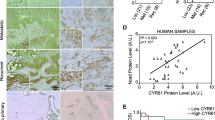
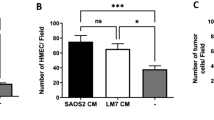
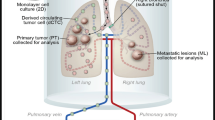
Abstract
Osteosarcoma (OS) is the most frequent malignant bone tumor, affecting predominantly children. Metastases represent a major clinical challenge and an estimated 80% would present undetectable micrometastases at diagnosis. The identification of metastatic traits and molecules would impact in micrometastasis management. We demonstrated that OS LM7 metastatic cells secretome was able to induce microvascular endothelium cell rearrangements, an angiogenic-related trait. A proteomic analysis indicated a gain in angiogenic-related pathways in these cells, as compared to their parental-non-metastatic OS SAOS2 cells counterpart. Further, factors with proangiogenic functions like VEGF and PDGF were upregulated in LM7 cells. However, no differential angiogenic response was induced by LM7 cells in vivo. Regulation of the Fas–FasL axis is key for OS cells to colonize the lungs in this model. Analysis of the proteomic data with emphasis in apoptosis pathways and related processes revealed that the percentage of genes associated with those, presented similar levels in SAOS2 and LM7 cells. Further, the balance of expression levels of proteins with pro- and antiapoptotic functions in both cell types was subtle. Interestingly and of relevance to the model, Fas associated Factor 1 (FAF1), which participates in Fas signaling, was present in LM7 cells and was not detected in SAOS2 cells. The subtle differences in apoptosis-related events and molecules, together with the reported cell-survival functions of the identified angiogenic factors and the increased survival features that we observed in LM7 cells, suggest that the gain in angiogenesis-related pathways in metastatic OS cells would relate to a prosurvival switch rather to an angiogenic switch as an advantage feature to colonize the lungs. OS metastatic cells also displayed higher adhesion towards microvascular endothelium cells suggesting an advantage for tissue colonization. A gain in angiogenesis pathways and molecules does not result in major angiogenic potential. Together, our results suggest that metastatic OS cells would elicit signaling associated to a prosurvival phenotype, allowing homing into the hostile site for metastasis. During the gain of metastatic traits process, cell populations displaying higher adhesive ability to microvascular endothelium, negative regulation of the Fas–FasL axis in the lung parenchyma and a prosurvival switch, would be selected. This opens a new scenario where antiangiogenic treatments would affect cell survival rather than angiogenesis, and provides a molecular panel of expression that may help in distinguishing OS cells with different metastatic potential.




Similar content being viewed by others
References
Cortini M, Avnet S, Baldini N (2017) Mesenchymal stroma: role in osteosarcoma progression. Cancer Lett 405:90–99
Kager L, Zoubek A, Dominkus M, Lang S, Bodmer N, Jundt G, Klingebiel T, Jürgens H, Gadner H, Bielack S, COSS Study Group (2010) Osteosarcoma in very young children. Cancer Lett 116(22):5316–5324
Bielack S, Kempf-Bielack B, Delling G, Exner GU, Flege S, Helmke K, Kotz R, Salzer-Kuntschik M, Werner M, Winkelmann W, Zoubek A, Jürgens H, Winkler K (2002) Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol 20(3):776–790
Alfranca A, Martinez-Cruzado L, Tornin J, Abarrategi A, Amaral T, de Alava E, Menendez P, Garcia-Castro J, Rodriguez R (2015) Bone microenvironment signals in osteosarcoma development. Cell Mol Life Sci 72(16):3097–3113
Lee SH, Shin MS, Park WS, Kim SY, Dong SM, Lee HK, Park JY, Oh RR, Jang JJ, Lee JY, Yoo NJ (1999) Immunohistochemical analysis of Fas ligand expression in normal human tissues. J Pathol Microbiol Immunol 107(11):1013–1019
Jia S, Worth LL, Kleinerman ES (1999) A nude mouse model of human osteosarcoma lung metastases for evaluating new therapeutic strategies. Clin Exp Metastas 17(6):501–506
Lafleur EA, Koshkina NV, Stewart J, Jia SF, Worth LL, Duan X, Kleinerman ES (2004) Increased Fas Expression Reduces the Metastatic Potential of Human Osteosarcoma Cells. Clin Cancer Res 10(23):8114–8119
Yang Y, Huang G, Zhou Z, Fewell JG, Kleinerman ES (2018) miR-20a regulates Fas expression in osteosarcoma cells by modulating Fas promoter activity and can be therapeutically targeted to inhibit lung metastases. Mol Cancer Ther 17(1):130–139
Xu WT, Bian ZY, Fan QM, Li G, Tang TT (2009) Human mesenchymal stem cells (hMSCs) target osteosarcoma and promote its growth and pulmonary metastasis. Cancer Lett 281(1):32–41
Lambert AW, Pattabiraman DR, Weinberg RA (2017) Emerging biological principles of metastasis. Cell 168(4):670–691
Fidler IJ (2003) The pathogenesis of cancer metastasis: the “seed and soil” hypothesis revisited. Nat Rev Cancer 3(6):453–458
Strilic B, Offermanns S (2017) Intravascular survival and extravasation of tumor cells. Cancer Cell 32(3):282–293
Chambers AF, Groom AC, MacDonald IC (2002) Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer 2(8):563–572
Weis SM, Cheresh DA (2011) Tumor angiogenesis: molecular pathways and therapeutic targets. Nat Med 17(11):1359–1370
Carmeliet P (2000) Mechanisms of angiogenesis and arteriogenesis. Nat Med 6(4):389–395
Dvorak HF (2000) VPF/VEGF and the angiogenic response. Semin Perinatol 24(1):75–78
Hempel N, Bartling TR, Mian B, Melendez JA (2013) Acquisition of the metastatic phenotype is accompanied by H2O2-dependent activation of the p130Cas signaling complex. Mol Cancer Res 11(3):303–312
Almalki SG, Agrawal DK (2016) Effects of matrix metalloproteinases on the fate of mesenchymal stem cells. Stem Cell Res Ther 7(1):1–12
Bolontrade MF, Sganga L, Piaggio E, Viale DL, Sorrentino MA, Robinson A, Sevlever G, García MG, Mazzolini G, Podhajcer OL (2012) A specific subpopulation of mesenchymal stromal cell carriers overrides melanoma resistance to an oncolytic adenovirus. Stem Cells Dev 21(14):2689–2702
Passaniti A, Taylor RM, Pili R, Guo Y, Long PV, Haney JA, Pauly RR, Grant DS, Martin GR (1992) A simple, quantitative method for assessing angiogenesis and antiangiogenic agents using reconstituted basement membrane, heparin, and fibroblast growth factor. Lab Invest 67(4):519–528
Morales-Arias J, Meyers PA, Bolontrade MF, Rodriguez N, Zhou Z, Reddy K, Chou AJ, Koshkina NV, Kleinerman ES (2007) Expression of granulocyte-colony-stimulating factor and its receptor in human Ewing sarcoma cells and patient tumor specimens: potential consequences of granulocyte-colony-stimulating factor administration. Cancer 110(7):1568–1577
Weidner N, Semple JP, Welch WR, Folkman J (1991) Tumor angiogenesis and metastasis—correlation in invasive breast carcinoma. N Engl J Med 324(1):1–8
Weidner N, Folkman J, Pozza F, Bevilacqua P, Allred EN, Moore DH, Meli S, Gasparini G (1992) Tumor angiogenesis: a new significant and independent prognostic indicator in early-stage breast carcinoma. J Natl Cancer Inst USA 84(24):1875–1887
Bolontrade MF, Stern MC, Binder RL, Zenklusen JC, Gimenez-Conti IB, Conti CJ (1998) Angiogenesis is an early event in the development of chemically induced skin tumors. Carcinogenesis 19(12):2107–2113
Vitale DL, Spinelli FM, Del Dago D, Icardi A, Demarchi G, Caon I, García M, Bolontrade MF, Passi A, Cristina C, Alaniz L (2018) Co-treatment of tumor cells with hyaluronan plus doxorubicin affects endothelial cell behavior independently of VEGF expression. Oncotarget 9(93):36585
Calvo N, Carriere P, Martín MJ, Gigola G, Gentili C (2019) PTHrP treatment of colon cancer cells promotes tumor associated-angiogenesis by the effect of VEGF. Mol Cell Endocrinol 483:50–63
Folkman J (2006) Angiogenesis. Annu Rev Med 57:1–18
Garcia MG, Alaniz L, Lopes EC, Blanco G, Hajos SE, Alvarez E (2005) Inhibition of NF-kappaB activity by BAY 11–7082 increases apoptosis in multidrug resistant leukemic T-cell lines. Leuk Res 29(12):1425–1434
Barreiro Arcos ML, Sterle HA, Vercelli C, Valli E, Cayrol MF, Klecha AJ, Paulazo MA, Diaz Flaqué MC, Franchi AM, Cremaschi GA (2013) Induction of apoptosis in T lymphoma cells by long-term treatment with thyroxine involves PKCzeta nitration by nitric oxide synthase. Apoptosis 18(11):1376–1390
Angulski AB, Capriglione LG, Batista M, Marcon BH, Senegaglia AC, Stimamiglio MA, Correa A (2017) The protein content of extracellular vesicles derived from expanded human umbilical cord blood-derived CD133+ and human bone marrow-derived mesenchymal stem cells partially explains why both sources are advantageous for regenerative medicine. Stem Cell Rev Rep 13(2):244–257
Cox J, Mann M (2008) MaxQuant enables high peptide identification rates, individualized ppb-range mass accuracies and proteome-wide protein quantification. Nat Biotechnol 26(12):1367–1372
Heldin CH, Westermark B (1999) Mechanism of action and in vivo role of platelet-derived growth factor. Physiol Rev 79(4):1283–1316
Byrne AM, Bouchier-Hayes DJ, Harmey JH (2005) Angiogenic and cell survival functions of vascular endothelial growth factor (VEGF). J Cell Mol Med 9(4):777–794
Menges CW, Altomare DA, Testa JR (2009) FAS-Associated Factor 1 (FAF1): diverse functions and implications for oncogenesis. Cell Cycle 8(16):2528–2534
Guerra B, Boldyreff B, Issinger OG (2001) FAS-associated factor 1 interacts with protein kinase CK2 in vivo upon apoptosis induction. Int J Oncol 19(6):1117–1126
Valenzuela Alvarez M, Gutiérrez LM, Auzmendi J, Correa A, Lazarowski A, Bolontrade MF (2020) Acquisition of stem associated-features on metastatic osteosarcoma cells and their functional effects on mesenchymal stem cells. Biochim Biophys Acta 1864(4):129522
Hanahan D, Coussens LM (2012) Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 21(3):309–322
Mohan V, Das A, Sagi I (2020) Emerging roles of ECM remodeling processes in cancer. Semin Cancer Biol 62:192–200
Aird WC (2007) Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circ Res 100(2):158–173
Ades EW, Candal FJ, Swerlick RA, George VG, Summers S, Bosse DC, Lawley TJ (1992) HMEC-1: establishment of an immortalized human microvascular endothelial cell line. J Invest Dermatol 99(6):683–690
Carter NM, Ali S, Kirby JA (2003) Endothelial inflammation: the role of differential expression of N-deacetylase/N-sulphotransferase enzymes in alteration of the immunological properties of heparan sulphate. J Cell Sci 116(Pt 17):3591–3600
Langley RR, Ramirez KM, Tsan RZ, Van Arsdall M, Nilsson MB, Fidler IJ (2003) Tissue-specific microvascular endothelial cell lines from H-2K(b)-tsA58 mice for studies of angiogenesis and metastasis. Cancer Res 63(11):2971–2976
Ghosh S, Joshi MB, Ivanov D, Feder-Mengus C, Spagnoli GC, Martin I, Erne P, Resink TJ (2007) Use of multicellular tumor spheroids to dissect endothelial cell-tumor cell interactions: a role for T-cadherin in tumor angiogenesis. FEBS Lett 581(23):4523–4528
Harmey JH, Bouchier-Hayes D (2002) Vascular endothelial growth factor (VEGF), a survival factor for tumour cells: implications for anti-angiogenic therapy. BioEssays 24(3):280–283
Chavakis E, Dimmeler S (2002) Regulation of endothelial cell survival and apoptosis during angiogenesis. Arterioscler Thromb Vasc Biol 22(6):887–893
Jia SF, Guan H, Duan X, Kleinerman ES (2008) VEGF165 is necessary to the metastatic potential of Fas− osteosarcoma cells but will not rescue the Fas+ cells. J Exp Ther Oncol 7(2):89–97
Pidgeon GP, Barr MP, Harmey JH, Foley DA, Bouchier-Hayes DJ (2001) Vascular endothelial growth factor (VEGF) upregulates BCL-2 and inhibits apoptosis in human and murine mammary adenocarcinoma cells. Br J Cancer 85(2):273–278
Zachary I, Gliki G (2001) Signaling transduction mechanisms mediating biological actions of the vascular endothelial growth factor family. Cardiovasc Res 49(3):568–581
Chen XL, Nam JO, Jean C, Lawson C, Walsh CT, Goka E, Lim ST, Tomar A, Tancioni I, Uryu S, Guan JL, Acevedo LM, Weis SM, Cheresh DA, Schlaepfer DD (2012) VEGF-induced vascular permeability is mediated by FAK. Dev Cell 22(1):146–157
Hoang BH, Dyke JP, Koutcher JA, Huvos AG, Mizobuchi H, Mazza BA, Gorlick R, Healey JH (2004) VEGF expression in osteosarcoma correlates with vascular permeability by dynamic MRI. Clin Orthop Relat Res 426:32–38
Weis SM, Cheresh DA (2005) Pathophysiological consequences of VEGF-induced vascular permeability. Nature 437(7058):497–504
Daft PG, Yang Y, Napierala D, Zayzafoon M (2015) The growth and aggressive behavior of human osteosarcoma is regulated by a CaMKII-controlled autocrine VEGF signaling mechanism. PLoS ONE 10(4):e0121568
Wang L, Zhang W, Ding Y, Xiu B, Li P, Dong Y, Zhu Q, Liang A (2015) Up-regulation of VEGF and its receptor in refractory leukemia cells. Int J Clin Exp Pathol 8(5):5282–5890
Papadopoulos N, Lennartsson J (2018) The PDGF/PDGFR pathway as a drug target. Mol Aspects Med 62:75–88
Peng F, Dhillon N, Callen S, Yao H, Bokhari S, Zhu X, Baydoun HH, Buch S (2008) Platelet-derived growth factor protects neurons against gp120-mediated toxicity. J Neurovirol 14(1):62–72
Yilmaz A, Kliche S, Mayr-Beyrle U, Fellbrich G, Waltenberger J (2003) p38 MAPK inhibition is critically involved in VEGFR-2-mediated endothelial cell survival. Biochem Biophys Res Commun 306(3):730–736
Eppihimer MJ, Russell J, Langley R, Vallien G, Anderson DC, Granger DN (1998) Differential expression of platelet-endothelial cell adhesion molecule-1 (PECAM-1) in murine tissues. Microcirculation 5(2–3):179–188
Mammoto A, Mammoto T (2019) Vascular niche in lung alveolar development, homeostasis, and regeneration. Front Bioeng Biotechnol 7:318
Pezzella F, Pastorino U, Tagliabue E, Andreola S, Sozzi G, Gasparini G, Menard S, Gatter KC, Harris AL, Fox S, Buyse M, Pilotti S, Pierotti M (1997) Non-small-cell lung carcinoma tumor growth without morphological evidence of neo-angiogenesis. Am J Pathol 151(5):1417–1423
Sardari Nia P, Hendriks J, Friedel G, Van Schil P, Van Marck E (2007) Distinct angiogenic and non-angiogenic growth patterns of lung metastases from renal cell carcinoma. Histopathology 51(3):354–361
Matei D, Kelich S, Cao L, Menning N, Emerson RE, Rao J, Jeng MH, Sledge GW (2007) PDGF BB induces VEGF secretion in ovarian cancer. Cancer Biol Ther 6(12):1951–1959
Langley RR, Fan D, Tsan RZ, Rebhun R, He J, Kim SJ, Fidler IJ (2004) Activation of the platelet-derived growth factor-receptor enhances survival of murine bone endothelial cells. Cancer Res 64(11):3727–3730
Lee C et al (2010) Reduced levels of IGF-I mediate differential protection of normal and cancer cells in response to fasting and improve chemotherapeutic index. Cancer Res 70(4):1564–1572
Knowles J, Loizidou M, Taylor I (2005) Endothelin-1 and angiogenesis in cancer. Curr Vasc Pharmacol 3(4):309–314
Kim SW, Choi HJ, Lee HJ, He J, Wu Q, Langley RR, Fidler IJ, Kim SJ (2014) Role of the endothelin axis in astrocyte- and endothelial cell-mediated chemoprotection of cancer cells. Neuro Oncol 16(12):1585–1598
Xie F et al (2017) FAF1 phosphorylation by AKT accumulates TGF-β type II receptor and drives breast cancer metastasis. Nat Commun 8:15021
Zhang L, Zhou F, van Laar T, Zhang J, van Dam H, Ten Dijke P (2011) Fas-associated factor 1 antagonizes Wnt signaling by promoting β-catenin degradation. Mol Biol Cell 22(9):1617–1624
LaBiche RA, Tresslert RJ, Nicolson GL (1993) Selection for enhanced adhesion to microvessel endothelial cells or resistance to interferon-γ modulates the metastatic potential of murine RAW117 large-cell lymphoma cells. Clin Exp Metastasis 11(6):472–481
Hansen AG, Arnold SA, Jiang M, Palmer TD, Ketova T, Merkel A, Pickup M, Samaras S, Shyr Y, Moses HL, Hayward SW (2014) ALCAM/CD166 is a TGF-β-responsive marker and functional regulator of prostate cancer metastasis to bone. Cancer Res 74(5):1404–1415
Soto MS, Serres S, Anthony DC, Sibson NR (2014) Functional role of endothelial adhesion molecules in the early stages of brain metastasis. Neuro Oncol 16(4):540–551
Degen WG, van Kempen LC, Gijzen EG, van Groningen JJ, van Kooyk Y, Bloemers HP, Swart GW (1998) MEMD, a new cell adhesion molecule in metastasizing human melanoma cell lines, is identical to ALCAM (activated leukocyte cell adhesion molecule). Am J Pathol 152(3):805–813
Acknowledgements
We thank the Program for Technological Development in Tools for Health-PDTIS-FIOCRUZ for the use of the mass spectrometry facility.
Funding
This work was supported by a grant from the Agencia Nacional de Promoción Científica y tecnológica (ANPCyT) PICT N°1974.
Author information
Authors and Affiliations
Contributions
LMG and MVA performed experiments, analyzed data and designed figures; FS, MJC, MGG and YY performed experiments; AC conducted proteomic analysis and analyzed data; ESK, MGG and LA contributed with essential reagents and analyzed data; ESK provided OS cells. ESK and AC contributed with paper revision; MB conceived research and experiments and analyzed data. Authors discussed and commented the results on the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
Authors declare that no competing financial interests or conflicts of interest exist.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.

10495_2021_1677_MOESM1_ESM.jpg
Supplementary file1 (JPG 62 kb) Supplementary Figure 1. Kaplan–Meier metastasis-free survival curves using the GSE42352 dataset. Curves represent high (blue) and low (red) gene expression in pretreatment high grade OS biopsy samples. A) PDGFA Kaplan-Meier metastasis-free survival, higher expression is worse (p 0.085). B) PDGFB Kaplan–Meier metastasis-free survival, higher expression is worse (p 0.046). C) PDGFC Kaplan–Meier metastasis-free survival, higher expression is worse (p 0.061). D) PDGFD Kaplan–Meier metastasis-free survival, higher expression is worse (p =0.009). E) FAF1Kaplan–Meier metastasis-free survival, higher expression is worse (p =0.056). F) VEGF Kaplan–Meier metastasis-free survival, higher expression is worse (p 0.028).
10495_2021_1677_MOESM2_ESM.docx
Supplementary file2 (DOCX 16 kb) Supplementary Table 1. LFQ intensity fold change for GO for biological pathway and biological processes terms in CM and cells. Analyses considering the relative abundance of the proteins (LFQ normalized intensities) were carried out using Funrich software. LFQ: label free quantification; GO: gene ontology; CM: conditioned medium; CM-LM7: conditioned medium from LM7 OS cells; CM-SAOS: conditioned medium from SAOS2 OS cells.
10495_2021_1677_MOESM3_ESM.docx
Supplementary file3 (DOCX 23 kb) Supplementary Table 2. OS cells apoptosis- related proteins at the intracellular compartment, classified by their pro- or antiapoptotic effect. Proteins are expressed as relative intensity values (normalized LFQ). FAF: Fas associated factor; BAG: BCL-2 associated athanogene; BAX: BCL-2 associated X protein; BCL2L13: BCL-2 like 13; BCLAF1: BCL-2 associated transcription factor 1; API5: Apoptosis inhibitor 5; AATF: Apoptosis antagonizing transcription factor; AIFM1: Apoptosis inducing factor mitochondrial 1; CASP3: caspase 3
Rights and permissions
About this article
Cite this article
Gutiérrez, L.M., Valenzuela Alvarez, M., Yang, Y. et al. Up-regulation of pro-angiogenic molecules and events does not relate with an angiogenic switch in metastatic osteosarcoma cells but to cell survival features. Apoptosis 26, 447–459 (2021). https://doi.org/10.1007/s10495-021-01677-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-021-01677-x