Abstract
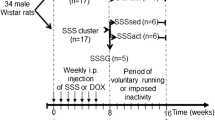
Apoptotic signaling pathways are involved in acute kidney injury (AKI) induced by the antineoplastic drug cisplatin (Cis). Mechanical stress is known to increase interleukin (IL) -11, a pleiotropic cytokine with antiapoptotic and antinecrotic effects. We compared the impact of high-intensity interval training (HIIT) with low-intensity continuous training (LICT) and moderate-intensity continuous training (MICT) on renal levels of IL-11 and the expression of apoptotic markers in female rats with nephrotoxicity induced by Cis. For that, the animals were divided into five groups (n = 7): control and sedentary (C + S); Cis and sedentary (Cis + S); Cis and LICT (Cis + LICT); Cis and MICT (Cis + MICT) and Cis and HIIT (Cis + HIIT). At the end of 8 weeks of treadmill running, the rats received a single injection of Cis (5 mg/kg), and 7 days later they were euthanized. Serum and kidney samples were collected to assess the blood urea nitrogen (BUN), gene expression of TNF receptor 1 (TNFR1) and 2 (TNFR2), caspase-3, (p38) MAPK (MAPK14), p53, Bax, Bak, Bcl-2, and Bcl-xL, renal levels of IL-11, IL-8, and p53, and immunolocalization of cleaved caspase-3, Bax, Bcl-2, and (p38) MAPK in renal tissue. Our data indicate that all trained groups showed a significant intensity-dependent increase in renal levels of IL-11 associated with reduced local expression of proapoptotic and increased antiapoptotic markers, but these effects were more pronounced with HIIT. So, HIIT appears to provide superior renoprotection than traditional continuous training by modulating apoptotic signaling pathways, and this effect can be related to the increase in renal levels of IL-11.





Similar content being viewed by others
Data availability
the datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Holditch SJ, Brown CN, Lombardi AM, Nguyen KN, Edelstein CL (2019) Recent advances in models, mechanisms, biomarkers, and Interventions in Cisplatin-Induced Acute kidney Injury. Int J Mol Sci 20:3011. https://doi.org/10.3390/ijms20123011
Volarevic V, Djokovic B, Jankovic MG, Harrell CR, Fellabaum C, Djonov V, Arsenijevic N (2019) Molecular mechanisms of cisplatin-induced nephrotoxicity: a balance on the knife edge between renoprotection and tumor toxicity. J Biomed Sci 26:1–14. https://doi.org/10.1186/s12929-019-0518-9
Dubey RK, Jackson EK (2001) Estrogen-induced cardiorenal protection: potential cellular, biochemical, and molecular mechanisms. Am J Physiol Renal Physiol 280:F365–F388. https://doi.org/10.1152/ajprenal.2001.280.3.F365
Pan JS, Sheikh-Hamad D (2019) Mitochondrial dysfunction in acute kidney injury and sex-specific implications. Med Res Arch. https://doi.org/10.18103/mra.v7i2.1898
Pezeshki Z, Nematbakhsh M, Mazaheri S, Eshraghi-Jazi F, Talebi A, Nasri H, Safari T, Mansouri A, Ashrafi F (2012) Estrogen abolishes Protective Effect of Erythropoietin against Cisplatin-Induced Nephrotoxicity in Ovariectomized rats. ISRN Oncol. https://doi.org/10.5402/2012/890310
Chen WY, Hsiao CH, Chen YC, Ho CH, Wang J-J, Hsing CH, Wang HY, Kan WC, Wu CC (2017) Cisplatin nephrotoxicity might have a sex difference. An analysis based on women’s sex hormone changes. J Cancer 8:3939–3944. https://doi.org/10.7150/jca.20083
Manohar S, Leung N (2018) Cisplatin nephrotoxicity: a review of the literature. J Nephrol 31:15–25. https://doi.org/10.1007/s40620-017-0392-z
Ma N, Wei W, Fan X, Ci X (2019) Farrerol attenuates Cisplatin-Induced nephrotoxicity by inhibiting the reactive oxygen species-mediated oxidation, inflammation, and apoptotic signaling pathways. https://doi.org/10.3389/fphys.2019.01419. Front Physiol
Malik S, Bhatia J, Suchal K, Gamad N, Dinda AK, Gupta YK, Arya DS (2015) Nobiletin ameliorates cisplatin-induced acute kidney injury due to its anti-oxidant, anti-inflammatory and anti-apoptotic effects. Exp Toxicol Pathol 67:427–433. https://doi.org/10.1016/j.etp.2015.04.008
Kim H-J, Park DJ, Kim JH, Jeong EY, Jung MH, Kim T-H, Yang JI, Lee G-W, Chung HJ, Chang S-H (2015) Glutamine protects against cisplatin-induced nephrotoxicity by decreasing cisplatin accumulation. J Pharmacol Sci 127:117–126. https://doi.org/10.1016/j.jphs.2014.11.009
Akassoglou K, Douni E, Bauer J, Lassmann H, Kollias G, Probert L (2003) Exclusive tumor necrosis factor (TNF) signaling by the p75TNF receptor triggers inflammatory ischemia in the CNS of transgenic mice. Proceedings of the National Academy of Sciences 100:709–714. https://doi.org/10.1073/pnas.0236046100
Ramesh G, Brian Reeves W (2003) TNFR2-mediated apoptosis and necrosis in cisplatin-induced acute renal failure. Am J Physiology-Renal Physiol 285:F610–F618. https://doi.org/10.1152/ajprenal.00101.2003
Yang Y-C, Hsu T-Y, Chen J-Y, Yang C-S, Lin R-H (2001) Tumour necrosis factor-α-induced apoptosis in cord blood T lymphocytes: involvement of both tumour necrosis factor receptor types 1 and 2. Br J Haematol 115:435–441. https://doi.org/10.1046/j.1365-2141.2001.03090.x
Naudé PJW, den Boer JA, Luiten PGM, Eisel ULM (2011) Tumor necrosis factor receptor cross-talk. FEBS J 278:888–898. https://doi.org/10.1111/j.1742-4658.2011.08017.x
Borghi A, Verstrepen L, Beyaert R (2016) TRAF2 multitasking in TNF receptor-induced signaling to NF-κB, MAP kinases and cell death. Biochem Pharmacol 116:1–10. https://doi.org/10.1016/j.bcp.2016.03.009
Al-Lamki RS, Mayadas TN (2015) TNF receptors: signaling pathways and contribution to renal dysfunction. Kidney Int 87:281–296. https://doi.org/10.1038/ki.2014.285
Yang S, Wang J, Brand DD, Zheng SG (2018) Role of TNF–TNF receptor 2 Signal in Regulatory T cells and its therapeutic implications. Front Immunol. https://doi.org/10.3389/fimmu.2018.00784
Tomar A, Vasisth S, Khan SI, Malik S, Nag TC, Arya DS, Bhatia J (2017) Galangin ameliorates cisplatin induced nephrotoxicity in vivo by modulation of oxidative stress, apoptosis and inflammation through interplay of MAPK signaling cascade. Phytomedicine 34:154–161. https://doi.org/10.1016/j.phymed.2017.05.007
Li RY, Zhang WZ, Yan XT et al (2019) Arginyl-fructosyl-glucose, a major Maillard reaction product of Red Ginseng, attenuates Cisplatin-Induced Acute kidney Injury by Regulating Nuclear factor κB and phosphatidylinositol 3-Kinase/Protein kinase B Signaling Pathways. J Agric Food Chem 67:5754–5763. https://doi.org/10.1021/acs.jafc.9b00540
Tsuruya K, Tokumoto M, Ninomiya T, Hirakawa M, Masutani K, Taniguchi M, Fukuda K, Kanai H, Hirakata H, Iida M (2003) Antioxidant ameliorates cisplatin-induced renal tubular cell death through inhibition of death receptor-mediated pathways. Am J Physiology-Renal Physiol 285:F208–F218. https://doi.org/10.1152/ajprenal.00311.2002
Jiang M, Yi X, Hsu S, Wang C-Y, Dong Z (2004) Role of p53 in cisplatin-induced tubular cell apoptosis: dependence on p53 transcriptional activity. Am J Physiology-Renal Physiol 287:F1140–F1147. https://doi.org/10.1152/ajprenal.00262.2004
Silva KA dos, dos Santos Silva S, da Silva Luiz KA R, et al (2012) Previous Exercise Training has a Beneficial Effect on Renal and Cardiovascular function in a model of diabetes. PLoS ONE 7:e48826. https://doi.org/10.1371/journal.pone.0048826
Faleiros CM, Francescato HDC, Papoti M, Chaves L, Silva CGA, Costa RS, Coimbra TM (2017) Effects of previous physical training on adriamycin nephropathy and its relationship with endothelial lesions and angiogenesis in the renal cortex. Life Sci 169:43–51. https://doi.org/10.1016/j.lfs.2016.11.014
Amaral LS, de Silva B, Correia FA VB et al (2016) Beneficial effects of previous exercise training on renal changes in streptozotocin-induced diabetic female rats. Experimental Biology and Medicine 241:437–445. https://doi.org/10.1177/1535370215609696
Amaral LS, de Souza B, Volpini CS RA et al (2018) Previous Exercise Training reduces markers of renal oxidative stress and inflammation in Streptozotocin-Induced Diabetic Female rats. J Diabetes Res 2018:1–9. https://doi.org/10.1155/2018/6170352
Souza CS, de Sousa Oliveira BS, Viana GN et al (2019) Preventive effect of exercise training on diabetic kidney disease in ovariectomized rats with type 1 diabetes. Experimental Biology and Medicine 244:758–769. https://doi.org/10.1177/1535370219843830
Leite AB, Lima HN, Flores CO et al (2021) High-intensity interval training is more effective than continuous training to reduce inflammation markers in female rats with cisplatin nephrotoxicity. Life Sci 266:118880. https://doi.org/10.1016/j.lfs.2020.118880
Kido S, Kuriwaka-Kido R, Imamura T, Ito Y, Inoue D, Matsumoto T (2009) Mechanical stress induces Interleukin-11 expression to stimulate osteoblast differentiation. Bone 45:1125–1132. https://doi.org/10.1016/j.bone.2009.07.087
Matsumoto T, Kuriwaka-Kido R, Kondo T, Endo I, Kido S (2012) Regulation of osteoblast differentiation by interleukin-11 via AP-1 and smad signaling [Review]. Endocr J 59:91–101. https://doi.org/10.1507/endocrj.ej11-0219
Du X, Williams DA (1997) Interleukin-11: review of molecular, cell biology, and clinical use. Blood 89:3897–3908. https://doi.org/10.1182/blood.v89.11.3897
Goldman SC, Bracho F, Davenport V, Slack R, Areman E, Shen V, Lenarsky C, Weinthal J, Hughes R, Cairo MS (2001) Feasibility study of IL-11 and granulocyte colony-stimulating factor after Myelosuppressive Chemotherapy to mobilize peripheral blood stem cells from heavily pretreated patients. J Pediatr Hematol Oncol 23:300–305. https://doi.org/10.1097/00043426-200106000-00013
Lai PC, Terence Cook H, Smith J, Keith JC, Pusey CD, Tam FWK (2001) Interleukin-11 attenuates nephrotoxic nephritis in Wistar Kyoto rats. J Am Soc Nephrol 12:2310–2320. https://doi.org/10.1681/asn.v12112310
Lee HT, Thomas Lee H, Park SW, Kim M, Ham A, Anderson LJ, Brown KM, D’Agati VD, Cox GN (2012) Interleukin-11 protects against renal ischemia and reperfusion injury. Am J Physiology-Renal Physiol 303:F1216–F1224. https://doi.org/10.1152/ajprenal.00220.2012
Kim JY, Kim M, Ham A, Brown KM, Greene RW, D’Agati VD, Thomas Lee H (2013) IL-11 is required for A1Adenosine receptor–mediated protection against ischemic AKI. J Am Soc Nephrol 24:1558–1570. https://doi.org/10.1681/asn.2013010114
Pham TD, MacLennan NK, Chiu CT, Laksana GS, Hsu JL, Lane RH (2003) Uteroplacental insufficiency increases apoptosis and alters p53 gene methylation in the full-term IUGR rat kidney. Am J Physiology-Regulatory Integr Comp Physiol 285:R962–R970. https://doi.org/10.1152/ajpregu.00201.2003
Francescato HDC, Almeida LF, Reis NG, Faleiros CM, Papoti M, Costa RS, Coimbra TM (2018) Previous Exercise Effects in Cisplatin-Induced Renal Lesions in rats. Kidney and Blood Pressure Research 43:582–593. https://doi.org/10.1159/000488964
Lima WV, Visona I, Schor N, Almeida WS (2019) Preconditioning by aerobic exercise reduces acute ischemic renal injury in rats. Physiological Rep. https://doi.org/10.14814/phy2.14176
Silveira MORda, da Silveira MOR, de Brito Amaral LS, de Souza SI, Ferraz HR, Dias JA, Rocha EC, de Abreu Silva F, de Magalhães ACM, de Jesus Soares T (2019) Effect of moderate exercise on renal changes and oxidative stress in ovariectomized rats with type 1 diabetes mellitus. Brazilian J Biol Sci 6:331–345. https://doi.org/10.21472/bjbs.061302
Bragado P, Armesilla A, Silva A, Porras A (2007) Apoptosis by cisplatin requires p53 mediated p38α MAPK activation through ROS generation. Apoptosis 12:1733–1742. https://doi.org/10.1007/s10495-007-0082-8
Miyagi MYS, Seelaender M, Castoldi A et al (2014) Long-Term Aerobic Exercise protects against Cisplatin-Induced nephrotoxicity by modulating the expression of IL-6 and HO-1. PLoS ONE 9:e108543. https://doi.org/10.1371/journal.pone.0108543
Chen KC, Peng CC, Hsieh CL, Peng RY (2013) Exercise ameliorates renal cell apoptosis in chronic kidney disease by intervening in the intrinsic and the extrinsic apoptotic pathways in a rat model. Evidence-Based Complement Altern Med 2013:1–13. https://doi.org/10.1155/2013/368450
Estrela GR, Wasinski F, Batista RO et al (2017) Caloric restriction is more efficient than physical Exercise to protect from Cisplatin Nephrotoxicity via PPAR-Alpha activation. Front Physiol. https://doi.org/10.3389/fphys.2017.00116
Noroozi J, Zeynali F, Nematbakhsh M, Pezeshki Z, Talebi A (2015) Nonpreventive role of Aerobic Exercise against Cisplatin-induced nephrotoxicity in female rats. Int J Prev Med 6:58. https://doi.org/10.4103/2008-7802.160333
Tucker PS, Scanlan AT, Vella RK, Dalbo VJ (2016) Genomic Integrity is favourably affected by high-intensity interval training in an animal model of early-stage chronic kidney disease. https://doi.org/10.1186/s40798-016-0055-y. Sports Medicine - Open
Tucker PS, Briskey DR, Scanlan AT, Coombes JS, Dalbo VJ (2015) High intensity interval training favourably affects antioxidant and inflammation mRNA expression in early-stage chronic kidney disease. Free Radic Biol Med 89:466–472. https://doi.org/10.1016/j.freeradbiomed.2015.07.162
Almeida AA, Correia TML, Pires RA et al (2022) Nephroprotective effect of exercise training in cisplatin-induced renal damage in mice: influence of training protocol. Braz J Med Biol Res 55:e12116. https://doi.org/10.1590/1414-431X2022e12116
Zhu M, Lu B, Cao Q, Wu Z, Xu Z, Li W, Yao X, Liu F (2015) IL-11 attenuates Liver Ischemia/Reperfusion Injury (IRI) through STAT3 signaling pathway in mice. PLoS ONE 10:e0126296. https://doi.org/10.1371/journal.pone.0126296
Acknowledgements
authors are grateful to professor Regiane Yatsuda for donating experimental animals, and Luiza Brito Amaral for her artistic contribution.
Funding
this work was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), grant number: 433542/2018-7, and Fundação de Amparo à Pesquisa do Estado da Bahia (FAPESB), grant number: 1144/2021.
Author information
Authors and Affiliations
Contributions
LSBA designed the study, supervised, and coordinated the project. LSBA and CAO wrote the original manuscript. LSBA, CAO, TJS, and FFM revised the paper. CAO, JMB, LMB, AVBS, and JAGC conducted animal experiments. CAO, EABM, FSP, FFM, and MVO conducted laboratory experiments. LSBA, ACMM, and TJS discussed the results and analyzed the data. All authors read and approved this manuscript.
Corresponding author
Ethics declarations
Statements and declarations
all authors of this manuscript have read the journal’s authorship agreement and policy and declare no conflict of interest with respect to the research, authorship, and publication of this article.
Ethical approval
the Ethics Committee on Animal Experimentation of the Federal University of Bahia - Multidisciplinary Institute of Health approved this experimental protocol (056/2018), and all experimental procedures were conducted in accordance with the recommendations of the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Oliveira, C.A., Mercês, É.A.B., Portela, F.S. et al. Benefits of high-intensity interval training compared to continuous training to reduce apoptotic markers in female rats with cisplatin nephrotoxicity – possible modulatory role of IL-11. Apoptosis 28, 566–575 (2023). https://doi.org/10.1007/s10495-023-01816-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-023-01816-6