Abstract
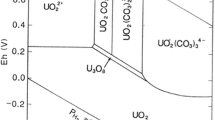
The desorption of uranium from a U(VI)-contaminated C-S-H matrix has been investigated as a function of the EDTA concentration in solution under N2- and ambient atmosphere. The Kd values evaluated from the experimental data indicate that U(VI) is retarded in the solid phase due to sorption of EDTA through interaction with U(VI) at the C-S–H surface. On the other hand, under ambient conditions the formation of stable U(VI)-carbonato species results in higher U(VI) concentration in solutions compared to corresponding systems under N2-atmosphere.



Similar content being viewed by others
References
Grambow B (2016) Geological disposal of radioactive waste in clay. Elements 12:239–245
Richardson IG (2008) The calcium silicate hydrates. Cem Concr Res 38:137–158
Tits J, Stumpf T, Rabung T, Wieland E, Fanghänel T (2003) Uptake of Cm(III) and Eu(III) by calcium silicate hydrates: a solution chemistry and time-resolved laser fluorescence spectroscopy. Study Environ Sci Technol 37:3568–3573
Tits J, Walther C, Stumpf T, Mace N, Wieland E (2015) A luminescence line-narrowing spectroscopic study of the uranium(VI) interaction with cementitious materials and titanium dioxide. Dalton Trans 44:966–976
Ochs M, Mallants D, Wang L (2016) Radionuclide and metal sorption on cement and concrete. Springer, Switzerland. DOI https://doi.org/10.1007/978-3-319-23651-3
Pashalidis I, Runde W, Kim IJ (1993) A study of solid-liquid phase equilibria of Pu (VI) and U (VI) in aqueous carbonate systems. Radiochim Acta 61:141–146
Zhang W, Wang J (2017) Leaching performance of uranium from the cement solidified matrices containing spent radioactive organic solvent. Ann Nucl Energy 101:31–35
Atkins M, Glasser FP (1992) Application of portland cement-based materials to radioactive waste immobilization. Waste Manage 12:105–131
Hongbin T, Yuxiang L (2005) The existence state of uranium(VI) in portland cement matrix material immobilization body. Uranium Min Metall 38:86–90
Wang F, Chen G, Ji L, Yuan Z (2020) Preparation and mechanical properties of cemented uranium tailing backfill based on alkali-activated slag. Adv Mater Sci Eng 2020:1–7
Ochs M, Vriens B, Tachi Y (2018) Retention of uranium in cement systems: effects of cement degradation and complexing ligands. Prog Nucl Sci Technol 5:208–212
Macé N, Wieland E, Dähn R, Tits J, Scheinost AC (2013) EXAFS investigation on U(VI) immobilization in hardened cement paste: influence of experimental conditions on speciation. Radiochim Acta 101:379–389
Tits J, Geipel G, Macé N, Eilzer M, Wieland E (2011) Determination of uranium(VI) sorbed species in calcium silicate hydrate phases: a laser-induced luminescence spectroscopy and batch sorption study. J Colloid Interface Sci 359:248–256
Androniuk I, Landesman C, Henocq P, Kalinichev GA (2017) Adsorption of gluconate and uranyl on C-S-H phases: combination of wet chemistry experiments and molecular dynamics simulations for the binary systems. Phys Chem Earth 99:194–203
Maragkou E, Pashalidis I (2021) Investigations on the interaction of EDTA with calcium silicate hydrate and its impact on the U(VI) sorption. Coatings 11:1037
du Bois de Maquillé L, Renaudin L, Goutelard F, Jardy A, Vial J, Thiébaut D (2013) Determination of ethylenediaminetetraacetic acid in nuclear waste by high-performance liquid chromatography coupled with electrospray mass spectrometry. J Chromatogr A 1276:20–25
Paschalidou P, Pashalidis I (2019) Alpha-spectroscopic analysis of uranium in ground- and seawater samples after EDTA-masking of interfering cations. J Radioanal Nucl Chem 321:973–975
Paschalidou P, Pashalidis I (2019) Selective separation and determination of uranium in calcite and gypsum after EDTA-mediated sample dissolution and cation-exchange. J Radioanal Nucl Chem 320:807–812
Paschalidou P, Pashalidis I (2019) Recovery of uranium from phosphate rock with EDTA-mediated dissolution and cation exchange. Hydrometallurgy 189:105118
Maddalena R, Li K, Chater AP, Michalik S, Hamilton A (2019) Direct synthesis of a solid calcium-silicate-hydrate (C-S-H). Constr Build Mater 223:554–565
Kiliari T, Pashalidis I (2010) Simplified alpha-spectroscopic analysis of uranium in natural waters after its separation by cation-exchange. Radiat Meas 45:966–968
Grangeon S, Claret F, Linard Y, Chiaberge C (2013) X-ray diffraction: a powerful tool to probe and understand the structure of nanocrystalline calcium silicate hydrates. Acta Crystallogr B 69:465–473
Matsumoto K, Nonaka R, Wang Y, Veryasov G, Hagiwara R (2017) Formation of a solid solution between [N(C2H5)4][BF4] and [N(C2H5)4][PF6] in crystal and plastic crystal phases. Phys Chem Chem Phys 19:2053–2059
Nalet C, Nonat A (2016) Ionic complexation and adsorption of small organic molecules on calcium silicate hydrate: relation with their retarding effect on the hydration. Cem Concr Res 89:97–108
Pashalidis I, Czerwinski KR, Fanghaenel T, Kim JI (1997) A study of solid–liguid phase equilibria of Pu(VI) and U(VI) in aqueous carbonate systems. determination of the carbonate stability constants. Radiochim Acta 76:55–62
Acknowledgements
The project leading to this application has received funding from the European Union’s Horizon 2020 Research and Innovation Programme under Grant Agreement No. 847593.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Maragkou, E., Pashalidis, I. The effect of EDTA on the desorption of uranium from calcium silicate hydrate matrices. J Radioanal Nucl Chem 331, 507–510 (2022). https://doi.org/10.1007/s10967-021-08089-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-021-08089-w