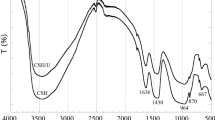
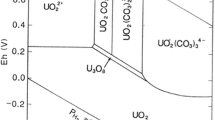
Abstract
The effect of Mg-, Ca-, and Sr–Uranyl-Carbonato complexes with respect to sorption on quartz was studied by means of batch experiments with U(VI) concentration of 0.126 × 10−6 M in the presence and absence of Mg, Ca, and Sr (each 1 mM) at pH from 6.5 to 9. In the absence of alkaline earth elements, 90% of the U(VI) sorbed on the quartz surface at all pH. In the presence of Mg, Ca, and Sr, the sorption of U(VI) on quartz decreased to 50, 10, and 30%, respectively. Sorption kinetics of U(VI) on quartz is faster in the absence of alkaline earth elements and reached equilibrium after 12 h, whereas in the presence of Mg, Ca and Sr, the kinetics of U(VI) sorption on quartz is pH dependent and attained equilibrium after 24 h. Aqueous speciation calculations for alkaline earth uranyl carbonates were carried out by using PHREEQC with the Nuclear Energy Agency thermodynamic database (NEA_2007) by adding constants for MUO2(CO3) 2−3 and M2UO2(CO3) 03 (M = Ca, Mg, Sr). This study reveals that alkaline earth elements can have a significant effect on the aqueous speciation of U(VI) under neutral to alkaline pH conditions and subsequently sorption behavior and mobility of U(VI) in aqueous environments.



Similar content being viewed by others
References
Abdelouas A, Lutze W, Nuttall E (1998) Chemical reactions of uranium in ground water at a mill tailings site. J Contam Hydrol 34:343–361
Barisic D, Lulic S, Miletic P (1992) Radium and uranium in phosphate fertilizers and their impact on the radioactivity of waters. Water Res 26:607–611
Benedikt G (2007) 797 VA Computrace—voltammetric trace determination of uranium(VI) in drinking and mineral water. Metrohm Information Issue 2/2007. Metrohm Ltd., CH-9101 Herisau, Switzerland. p 36
Bernhard G (2005) Speciation of uranium in environmental relevant compartments. Landbauforschung Volkenrode 55:139–148
Bernhard G, Geipel G, Brendler V, Nitsche H (1996) Speciation of uranium in seepage waters of a mine tailing pile studied by time-resolved laser-induced fluorescence spectroscopy (TRLFS). Radiochimica Acta 74:87–91
Bernhard G, Geipel G, Reich T, Brendler V, Amayri S, Nitsche H (2001) Uranyl(VI) carbonate complex formation: validation of the Ca2UO2(CO3)(3)(aq.) species. Radiochimica Acta 89:511–518
Curtis GP, Davis JA, Naftz DL (2006) Simulation of reactive transport of uranium(VI) in groundwater with variable chemical conditions. Water Resour Res 42
Davis JA, Meece DE, Kohler M, Curtis GP (2004) Approaches to surface complexation modeling of uranium(VI) adsorption on aquifer sediments. Geochimica Et Cosmochimica Acta 68:3621–3641
Dong WM, Brooks SC (2006) Determination of the formation constants of ternary complexes of uranyl and carbonate with alkaline earth metals (Mg2+, Ca2+, Sr2+, and Ba2+) using anion exchange method. Environ Sci Technol 40:4689–4695
Dong WM, Brooks SC (2008) Formation of aqueous MgUO2(CO3) 2−3 complex and uranium anion exchange mechanism onto an exchange resin. Environ Sci Technol 42:1979–1983
Fox PM, Davis JA, Zachara JM (2006) The effect of calcium on aqueous uranium(VI) speciation and adsorption to ferrihydrite and quartz. Geochimica Et Cosmochimica Acta 70:1379–1387
Gabriel U, Charlet L, Schlapfer CW, Vial JC, Brachmann A, Geipel G (2001) Uranyl surface speciation on silica particles studied by time-resolved laser-induced fluorescence spectroscopy. J Colloid Interface Sci 239:358–368
Geipel G, Amayri S, Bernhard G (2008) Mixed complexes of alkaline earth uranyl carbonates: a laser-induced time-resolved fluorescence spectroscopic study. Spectrochimica Acta Part A-Mol Biomol Spectrosc 71:53–58
Grenthe I, Fuger J, Konings R, Lemire RJ, Muller AB, Wanner J (2007) The chemical thermodynamics of uranium. Elsevier, New York
Hsi CKD, Langmuir D (1985) Adsorption of uranyl onto ferric oxyhydroxides—application of the surface complexation site-binding model. Geochimica Et Cosmochimica Acta 49:1931–1941
Huber F, Lützenkirchen J (2009) Uranyl retention on quartz—new experimental data and blind prediction using an existing surface complexation model. Aquatic Geochem
Kalmykov SN, Choppin GR (2000) Mixed Ca2+/UO22+/CO32—complex formation at different ionic strengths. Radiochimica Acta 88:603–606
Kelly SD, Kemner KM, Brooks SC (2007) X-ray absorption spectroscopy identifies calcium-uranyl-carbonate complexes at environmental concentrations. Geochimica Et Cosmochimica Acta 71:821–834
Kohler M, Curtis GP, Kent DB, Davis JA (1996) Experimental investigation and modeling of uranium(VI) transport under variable chemical conditions. Water Resour Res 32:3539–3551
Kumar R, Mahur AK, Sengupta D, Prasad R (2005) Radon activity and exhalation rates measurements in fly ash from a thermal power plant. Radiat Meas 40:638–641
Lieser KH, Quandtklenk S, Thybusch B (1992) Sorption of uranyl ions on hydrous silicon dioxide. Radiochimica Acta 57:45–50
Meinrath G, Volke P, Helling C, Dudel EG, Merkel BJ (1999) Determination and interpretation of environmental water samples contaminated by uranium mining activities. Fresenius J Anal Chem 364:191–202
Merkel BJ, Hasche-Berger A (2005) Uranium in the environment. Uranium mining and hydrogeology. Springer, Freiberg, Germany
Merkel BJ, Planer-Friedrich B, Wolkersdorfer C (2002) Uranium in the aquatic environment international conference uranium mining and hydrogeology III and the international mine water association symposium, Freiberg, Germany
Pabalan RT, Bertetti FP, Prikryl JD, Turner DR (1996) Uranium(VI) sorption onto selected mineral surfaces: key geochemical parameters. Abstracts of papers of the American Chemical Society 211:55-Geoc
Papp Z, Dezso Z, Daroczy S (2002) Significant radioactive contamination of soil around a coal-fired thermal power plant. J Environ Radioact 59:191–205
Parkhurst DL, Appelo CA (1999) User’s guide to PHREEQC (version 2). A computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculation. U.S.G.S., Water Resources Investigation Report 99–4259
Prikryl JD, Jain A, Turner DR, Pabalan RT (2001) Uranium(VI) sorption behavior on silicate mineral mixtures. J Contam Hydrol 47:241–253
Smith JT, Wright SM, Cross MA, Monte L, Kudelsky AV, Saxen R, Vakulovsky SM, Timms DN (2004) Global analysis of the riverine transport of Sr-90 and Cs-137. Environ Sci Technol 38:850–857
Stamberg K, Venkatesan KA, Rao PRV (2003) Surface complexation modeling of uranyl ion sorption on mesoporous silica. Colloids Surf A-Physicochem Eng Aspects 221:149–162
Standring WJF, Oughton DH, Salbu B (2002) Potential remobilization of Cs-137, Co-60, Tc-99 and Sr-90 from contaminated Mayak sediments river and estuary environments. Environ Sci Technol 36:2330–2337
Sylwester ER, Hudson EA, Allen PG (2000) The structure of uranium (VI) sorption complexes on silica, alumina, and montmorillonite. Geochimica Et Cosmochimica Acta 64:2431–2438
Zheng ZP, Tokunaga TK, Wan JM (2003) Influence of calcium carbonate on U(VI) sorption to soils. Environ Sci Technol 37:5603–5608
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nair, S., Merkel, B.J. Impact of Alkaline Earth Metals on Aqueous Speciation of Uranium(VI) and Sorption on Quartz. Aquat Geochem 17, 209–219 (2011). https://doi.org/10.1007/s10498-011-9120-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10498-011-9120-9