocrit values, hemoglobin content and White Blood Cell (WBC) count are severely affected by AFB1 (Tuan et al. 2002; Mohapatra et al. 2011). Mycotoxins exhibit a variety of toxic effects in animals, primarily in the liver and kidney (Kovács 2004). Chronic aflatoxicosis produced remarkable gross changes and partial damage in the liver of Nile tilapia (El-Banna et al. 1992; Chávez-Sánchez et al. 1994; Tuan et al. 2002). One of the observed signs of aflatoxin toxicity is reduction in immune function (Celik et al. 2000). Total serum protein, albumin and globulin levels were significantly lower in hybrid tilapia and rohu exposed to AFB1 (Deng et al. 2010; Mohapatra et al. 2011). Furthermore, the bacterial agglutination titer and relative levels of protection were significantly suppressed due to AFB1 exposure (Sahoo and Mukherjee 2001, 2003).
Animal tissues can retain aflatoxin residues leading to suspected public health risks due to human consumption of contaminated food (Puschner 2002; Murjani 2003; Wu 1998; Boonyaratpalin et al. 2001). Prolonged exposure to AFB1 produced accumulation of toxic residues in the musculature of walleye fish and sea bass (Hussain et al. 1993; El-Sayed and Khalil 2009). In Nile tilapia, AFB1 residues were detected in the liver after exposure to even smaller doses of toxin (less than 2 mg/kg) (Deng et al. 2010). Abdelhamid et al. (2004) reported that AFB1 residues in flesh showed a cumulative effect based on the dietary AFB1 exposure level and duration.
Several methods have been used in an attempt to decrease the bioavailability of toxin-producing fungi. One of the most practical approaches is the use of adsorbing or binding agents that specifically bind mycotoxins in contaminated feed. However, some adsorbents have been shown to impair nutrient utilization and mineral absorption (Kubena et al. 1993a; Chestnut et al. 1992). Hydrated sodium calcium aluminosilicate (HSCAS) is an efficient aflatoxin absorbent (Phillips et al. 1988; Diaz and Smith 2005; Kabak et al. 2006). The use of Saccharomyces cerevisiae (S.C.), a growth promoter and immunostimulant, has resulted in significant improvements against aflatoxicosis in poultry (Parlat et al. 2001; Yildirim and Parlat 2003). Furthermore, esterified glucomannan (EGM), derived from the cell wall of Saccharomyces cerevisiae, showed high binding ability to aflatoxins in contaminated poultry feed (Devegowda et al. 1998; Diaz et al. 2002).
The current study suggests the ability of selected mycotoxin adsorbents to alleviate the toxic AFB1 effects in Oreochromis niloticus. Although HSCAS, S.C. and its derivative EGM show anti-mycotoxin activity, their use in aquaculture needs to be thoroughly investigated. The impacts of these strategies on growth performance, hematological parameters, immune function and residual content in the Nile tilapia musculature after exposure to AFB1-contaminated feed were evaluated.
Materials and methods
Fish
A total of 240 fingerlings of Nile tilapia, Oreochromis niloticus (15 ± 2 g), were obtained from a private fish farm at Borg-El-Arab, Alexandria, Egypt. The fish were acclimated to their new housing conditions for 2 weeks before the start of the experiment. The water temperature, pH, dissolved oxygen and salinity were recorded daily. Carbon dioxide, carbonate hardness, ammonia-N, nitrite-N and nitrate–N levels were recorded once a week following the standard APHA method (1998). All water parameters were maintained within recommended ranges during the experimental period. A basal diet (Table 1) was formulated from commercial ingredients and was compressed by a pelletization machine (1 mm size). Food was provided twice daily (8 am and 2 pm) at the rate of 3 % of the fish biomass.
Production of aflatoxin B1 (AFB1)
Aflatoxin B1 was produced from Aspergillus flavus using polished raw rice as a substrate for growth (Shotwell et al. 1966; Mehrim et al. 2006 with little modifications). This fungus was previously identified in the Poultry and Fish Disease department, Faculty of Veterinary Medicine, Alexandria University. The fungus was purified by single spore isolation and culturing on Sabaruoud’s dextrose agar media at 25 °C for 4 days. Polished raw rice was autoclaved 3 times for 3 successive days. A block of fungus from the edge of growing colonies was inoculated into 2 kg of sterilized rice and incubated at 25 °C for 7 days. The rice was autoclaved twice to kill the fungus. The contaminated rice was dried in an oven at 56 °C for 24 h. Next, the amount of AFB1 in the rice was measured by thin layer chromatography apparatus. Contaminated rice that contained 2200 ppb AFB1 was incorporated into diets T2, T3, T4 and T5 for a final concentration of 200 ppb.
Experimental design
After an acclimation period of 2 weeks, the O. niloticus fingerlings were randomly divided into eight experimental groups (30 fish per group) (Table 2). Each group was placed in a fully prepared and continuously aerated aquarium (60 × 60 × 50 cm) containing dechlorinated tap water. The water in the glass aquaria was partially changed daily to avoid metabolite accumulation. Two control and six test groups were selected for the 10-week experiment. Each of the treatments was assigned to triplicate aquaria. Groups T1 and T2 constituted the negative control fed a basal diet and the positive control fed an AFB1-contaminated diet (200 ppb), respectively. Groups T3, T4 and T5 were fed an AFB1-contaminated diet (200 ppb) supplemented with 0.5 % HSCAS (EL-NASR Co., Egypt), 0.25 % Saccharomyces cerevisiae (S.C.) (Orgasel Zinc, Microbiotic, USA) or 0.25 % esterified glucomannan (EGM) (Media Vet, Garlien, Egypt), respectively. Groups T6, T7 and T8 were fed a basal diet supplemented with 0.5 % HSCAS, 0.25 % S.C. or 0.25 % EGM, respectively.
Growth performance and body weight
Fish groups in aquaria were counted and weighed every 2 weeks. At the end of feeding period (10 weeks), the final body weight per fish, weight gain, average daily gain (ADG), specific growth rate (SGR) and survival rates were determined.
Blood parameters
Fish were anesthetized with 120 mg/l amino-benzoic acid (Sigma–Aldrich) before the drawing of blood. The body surface of the peduncle region was cleaned and dried using adsorbent paper. Blood samples were collected from the caudal blood vessels every 2 weeks using a disposable 1 cc tuberculin syringe. Whole blood was collected in a small sterile vial containing EDTA as an anticoagulant. Samples were used to determine the hemoglobin (Hb) content using a commercial kit (Diamond Diagnostic, Egypt), and the total erythrocyte (RBC) and leukocyte (WBC) counts using an Ao Bright–Line Haemocytometer (Neubauer improved, Precicolor HBG, Germany) were obtained according to the methods described by Jain (1993). Other blood samples for serum separation were collected without the addition of anticoagulants and then centrifuged at 3000 g for 10 min. The activity of serum aspartate transferase (AST), alanine transferase (ALT) and creatinine was estimated according to the methods of Reitman and Frankel (1957) and Henry et al. (1974). In addition, serum total protein, albumin and globulin were determined spectrophotometrically using the methods of Doumas et al. (1981), Reinhold (1953) and Coles (1986).
Antibody titration against Aeromonas hydrophila
After 10 weeks, fish groups were given an intraperitoneal injection of a prepared bacterin adjuvant from A. hydrophila. Serum was separated from the clotted blood by centrifugation at 3,000 g for 10 min and stored at −20 °C before future use. An antibody agglutination titer against the isolate of A. hydrophila was determined for each fish using a modified agglutination assay (Roberson 1990). Serum was serially diluted with equal volumes of PBS (pH 7.2) and a heat-inactivated cell-free suspension of A. hydrophila (approximately 109 cfu/ml). The 96-round-well microplates were incubated overnight at room temperature and then examined microscopically for agglutination. The antibody titer was calculated as the reciprocal of the highest dilution of serum that was positive for agglutination.
Challenge infection test
After 28 days post-immunization, the immunized and control groups were given a 0.2 cc intraperitoneal injection of a virulent strain of A. hydrophila (107 cfu/ml) according to Schaperclaus et al. (1992). All groups were kept under observation for 2 weeks to record clinical signs, postmortem lesions and daily mortality rates. Samples (blood, liver and kidney) from dead and clinically infected fish were taken to confirm the cause of disease using specific media from A. hydrophila. The relative level of protection (RLP) was calculated according to the following formula developed by Newman and Majnarich (1982):
At the end of the fourth challenge week, agglutinating antibody titers were assayed in sera samples according to Plumb and Areechon (1990).
Analysis of aflatoxin B1 in fish musculature
Muscle samples from three fish (about 50 g) of the same group were pooled and thoroughly homogenized in a mortem. Aflatoxin B1 was extracted, filtrated and quantitatively analyzed by HPLC (AOAC 2000) with a reverse phase column. The mobile phase consisted of 45 % methanol and was pumped through the system at a flow rate of 1 ml/min. The column temperature was set to 40 °C, and analytes were detected using a fluorescence detector. Aflatoxin standards were purchased from Sigma-Aldrich (USA).
Statistical analysis
Analysis of variance (ANOVA) with Duncan’s multiple comparisons when appropriate as post hoc test was used to determine the significant variation among the different experimental groups. One-way ANOVA was used to analyze data of growth performance, survival rate and relative level of protection and agglutinating Ab titer against Aeromonas hydrophila as well as residue of AFB1. On the other hand, two-way ANOVA was used to analyze data of hematological and biochemical parameters as well as plasma protein. All of the statistical analyses were performed using SPSS version 14 (SPSS, Chicago, IL, USA). A P value of <0.05 was considered statistically significant.
Results
Growth performance and survival rate
The results of growth performance and survival rate of tilapia (O. niloticus) treated with different mycotoxin adsorbents are presented in Table 3. There was no mortality among the fish fed the basal diet. The highest survival rates among the AFB1-treated groups were 87.66 ± 0.08, 75.66 ± 0.06 and 75.66 ± 0.06 in T3, T4 and T5, respectively. The highest mortality rate of 34.34 % occurred in group T2. The groups not exposed to AFB1 (T1, T6, T7 and T8) showed a significant (P < 0.05) increase in total weight gain (TWG) of over 200 %. AFB1-treated groups (T2, T3, T4 and T5) showed a decreased growth rate. The lowest significant growth rate was observed in T2. Within the AFB1 and adsorbent-treated groups, T3 showed the highest growth rate, followed by T4 and T5, respectively. The ADG values were significantly higher (P < 0.05) in groups not exposed to AFB1. Group T3 showed the largest decrease in ADG; in addition, ADG was significantly lower (P < 0.05) in T4, T5 and T2, respectively. Among all fish groups, only the control AFB1 group (T2) showed a significantly lower SGR.
Hematological parameters
The addition of AFB1 to the O. niloticus diet (T2, T3, T4 and T5) led to a significant decrease (P < 0.05) in the total erythrocyte count (Fig. 1a), hemoglobin content (Fig. 1b) and total leukocyte count (Fig. 1c). Fish exposed to AFB1 in combination with mycotoxin adsorbents (T3, T4 and T5) showed higher RBC counts and hemoglobin content when compared with fish exposed to AFB1 only (T2). The RBC counts did not show a significant increase in T6, T7 and T8 when compared with the control group (T1). RBC counts and hemoglobin content in T3, T4 and T5 showed significant descending decreases, while T6, T7 and T8 showed significant ascending increases in these parameters. The toxic effects of AFB1 (T2) on RBC count and hemoglobin content were observed at 2 weeks post-treatment with a peak effect at week 6 and 8, respectively.
Effect of AFB1 and mycotoxin adsorbents on hematological parameters in Oreochromis niloticus. a Bars indicate the total erythrocyte count (RBC count × 106/mm3). b Bars indicate the hemoglobin content (g/ml). c Bars indicate the total leukocyte count. Groups with different superscripts (a, b, c and d) are significantly different (P < 0.05, using two-way ANOVA; Post hoc: Duncan’s multiple comparisons)
Aflatoxin B1-treated fish showed a significant decrease in WBC number (P < 0.05), especially after 10 weeks of exposure, when compared with non-treated fish. Group T3 showed a significantly higher WBC counts when compared with groups T4 and T5.
Serum biochemical parameters
The activity of serum AST and ALT increased in AFB1-exposed groups (Fig. 2a, b). The use of mycotoxin adsorbents (T3, T4 and T5) significantly reduced serum AST and ALT activity. Aflatoxin B1-exposure led to a highly significant increase in serum creatinine levels (Fig. 2c). Interestingly, the three mycotoxin adsorbents significantly altered creatinine levels when compared with T1 or T2. In group T2, the serum activity of ALT and AST and creatinine levels peaked after 6 weeks post-toxin exposure. Use of mycotoxin adsorbents (T6, T7 and T8) did not have a significant influence on serum AST and ALT activity or creatinine levels when compared with the control (T1). The lowest activity of both enzymes and creatinine levels was found in group T3.
Effect of AFB1 and mycotoxin adsorbents on serum biochemical parameters in Oreochromis niloticus. a Bars indicate the values of serum AST (lu/l). b Bars indicate the values of serum ALT (lu/l). c Bars indicate the concentration of creatinine (mg/dl). Groups with different superscripts (a, b, c, d and e) are significantly different (P < 0.05, using a two-way ANOVA; Post hoc: Duncan’s multiple comparisons)
Total serum protein, albumin and globulin were significantly (P < 0.05) reduced after addition of AFB1 (T2) in the diet (Fig. 3a–c). T3 and T4 showed significantly (P < 0.05) higher values of total serum protein, albumin and globulin when compared with T2; in addition, these groups show significant lower total serum protein and globulin when compared with T1. Group T5 did not show significant changes in total protein and albumin values; however, the globulin levels were significantly higher when compared with T2. Interestingly, T3, T4, T6, T7 and T8 showed higher values of total albumin (significant only in T3) when compared with T1.
Effect of AFB1 and mycotoxin adsorbents on serum plasma proteins in Oreochromis niloticus. a Bars indicate the values of serum total protein (g/dl). b Bars indicate the values of serum albumin (g/dl). c Bars indicate the values of serum globulin (g/dl). Groups with different superscripts (a, b, c and d) are significantly different (P < 0.05, using two-way ANOVA; Post hoc: Duncan’s multiple comparisons)
Protection and agglutinating antibody titer
The mortality percentages due to Aeromonas hydrophila infection were significantly reduced in immunized groups that were not exposed to AFB1 (T1, T6, T7 and T8) (Fig. 4a). The highest mortality rate and lowest relative level of protection were found in T2. T3 showed a lower mortality rate when compared with T4 and T5.
Effect of AFB1 and mycotoxin adsorbents on the specific immune response of immunized Oreochromis niloticus. a Relative level of protection percentage against Aeromonas hydrophila infection. b Agglutinating antibody titers against A. hydrophila. Groups with different superscripts (a, b, c and d) are significantly different (P < 0.05, using one-way ANOVA; Post hoc: Duncan’s multiple comparisons)
Aflatoxin B1 exposure reduced the antibody production against A. hydrophila (Fig. 4b). The values of A. hydrophila agglutinating antibody titers were highest in T1 and T6 followed by T7 and T8 when compared with AFB1-exposed groups (P < 0.05). Among adsorbent-treated groups, T3 showed the highest significant antibody titer against A. hydrophila infection, followed by T4 and T5.
Residual AFB1 in musculature
The bioaccumulation of AFB1 in musculature of O. niloticus was measured every 2 weeks in fish exposed to AFB1 and the negative control group (T2, T3, T4, T5 and T1) (Fig. 5). There was no AFB1 residue found in the control group (T1). The residue in all AFB1-treated groups showed significant increases from weeks 2 to 10. The lowest residue levels were found in T3 followed by T4 and T5.
Effect of mycotoxin adsorbents on AFB1 residues in the musculature of Oreochromis niloticus. Bars indicate the values of residues in musculature (ppb). Groups with different superscripts (a, b, c and d) are significantly different (P < 0.05, using one-way ANOVA; Post hoc: Duncan’s multiple comparisons)
Discussion
Aflatoxin contamination is a common problem of aquaculture worldwide, especially in humid tropical and subtropical areas (Murjani 2003). Fish are susceptible to the AFB1 effects due to the increased use of plant-based additives in aquaculture feeds. The main clinical signs of AFB1 toxicity are chronic in nature, including impaired liver function, lower growth rate, loss of body weight, increased disease susceptibility, internal organ dysfunction and increased mortality (Murjani 2003; Santacroce et al. 2008). To our knowledge, our present work is the first study to focus on AFB1 adsorption by HSCAS, S.C. and EGM on Oreochromis niloticus fingerlings.
We found that exposure to AFB1 at a daily concentration of 200 μg/kg diet for 10 weeks showed a 34.34 % mortality rate in Nile tilapia. Previously, an intraperitoneal AFB1 injection at a dose of 0.5 μg/kg body weight and exposure to 100 mg/kg diet led to 65 and 60 % mortality, respectively (El-Enbaawy et al. 1994; Tuan et al. 2002). Lower doses of AFB1 (30 mg/kg diet or less) did not induce mortalities in Tilapia (Chávez-Sánchez et al. 1994; Tuan et al. 2002; Deng et al. 2010). Aflatoxicosis in Nile tilapia is dependent on the toxin concentration in the feed, the duration of exposure and the route of administration (Bauer et al. 1969; Deng et al. 2010).
Ingestion of low to moderate doses of AFB1 over a long period of time produces a reduction in weight gain and feed efficacy in a concentration-dependent manner (Tuan et al. 2002; Cagauan et al. 2004; Zaki et al. 2008). The results of mortality, growth performance and blood parameters suggest that tilapia is a species with moderate AFB1 susceptibility, especially when compared with channel catfish fed similar concentrations of AFB1 (Jantrarotai and Lovell 1990; Tuan et al. 2002).
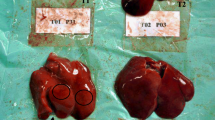
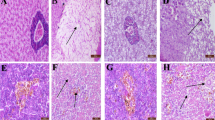
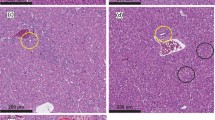
Our results confirmed that erythrocyte number, hemoglobin content and leukocyte number are severely affected by AFB1. In Nile tilapia, exposure to AFB1 at low concentrations for long durations led to marked anemia and leukopenia (Rizkalla et al. 1997, Hussein et al. 2000). Similar to channel catfish and common carp, this toxicity may be attributed to the damage of the hematopoietic tissue and the anterior kidney (Jantrarotai and Lovell 1990; Jantrarotai et al. 1990; Pepeljnjak et al. 2003). Our results showed that the creatinine levels were elevated. These data indicate a toxic effect on the kidney, which was confirmed pathologically (data not shown). Aflatoxin B1 is a hepatotoxin in several fish species, including Nile tilapia (Hendricks 1994; Tuan et al. 2002). Aflatoxin B1 significantly changed the stability of the lysosomal membrane, leading to a disorder of hepatocyte permeability and pathological changes in the liver of Oreochromis mossambicus (Varior and Philip 2012). This effect can be confirmed by high levels of ALT and AST enzymes in the blood. In the present study, we found significant increases in serum ALT and AST, confirming hepatotoxicity. Similarly, Deng et al. (2010) reported an AFB1-induced hepatic disorder in hybrid tilapia characterized by decreased lipid content, hepatosomatic index and abnormal hepatic morphology.
The immune system is a defensive mechanism against pathogens, and impaired immune functions will decrease resistance to infectious diseases. Aflatoxins generate a long-term dysfunction in the specific and non-specific immune response of aquatic organisms (Rodriguez-Cervantes et al. 2010). In our study, AFB1 reduced the serum levels of total protein, albumin and globulin. Similar reductions were reported in sea bass and rohu (El-Sayed and Khalil 2009; Mohapatra et al. 2011). Plasma proteins are used for energy production during times of toxicity or protein catabolism induced by stress (Pfeifer and Weber 1979). Moreover, aflatoxin hepatotoxicity leads to alterations in protein synthesis and cellular integrity of the liver by the binding of aflatoxin adducts to cellular macromolecules. This binding results in the reduction in total protein and albumin in serum (Patterson 1976; Jindal et al. 1994; Abo-Norag et al. 1995). However, globulin reduction may be related to hemopoietic toxicity (anterior kidney and spleen) and lymphocytolysis (Sahoo et al. 2001). Similar to our findings, Sahoo and Mukherjee (2002) reported that globulin was the most susceptible plasma protein to aflatoxin exposure in Labeo rohita. AFB1 treatment led to a significant decrease in the bacterial agglutination titers and relative levels of protection against Aeromonas hydrophila. In addition, Aflatoxin B1 induced a significant suppression of B-cell memory, immunoglobulin production and lymphocyte proliferation in rainbow trout (Ottinger and Kaattari 1998, 2000). Suppression of serum bactericidal activity, macrophage phagocytic activity, neutrophil function and humoral immune response levels were previously observed in Nile tilapia (El-Enbaawy et al. 1994). In the present study, the agglutinating antibody titer in fish exposed to AFB1 was higher than that of AFB1-injected Indian major carp (Sahoo and Mukherjee 2001). Chronic exposure to aflatoxin suppressed the release of lysozymes and decrease bactericidal activity, leading to increases in the susceptibility of fish to infections and mortality (Sahoo and Mukherjee 2003). Therefore, AFB1–induced immune dysfunction may be responsible for the higher mortality rates from infectious diseases. Further studies are needed to differentiate if AFB1 effects are directed to specific or humeral immunity.
Sustainable mycotoxin residue in fish flesh is a food safety concern (El-Banna et al. 1992). The results of this study show that exposure to AFB1 at a dose of 200 μg/kg diet for 10 weeks will lead to accumulation in Nile tilapia flesh at levels ranging from 23 to 94 ppb. Deng et al. (2010) detected AFB1 residues in hybrid tilapia livers but not the musculature when fish exposed to AFB1 at a dose of 1.641 mg/kg in the diet. The lack of observed effects may be attributed to the low concentration of exposure. The majority of AFB1 residues were found in the liver, rather than the musculature, in sea bass and sturgeon (El-Sayed and Khalil 2009; Raghavan et al. 2011). These data suggest that the liver plays an important role in metabolism and excretion of AFB1, activation of toxic metabolites or detoxification (Guengerich et al. 1998; Takahashi et al. 1995).
Protection of aflatoxicosis through the use of adsorbents is economical. Many adsorbents are available, such as aluminosilicates (Bentonites, Montmorillonites, Zeolite and HSCAS) and yeast and its cell wall (Saccharomyces cervisiae). However, the efficacy and action of these products is not thoroughly understood. In addition, very few studies have tested these substances in aquaculture. The use of aluminum silicates as a mycotoxin adsorbent in feed has yielded positive results in chicken and turkey (Kubena et al. 1993a, b, 1998; Ledoux et al. 1999), swine (Harper et al. 2010), rats (Mayura et al. 1998, Abdel-Wahhab et al. 2002) and dogs (Bingham et al. 2004). The efficacy of HSCAS depends on the source and surface of the clay; more specifically, the larger the surface area, the more effective the HSCAS is the clay. HSCAS binds AFB1 in the GI tract, thereby reducing overall bioavailability to the bloodstream (Phillips et al. 1990). Here, HSCAS did not alter ADG and SGR. However, the positive effect of HSCAS was confirmed by the reduction of toxin residues in musculature and lower mortality in challenged fish. These results indicate that the significant anti-mycotoxin effects of feed supplemented by 0.5 % HSCAS are likely due to the adsorption of AFB1 on the clay surface, which greatly reduces its distribution. Addition of 2 % dietary bentonite into rainbow trout diet resulted in the stopping of intestinal absorption, reduction in liver and kidney loads and increase in excretion through feces (Ellis et al. 2000). Another clay-based adsorbing agent, an Egyptian montmorillonite, protected against the toxicity of sterigmatocystin in Nile tilapia at a dose of 0.5 μg/kg body weight (1.6 μg/kg body weight twice a week) (Abdel-Wahhab et al. 2005). Similar to results from Huff et al. (1992) in chicken, addition of HSCAS alone did not significantly improve any of blood parameters tested.
Saccharomyces cerevisiae contains various immunostimulating compounds, such as β-glucans, nucleic acids and mannan oligosaccharides. Therefore, this strain of yeast can be used as a growth promoter and immune stimulant in some fish species (Siwicki et al. 1994; Sakai 1999; Oliva-Teles and Gonçalves 2001; Li and Gatlin 2004). In the poultry industry, S.C. has been used as general performance promoter and anti-mycotoxin because of its adsorbent effect on AFB1 (Celik et al. 2000; Celyk et al. 2003). An esterified glucomannan, a fibrous material from the yeast cell wall, was shown to have the potential to bind several mycotoxins (Devegowda et al. 1998). In fish treated with S.C. and EGM, growth performance, blood parameters, liver enzymes, creatinine, plasma proteins and immune status were all improved when compared with the AFB1-exposed group. These data indicate significant mycotoxin-adsorbing effects. In a chicken diet, S.C. bound as much as 77 % of mycotoxins and modified mannan oligosaccharides (derived from the S. cerevisiae cell) were found to bind up to 95 % of mycotoxins (Devegowda et al. 1996; Raju and Devegowda 2000).
Aflatoxin B1 severely depresses weight gain and induces a high mortality rate. Nile tilapia fed on AFB1 had anemia and leukopenia, as well as decreased serum globulin level. Aflatoxin B1 exposure led to liver dysfunction manifested by high serum levels of AST and ALT and kidney damage as shown by high creatinine levels. After challenge with Aeromonas hydrophila, AFB1 produced a low level of agglutinating antibody titer and a scant relative level of protection. HSCAS, S. cerevisiae and EGM effectively ameliorated AFB1 toxicity. HSCAS was more effective than S. cerevisiae and its derivative EGM. Our study provides new perspectives toward the understanding of AFB1 adsorbents; however, their interactions with each other need to be investigated.
References
Abdelhamid AM, Abdelkhalek AE, Mehrm AI, Khalil FF (2004) An attempt to alleviate aflatoxicosis on Nile tilapia fish by dietary supplementations with chicken-hatchery by-products (egg shells) and shrimp processing wastes (shrimp shells) 2-On clinical, blood and histological parameters. J Agric Sci Mansoura Univ 29:6175–6196
Abdel-Wahhab MA, Nada SA, Khalil FA (2002) Physiological and toxicological responses in rats fed aflatoxin-contaminated diet with or without sorbent materials. Anim Feed Sci Technol 10740:1–11
Abdel-Wahhab MA, Hassan AM, Aly SE, Mahrous KF (2005) Adsorption of sterigmatocystin by montmorillonite and inhibition of its genotoxicity in the Nile tilapia (Oreochromis niloticus). Mutat Res 582:20–27
Abo-Norag M, Edrington S, Kubena F, Harvey B, Phillips D (1995) Influence of a hydrated sodium calcium aluminosilicate and virginiamycin on aflatoxicosis in broiler chicks. Poult Sci 74:626–632
Alpert M, Hutt M, Wogan N, Davidson C (1971) Association between aflatoxin content of food and hepatoma frequency in Uganda. Cancer 28:253–260
AOAC (2000) Official methods of analysis, 15th Ed. Association of Official Analysis of Chemist, Washington
APAH (1998) American Water Works Association and Water Pollution Control Pollution Federation. In: Franson MAH (ed) Standard methods for examination of water and wastewater, 16th edn. American Public Health Association, Wasington
Bauer DH, Lee DJ, Sinnhuber RO (1969) Acute toxicity of aflatoxin B1 and G1 in the rainbow trout (Salmo gairdneri). Toxicol Appl Pharmcol 15:415–419
Bingham AK, Huebner HJ, Phillips TD, Bauer JE (2004) Identification and reduction of urinary aflatoxin metabolites in dogs. Food Chem Toxicol 42(11):1851–1858
Boonyaratpalin M, Supamattaya K, Verakunpiriya V, Suprasert D (2001) Effects of aflatoxin B1 on growth performance, blood components, immune function and histopathological changes in black tiger shrimp (Penaeus monodon Fabricius). Aquacult Res 32:388–398
Cagauan GA, Tayaban RH, Somga JR, Bartolome RM (2004). Effect of Aflatoxin contaminated feed in Nile tilapia (Oreochromis niloticus L.). In: Remedios RB, Mair GC, Fitzsimmons K (eds) Proceedings of the 6th international symposium on tilapia aquaculture, pp 172–178
Celik I, Oguz H, Demet O, Donmez HH, Boydak M, Sur E (2000) Efficacy of polyvinyl-polypyrrolidone in reducing the immunotoxicity of aflatoxin in growing broilers. Br Poult Sci 41:430–439
Celyk K, Denly M, Savas T (2003) Reduction of toxic effects of aflatoxin by using baker yeast (Saccharomyces cerevisiae) in growing broiler chicken diets. Revista Brasileira de Zootecnia 32:615–619
Chávez-Sánchez MC, Martínez Palacios CA, Osorio Moreno I (1994) Pathological effects of feeding young Oreochromis niloticus diets supplemented with different levels of aflatoxin B1. Aquaculture 127:49–60
Chestnut AB, Anderson PD, Cochran MA, Fribourg HA, Twinn KD (1992) Effects of hydrated sodium calcium aluminosilicate on fescue toxicosis and mineral absorption. J Anim Sci 70:2838–2846
Coles EH (1986) Veterinary clinical pathology, WB Saunders, Philadelphia, pp 10–42
Deng SX, Tian LX, Liu FJ, Jin SJ, Liang GY, Yang HJ, Du ZY, Liu YJ (2010) Toxic effects and residue of aflatoxin B1 in tilapia (Oreochromis niloticus × O. aureus) during long-term dietary exposure. Aquaculture 307:233–240
Devegowda G, Arvind IR, Morton MG (1996) Saccharomyces cerevisiae and mannanoligosaccharides to counteract aflatoxicosis in broilers. In: Proceedings of Australian poultry science symposium Sydney
Devegowda G, Raju LN, Afzali N, Swamy LN (1998) Mycotoxin picture worldwide: novel solutions for their counteraction. In: Proceedings of Alltech’s 14th Annual Symposium on Biotechnology in Feed Industry. Nottingham University Press, Nottingham, pp 241–255
Diaz DE, Smith TK (2005) Mycotoxin sequestering agents: practical tools for the neutralisation of mycotoxins
Diaz DE, Hagler WM, Hopkins BA, Whitlow LW (2002) Aflatoxin binders I: in vitro binding assay for aflatoxin B1 by several potential sequestering agents. Mycopathologia 156:223–226
Doumas T, Bayso DD, Carter RJ, Peters RS (1981) Determination of total serum protein. Clin Chem 27:1642–1843
El-Banna R, Teleb HM, Hadi MM, Fakhry FM (1992) Performance and tissue residue of tilapias fed dietary aflatoxin. Vet Med J 40:17–23
El-Enbaawy M, Adel M, Marzouk MS, Salem AA (1994) The effects of acute and chronic aflatoxicosis on the immune functions of Oreochromis niloticus in Egypt. Vet Med J Giza 42:47–52
Ellis RW, Clement M, Tibbetts A, Winfree R (2000) Reduction of the bioavailability of 20 μg/kg aflatoxin in trout feed containing clay. Aquaculture 183:179–188
El-Sayed YS, Khalil RH (2009) Toxicity, biochemical effects and residue of aflatoxin B1 in marine water reared sea bass (Dicentrarchus labrax L.). Food Chem Toxicol 47:1606–1609
Guengerich FP, Johnson WW, Shimada T, Ueng YF, Yamazaki H, Langouet S (1998) Activation and detoxication of aflatoxin B1. Mutat Res 402:121–128
Han XY, Huang QC, Li WF, Xu ZR (2008) Changes in growth performance, digestive enzyme activities and nutrient digestibility of cherry valley ducks in response to aflatoxin B1 levels. Livest Sci 119:216–220
Harper AF, Estienne MJ, Meldrum JB, Harrell RJ, Diaz DE (2010) Assessment of a feed anti-caking agent and antioxidant blend for amelioration of aflatoxicosis in pigs. J Swine Health Prod 18(6):282–289
Hendricks JD (1994) Carcinogenicity of aflatoxins in nonmammalian organisms. In: Eaton DL, Groopman JD (eds) Toxicology of aflatoxins: human health. Veterinary and agricultural significance. Academic Press, San Diego, pp 103–136
Hendricks JD, Bailey GS (1989) Adventitious toxins. In: Halver JE (ed) Fish nutrition, 2nd edn. Academic Press Inc., New York, pp 605–651
Henry RL, Cannon DC, Winklemen JW (1974) Clinical chemistry: principles and techniques, 2nd (ed) Harper and Row Hagerstown, New York, pp 726–727
Horn CW, Boleman LL, Coffman CG, Deton JH, Lawhorn DB (1989) Mycotoxins in feed and food producing crops college state Texas. Texas Vet Med Diagnostic, The National Dairy Database (1992)
Huff WE, Kubena LF, Harvey RB, Phillips TD (1992) Efficacy of a hydrated sodium calcium aluminosilicate to reduce the individual and combined toxicity of aflatoxin and ochratoxin A. Poult Sci 71:64–69
Hussain M, Gabal MA, Wilson T, Summerfelt RC (1993) Effect of aflatoxin-contaminated feed on morbidity and residues in walleye fish. Vet Hum Toxicol 35(5):396–398
Hussein H, Brasel JM (2001) Toxicity, metabolism and impact of mycotoxins on humans and animals. Toxicology 167:101–134
Hussein SY, Mekkawy IA, Moktar ZZ, Mubarak M (2000) Protective effect of Nigella sativa seed against aflatoxicosis in Oreochromis niloticus. Mycotoxin Conf Mycotoxins Environ Poland Bydgoszez 25–27:109–130
Jain NC (1993) Essential of veterinary hematology copyrights by Lea and Febiger Philadelphia, USA
Jantrarotai IV, Lovell RT (1990) Subchronic toxicity of dietary aflatoxin B1 to channel catfish. J Aqua Anim Health 2:248–254
Jantrarotai IV, Lovell RT, Grizzle JM (1990) Acute toxicity of AFB1 to channel catfish. J Aqua Anim Health 2:237–247
JECFA (1998) Joint FAO/WHO expert committee on food additives. Safety evaluation of certain food additives and contaminants. World Health Organization, Geneva
Jindal N, Manipal SK, Mahajan NK (1994) Toxicity of aflatoxin B1 in broiler chicks and its reduction by activated charcoal. Res Vet Sci 56:37–40
Kabak B, Dobson ADW, Var I (2006) Strategies to prevent mycotoxin contamination of food and animal feed: A review. Crit Rev Food Sci Nutr 46:593–619
Kennedy D, Delaney A, Koren G (1998) Mutagens, carcinogens and teratogens. In: Goldfrank L (ed) Goldfrank’s toxicologic emergencies, 6th edn. Appleton & Lange, Stanford, pp 262–273
Kovács M (2004) Nutritional health aspects of mycotoxins. Orv Hetil 22145(34):1739–1746
Kubena LF, Harvey RB, Huff WE, Ellisalde MH, Yersin AG, Phillips TD, Rottinghaus G (1993a) Efficacy of a hydrated sodium calcium aluminosilicate to reduce the toxicity of aflatoxin and diacetoxyscirpenol. Poult Sci 72:51–59
Kubena LF, Harvey RB, Phillips TD, Clement BA (1993b) Effect of hydrated sodium calcium aluminosilicates on aflatoxicosis in broiler chicks. Poult Sci 72:651–657
Kubena LF, Harvey RB, Bailey RH, Buckley SA, Rottinghaus GE (1998) Effects of a hydrated sodium calcium aluminosilicate (T-Bind) on mycotoxicosis in young broiler chickens. Poult Sci 77:1502–1509
Ledoux DR, Rottinghaus GE, Bermudez AJ, Alonso-Debolt M (1999) Efficacy of a hydrated sodium calcium aluminosilicate to ameliorate the toxic effects of aflatoxin in broiler chicks. Poult Sci 78:204–210
Li P, Gatlin DM III (2004) Dietary brewer’s yeast and the prebiotic GroBiotick™ AE influence growth performance, immune responses and resistance of hybrid striped bass (Morone chrysops × M. saxatilis) to Streptococcus iniae infection. Aquaculture 231:445–456
Mayura K, Abdel-Wahhab MA, McKenzie KS, Sarr AB, Edwards JF, Naguib K, Phillips TD (1998) Prevention of maternal and developmental toxicity in rats via dietary inclusion of common aflatoxin sorbents: potential for hidden risks. Toxicol Sci 41:175–182
Mehrim AI, Abdelhamid AM, Abou-Shousha A, Salem MF, El-Sharawy MA (2006) Nutritious attempts to detoxify aflatoxic diets of tilapia fish: clinical, biochemical and histological parameters. J Arab Aqua Soci 1(2):69–90
Mohapatra S, Sahu NP, Pal AK, Prusty AK, Kumar V, Kumar S (2011) Haemato-immunology and histo-architectural changes in Labeo rohita fingerlings: effect of dietary aflatoxin and mould inhibitor. Fish Physiol Biochem 37:177–186
Murjani G (2003) Chronic aflatoxicosis in fish and its relevance to human health. Central Institute of Freshwater Aquaculture, India
Newman SG, Majnarich JJ (1982) Direct immersion vaccination of Juvenile rainbow trout, Salmo gairdneri and Juvenile Coho salmon, Oncorhynchus Kisutch with Yersinia ruckeri bacterin. J Fish Dis 3:339–341
Oliva-Teles A, Gonçalves P (2001) Partial replacement of fishmeal by brewers yeast Saccharomyces cerevisiae in diets for sea bass, Dicentrarchus labrax, juveniles. Aquaculture 202:269–278
Ottinger CA, Kaattari SL (1998) Sensitivity of rainbow trout leucocytes to aflatoxin B1. Fish Shell Fish Immunol 8:515–520
Ottinger CA, Kaattari SL (2000) Long-term immune dysfunction in rainbow trout (Oncorhynchus mykiss) exposed as embryos to aflatoxin B1. Fish Shellfish Immunol 10(1):101–106
Parlat SS, Ozcan M, Oguz H (2001) Biological suppression of aflatoxicosis in Japanese quail (Coturnix coturnix japonica) by dietary addition of yeast (Saccharomyces cerevisiae). Res Vet Sci 71:207–211
Patterson SP (1976) Structure, metabolism and toxicity of aflatoxin. Cab Nutr Diet 2:71–78
Pepeljnjak S, Petrinec Z, Kovacic S, Segvic M (2003) Screening toxicity study in young carp (Cyprinus carpio L.) on feed amended with fumonisin B1. Mycopathologia 156(2):139–145
Pfeifer KF, Weber LJ (1979) The effect of carbon tetrachloride on the total protein concentration of rainbow trout, Salmo gairdneri. Com Bioch Physiol 64(C):37–42
Phillips TD, Kubena LS, Harvey RB, Taylor BS, Heidelbaugh ND (1988) Hydrated sodium calcium aluminate silicate: a high affinity sorbent for aflatoxin. Poult Sci 67:243–247
Phillips TD, Clement BA, Kubena LF, Harvey RB (1990) Detection and detoxification of aflatoxins: prevention of aflatoxicosis and aflatoxin residues with hydrated sodium calcium aluminosilicate. J Vet Hum Toxicol 32:15–19
Plakas SM, Loveland PM, Bailey GS, Blazer VS, Wilson GL (1991) Tissue deposition and excretion of 14C-labeled aflatoxin B1 after oral administration in channel catfish. Food Chem Toxicol 29:805–808
Plumb JA, Areechon N (1990) Effect of malathion on humoral immune response of channel catfish. Dev Comp Immunol 14:355–358
Puschner B (2002) Mycotoxins. Vet Clin Small Anim 32:409–419
Raghavan PR, Zhu X, Lei W, Han D, Yang Y, Xie S (2011) Low levels of aflatoxin B1 could cause mortalities in juvenile hybrid sturgeon, Acipenser ruthenus ♀ × A. baerii ♂. Aquacult Nutr 13:39–47
Raju LN, Devegowda G (2000) Influence of esterified-glucomannan on performance and organ morphology, serum biochemistry and haematology in broilers exposed to individual and combined mycotoxicosis (aflatoxin, ochratoxin and T-2 toxin). Br Poult Sci 41:640–650
Reinhold RR (1953) Determination of serum albumin. Clin Chem 21:1370–1372
Reitman S, Frankel S (1957) A colorimetric methods for the determination of serum glutamic oxaloacetic and glutamic pyruvic transaminases. Am J Clin Path 28(1):56–63
Rizkalla EH, Zahra MH, Deab A, Farag MF (1997) Hematological responsiveness of Oreochromis aureus to dietary aflatoxins contamination and the role of selenium and vitamin E. Alex J Vet Sci 13(4):423–438
Roberson BS (1990) Bacterial agglutination. In: Stolen JS, Fletcher GC, Anderson DP, Roberson BS, van Muiswinkel WB (eds) Techniques in fish immunology. SOS Publications, Fair Haven, pp 137–154
Rodriguez-Cervantes CH, Giron-Perez MI, Robledo-Marenco ML, Marin S, Velazquez-Fernandez JB, Medina-Diaz IM, Rojas-Garcia AE, Ramos AJ (2010) Aflatoxin B1 and its toxic effects on immune response of teleost fishes: a review. World Mycotoxin J 3(2):193–199
Royes JB, Yanong RP (2002) Molds in fish feeds and aflatoxicosis. Copyright by the University of Florida, Institute of Agricultural Science (UF/IFAS)
Sahoo PK, Mukherjee SC (2001) Immunosuppressive effects of aflatoxin B1 in Indian major carp (Labeo rohita). Comp Immun Microbiol Infect Dis 24:143–149
Sahoo PK, Mukherjee SC (2002) Effect of dietary immunomodulation upon Edwardsiella tarda vaccination in healthy and immunocompromised Indian major carp, (Labeo rohita). Fish Shellfish Immunol 12:1–16
Sahoo PK, Mukherjee SC (2003) Immunomodulation by dietary vitamin C in healthy and aflatoxin B1-immunocompromised rohu (Labeo rohita). Comp Immun Microbiol Infect Dis 26:65–76
Sahoo PK, Mukherjee SC, Jain AK, Mukherjee A (2001) Histopathological and electron microscopic studies of gills and opisthonephros of Rohu, Labeo rohita to acute and subchronic aflatoxin B1 toxicity. Asian Fish Sci 16:257–268
Sakai M (1999) Current research status of fish immunostimulants. Aquaculture 172:63–92
Santacroce MP, Conversano MC, Casalino E, Lai O, Zizzadoro C, Centoducati G, Crescenzo G (2008) Aflatoxins in aquatic species: metabolism, toxicity and perspectives. Rev Fish Biol Fish 18:99–130
Schaperclaus W, Kulow H, Schreckenbach K (1992) Fish disease. AA Balkema, Rotterdam
Schoental R (1967) Aflatoxins. Ann Rev Pharmacol 7:343–356
Shane S (1993) Economic issues associated with aflatoxins. In: Eaton DL, Groopman JD (eds) The toxicology of aflatoxins: human health, veterinary, and agricultural significance. Academic Press, London, pp 513–527
Shotwell OL, Hesseltine CW, Stubblefield RD, Sorenson WG (1966) Production of aflatoxin on rice. Appl Microb 14(3):425–428
Siwicki AK, Anderson DP, Rumsey GL (1994) Dietary intake of immunostimulants by rainbow trout affects non-specific immunity and protection against furunculosis. Vet Immunol Immunopathol 41:125–139
Takahashi N, Stresser DM, Williams DE, Bailey GS (1995) Induction of hepatic CYP1A by indole-3-carbinol in protection against aflatoxin B1 hepatocarcinogenesis in rainbow trout. Food Chem Toxicol 33:841–850
Tsai HW (1996) Evaluation of zebrafish (Brachydanio rerio) as a model for carcinogenesis. Doctoral dissertation, Oregon State University
Tuan NA, Grizzle JM, Lovell RT, Manning BB, Rottinghaus GE (2002) Growth and hepatic lesions of Nile tilapia (Oreochromis niloticus) fed diets containing aflatoxin B1. Aquaculture 212:311–319
Varior S, Philip B (2012) Aflatoxin B1 induced alterations in the stability of the lysosomal membrane in Oreochromis mossambicus (Peters 1852). Aqua Res 43(8):1170–1175
Wu FC (1998) Retention of diet-related mycotoxins in tissues of channel catfish. Doctoral dissertation, Auburn University, Auburn, Alabama
Yildirim I, Parlat SS (2003) Effects of dietary addition of live yeast (Saccharomyces cerevisiae) on hatching traits, testis and ovary weights of Japanese quail (Coturnix coturnix japonica) in aflatoxicosis. Arch Geflugelk 67(5):208–211
Zaki MS, Sharaf NE, Rashad H, Mastala SO, Fawzy QM (2008) Diminution of aflatoxicosis in tilapia nilotica fish by dietary supplementation with fix in toxin and Nigella sativa oil. Am Eurasian J Agric Environ Sci 3:211–215
Zychowski KE, Pohlenz C, Mays T, Romoser A, Hume M, Buentello A, Gatlin DM III, Phillips TD (2013) The effect of NovaSil dietary supplementation on the growth and health performance of Nile tilapia (Oreochromis niloticus) fed aflatoxin-B1 contaminated feed. Aquaculture 376:117–123
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Selim, K.M., El-hofy, H. & Khalil, R.H. The efficacy of three mycotoxin adsorbents to alleviate aflatoxin B1-induced toxicity in Oreochromis niloticus . Aquacult Int 22, 523–540 (2014). https://doi.org/10.1007/s10499-013-9661-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-013-9661-6