Abstract
Background
Osteosarcoma (OS) stands out as the most common bone tumor, with approximately 20% of the patients receiving a diagnosis of metastatic OS at their initial assessment. A significant challenge lies in the frequent existence of undetected metastases during the initial diagnosis. Mesenchymal stem cells (MSCs) possess unique abilities that facilitate tumor growth, and their interaction with OS cells is crucial for metastatic spread.
Methods and results
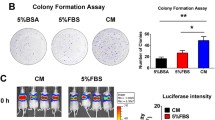
We demonstrated that, in vitro, MSCs exhibited a heightened migration response toward the secretome of non-metastatic OS cells. When challenged to a secretome derived from lungs preloaded with OS cells, MSCs exhibited greater migration toward lungs colonized with metastatic OS cells. Moreover, in vivo, MSCs displayed preferential migratory and homing behavior toward lungs colonized by metastatic OS cells. Metastatic OS cells, in turn, demonstrated an increased migratory response to the MSCs’ secretome. This behavior was associated with heightened cathepsin D (CTSD) expression and the release of active metalloproteinase 2 (MMP2) by metastatic OS cells.
Conclusions
Our assessment focused on two complementary tumor capabilities crucial to metastatic spread, emphasizing the significance of inherent cell features. The findings underscore the pivotal role of signaling integration within the niche, with a complex interplay of migratory responses among established OS cells in the lungs, prometastatic OS cells in the primary tumor, and circulating MSCs. Pulmonary metastases continue to be a significant factor contributing to OS mortality. Understanding these mechanisms and identifying differentially expressed genes is essential for pinpointing markers and targets to manage metastatic spread and improve outcomes for patients with OS.





Similar content being viewed by others
Data availability
All relevant data supporting the findings of this study are available within the paper and it’s Supporting Information files.
Abbreviations
- BM:
-
Bone marrow
- CCL2:
-
C–C motif chemokine ligand 2
- CCR2:
-
C–C motif chemokine receptor 2
- CM:
-
Conditioned medium
- CTSA:
-
Cathepsin A
- CTSD:
-
Cathepsin D
- CTSs:
-
Cathepsins
- CXCR4:
-
C–X–C motif chemokine receptor 4
- CXCL12:
-
C–X–C motif chemokine ligand 12
- DAPI:
-
4′,6-Diamidino-2-phenylindole dihydrochloride
- DiR:
-
1,1′-Dioctadecyl-3,3,3′,3′-tetramethylindotricarbocyanine iodide
- DMEM:
-
Dulbecco’s modified eagle’s medium
- DMEM/F12:
-
Dulbecco’s modified eagle’s medium/nutrient mixture F12
- ECM:
-
Extracellular matrix
- FBS:
-
Fetal bovine serum
- FI:
-
Fluorescence intensity
- GAPDH:
-
Glyceraldehyde-3-phosphate dehydrogenase
- GEO:
-
Gene expression omnibus
- HMEC-1:
-
Human microvascular endothelial cells
- IACUC:
-
Institutional Animal Care and Use Committee
- IL-1:
-
Interleukin 1 alpha
- i.v.:
-
Intravenously
- MMPs:
-
Metalloproteinases
- MMP2:
-
Metalloproteinase 2
- MMP9:
-
Metalloproteinase 9
- MSCs:
-
Mesenchymal stem/stromal cells
- NIH:
-
National Institutes of Health
- qPCR:
-
Real time polymerase chain reaction
- PBS:
-
Phosphate buffer solution
- PECAM:
-
Platelet–endothelial cell adhesion molecule 1
- OS:
-
Osteosarcoma
- SDS-PAGE:
-
Sodium dodecyl sulfate polyacrylamide gel electrophoresis
References
Hanahan D (2022) Hallmarks of cancer: new dimensions. Cancer Discov 12(1):31–46. https://doi.org/10.1158/2159-8290.CD-21-1059
Lazebnik Y (2010) What are the hallmarks of cancer? Nat Rev Cancer 10(4):232–233. https://doi.org/10.1038/nrc2827
Welch DR, Hurst DR (2019) Defining the hallmarks of metastasis. Cancer Res 79(12):3011–3027. https://doi.org/10.1158/0008-5472.CAN-19-0458
Vidak E, Javoršek U, Vizovišek M, Turk B (2019) Cysteine cathepsins and their extracellular roles: shaping the microenvironment. Cells 8(3):264. https://doi.org/10.3390/cells8030264
Niland S, Riscanevo AX, Eble JA (2022) Matrix metalloproteinases shape the tumor microenvironment in cancer progression. Int J Mol Sci 23:146. https://doi.org/10.3390/ijms23010146
Khan SU, Fatima K, Malik F, Kalkavan H, Wani A (2023) Cancer metastasis: molecular mechanisms and clinical perspectives. Pharmacol Ther 250:108522. https://doi.org/10.1016/j.pharmthera.2023.108522
Tulotta C, Stefanescu C, Chen Q, Torraca V, Meijer H, Snaar-Jagalska B (2019) CXCR4 signaling regulates metastatic onset by controlling neutrophil motility and response to malignant cells. Sci Rep 9(1):2399. https://doi.org/10.1038/s41598-019-38643-2
Xu M, Wang Y, Xia R, Wei Y, Wei X (2021) Role of the CCL2–CCR2 signalling axis in cancer: mechanisms and therapeutic targeting. Cell Prolif 54(10):e13115. https://doi.org/10.1111/cpr.13115
Lambert AW, Pattabiraman DR, Weinberg RA (2017) Emerging biological principles of metastasis. Cell 168(4):670–691. https://doi.org/10.1016/j.cell.2016.11.037
Marko TA, Diessner BJ, Spector LG (2016) Prevalence of metastasis at diagnosis of osteosarcoma: an international comparison. Pediatr Blood Cancer 63(6):1006–1011. https://doi.org/10.1002/pbc.25963
Sheng G, Gao Y, Yang Y, Wu H (2021) Osteosarcoma and metastasis. Front Oncol 11:780264. https://doi.org/10.3389/fonc.2021.780264
Strauss SJ, Ng T, Mendoza-Naranjo A, Whelan J, Sorensen PH (2010) Understanding micrometastatic disease and anoikis resistance in Ewing family of tumors and osteosarcoma. Oncologist 15(6):627–635. https://doi.org/10.1634/theoncologist.2010-0093
Bruland OS, Høifødt H, Saeter G, Smeland S, Fodstad O (2005) Hematogenous micrometastases in osteosarcoma patients. Clin Cancer Res 11(13):4666–73. https://doi.org/10.1158/1078-0432.CCR-05-0165
Odri GA, Tchicaya-Bouanga J, Yoon DJY, Modrowski D (2022) Metastatic progression of osteosarcomas: a review of current knowledge of environmental versus oncogenic drivers. Cancers 14:360. https://doi.org/10.3390/cancers14020360
Mao X, Mei R, Yu S, Shou L, Zhang W, Li K, Qiu Z, Xie T, Sui X (2022) Emerging technologies for the detection of cancer micrometastasis. Technol Cancer Res Treat 21:1–11. https://doi.org/10.1177/15330338221100355
Valenzuela Alvarez M, Gutierrez LM, Correa A, Lazarowski A, Bolontrade MF (2019) Metastatic niches and the modulatory contribution of mesenchymal stem cells and its exosomes. Int J Mol Sci 20(8):1946. https://doi.org/10.3390/ijms20081946
Frisbie L, Buckanovich RJ, Coffman L (2022) Carcinoma-associated mesenchymal stem/stromal cells: architects of the pro-tumorigenic tumor microenvironment. Stem Cells 40(8):705–715. https://doi.org/10.1093/stmcls/sxac036
Valenzuela Alvarez M, Gutiérrez LM, Auzmendi J, Correa A, Lazarowski A, Bolontrade MF (2020) Acquisition of stem associated-features on metastatic osteosarcoma cells and their functional effects on mesenchymal stem cells. Biochim Biophys Acta Gen Subj. https://doi.org/10.1016/j.bbagen.2020.129522
Gutiérrez LM, Valenzuela Alvarez M, Yang Y, Spinelli F, Cantero MJ, Alaniz L, García MG, Kleinerman ES, Correa A, Bolontrade MF (2021) Up-regulation of pro-angiogenic molecules and events do not relate with an angiogenic switch in metastatic osteosarcoma cells but to cell survival features. Apoptosis 26(7–8):447–459. https://doi.org/10.1007/s10495-021-01677-x.20
Xu M, Zhang T, Xia R, Wei Y, Wei X (2022) Targeting the tumor stroma for cancer therapy. Mol Cancer 21:208. https://doi.org/10.1186/s12943-022-01670-1
Dzobo K, Dandara C (2020) Architecture of cancer-associated fibroblasts in tumor microenvironment: mapping their origins, heterogeneity, and role in cancer therapy resistance. OMICS 24(6):314–339. https://doi.org/10.1089/omi.2020.0023
Bolontrade MF, Sganga L, Piaggio E, Viale DL, Sorrentino MA, Robinson A, Sevlever G, García MG, Mazzolini G, Podhajcer OL (2012) A specific subpopulation of mesenchymal stromal cell carriers overrides melanoma resistance to an oncolytic adenovirus. Stem Cells Dev 21:2689–702. https://doi.org/10.1089/scd.2011.0643
Sterle HA, Hildebrandt X, Valenzuela Álvarez M, Paulazo MA, Gutierrez LM, Klecha AJ, Cayrol F, Díaz Flaqué MC, Rosemblit C, Barreiro Arcos ML et al (2021) Thyroid status regulates the tumor microenvironment delineating breast cancer fate. Endocr Relat Cancer 28(7):403–418. https://doi.org/10.1530/ERC-20-0277
Jia S, Worth LL, Kleinerman ES (1999) A nude mouse model of human osteosarcoma lung metastases for evaluating new therapeutic strategies. Clin Exp Metastasis 17:501–506. https://doi.org/10.1023/a:1006623001465
Fritsche-Guenther R, Noske A, Ungethüm U, Kuban RJ, Schlag PM, Tunn PU, Karle J, Krenn V, Dietel M, Sers C (2010) De novo expression of EphA2 in osteosarcoma modulates activation of the mitogenic signalling pathway. Histopathology 57(6):836–850. https://doi.org/10.1111/j.1365-2559.2010.03713.x
Kuijjer ML, Rydbeck H, Kresse SH, Buddingh EP, Lid AB, Roelofs H, Bürger H, Myklebost O, Hogendoorn PC, Meza-Zepeda LA et al (2012) Identification of osteosarcoma driver genes by integrative analysis of copy number and gene expression data. Genes Chromosomes Cancer 51(7):696–706. https://doi.org/10.1002/gcc.21956
Bielack SS, Kempf-Bielack B, Delling G, Ulrich Exner G, Flege S, Helmke K, Kotz R, Salzer-Kuntschik M, Werner M, Winkelmann W et al (2002) Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol 20(3):776–790. https://doi.org/10.1200/JCO.2002.20.3.776. Corrected and republished in: J Clin Oncol 2023 Sep 20;41(27):4323–4337
Smeland S, Bielack SS, Whelan J, Bernstein M, Hogendoorn P, Krailo MD, Gorlick R, Janeway KA, Ingleby FC, Anninga J et al (2019) Survival and prognosis with osteosarcoma: outcomes in more than 2000 patients in the EURAMOS-1 (European and American Osteosarcoma Study) cohort. Eur J Cancer 109:36–50. https://doi.org/10.1016/j.ejca.2018.11.027
Reeves MQ, Balmain A (2023) Mutations, bottlenecks, and clonal sweeps: how environmental carcinogens and genomic changes shape clonal evolution during tumor progression. Cold Spring Harb Perspect Med. https://doi.org/10.1101/cshperspect.a041388
Caligiuri G, Tuveson DA (2023) Activated fibroblasts in cancer: perspectives and challenges. Cancer Cell 41(3):434–449. https://doi.org/10.1016/j.ccell.2023.02.015
Sharma P, Alsharif S, Fallatah A, Chung BM (2019) Intermediate filaments as effectors of cancer development and metastasis: a focus on keratins, vimentin, and nestin. Cells 8(5):497. https://doi.org/10.3390/cells8050497
Sznurkowska MK, Aceto N (2022) The gate to metastasis: key players in cancer cell intravasation. FEBS J 289(15):4336–4354. https://doi.org/10.1111/febs.16046
Ali N, Venkateswaran G, Garcia E, Landry T, McColl H, Sergi C, Persad A, Abuetabh Y, Eisenstat DD, Persad S (2019) Osteosarcoma progression is associated with increased nuclear levels and transcriptional activity of activated β-catenin. Genes Cancer 10(3–4):63–79. https://doi.org/10.18632/genesandcancer.191
Zhang C, Zhang M, Song S (2018) Cathepsin D enhances breast cancer invasion and metastasis through promoting hepsin ubiquitin-proteasome degradation. Cancer Lett 438:105–15. https://doi.org/10.1016/j.canlet.2018.09.021
Gemoll T, Epping F, Heinrich L, Fritzsche B, Roblick UJ, Szymczak S, Hartwig S, Depping R, Bruch HP, Thorns C et al (2015) Increased cathepsin D protein expression is a biomarker for osteosarcomas, pulmonary metastases and other bone malignancies. Oncotarget 6(18):16517–16526. https://doi.org/10.18632/oncotarget.4140
Mourão TC, Bezerra SM, de Almeida E, Paula F, Rocha MM, Santos VE, Brazão Junior ES, Abreu D, da Costa WH, Zequi SC (2023) Prognostic role of the immunohistochemical expression of proteins related to the renin-angiotensin system pathway in nonmetastatic clear cell renal cell carcinoma. Urol Oncol 41(8):359.e1-359.e13
Seo SU, Woo SM, Im SS, Jang Y, Han E, Kim SH, Lee H, Lee HS, Nam JO, Gabrielson E et al (2022) Cathepsin D as a potential therapeutic target to enhance anticancer drug-induced apoptosis via RNF183-mediated destabilization of Bcl-xL in cancer cells. Cell Death Dis 13(2):115. https://doi.org/10.1038/s41419-022-04581-7
Yip HYK, Papa A (2021) Signaling pathways in cancer: therapeutic targets, combinatorial treatments, and new developments. Cells 10(3):659. https://doi.org/10.3390/cells10030659
Langley RR, Fidler IJ (2011) The seed and soil hypothesis revisited-the role of tumor–stroma interactions in metastasis to different organs. Int J Cancer 128(11):2527–35. https://doi.org/10.1002/ijc.26031
Gao Y, Bado I, Wang H, Zhang W, Rosen JM, Zhang XH (2019) Metastasis organotropism: redefining the congenial soil. Dev Cell 49(3):375–391. https://doi.org/10.1016/j.devcel.2019.04.012
Risson E, Nobre AR, Maguer-Satta V, Aguirre-Ghiso JA (2020) The current paradigm and challenges ahead for the dormancy of disseminated tumour cells. Nat Cancer 1(7):672–680. https://doi.org/10.1038/s43018-020-0088-5
Fidler IJ (2016) Commentary on “Tumor heterogeneity and the biology of cancer invasion and metastasis.” Cancer Res 76(12):3441–2. https://doi.org/10.1158/0008-5472.CAN-16-1330
Bolamperti S, Villa I, Rubinacci A (2022) Bone remodeling: an operational process ensuring survival and bone mechanical competence. Bone Res 10:48. https://doi.org/10.1038/s41413-022-00219-8
Zhou T, Yang Y, Chen Q, Xie L (2019) Glutamine metabolism is essential for stemness of bone marrow mesenchymal stem cells and bone homeostasis. Stem Cells Int 2019:8928934. https://doi.org/10.1155/2019/8928934
Yamada T, Fukasawa K, Horie T, Kadota T, Lyu J, Tokumura K, Ochiai S, Iwahashi S, Suzuki A, Park G et al (2022) The role of CDK8 in mesenchymal stem cells in controlling osteoclastogenesis and bone homeostasis. Stem Cell Rep 17(7):1576–1588. https://doi.org/10.1016/j.stemcr.2022.06.001
Bussard KM, Spaeth E, Mutkus LA, Stumpf KA, Marini FC (2017) Mesenchymal stem cell transition to tumor-associated stromal cells contributes to cancer progression. In: Bolontrade MF, Garcia MG (eds) Mesenchymal stromal cells as tumor stromal modulators. Elsevier, Amsterdam, pp 253–73
Funding
This work was supported in part by the Fund for Scientific and Technological Research (FONCyT) PICT No. 1974 and by Florencio Fiorini Foundation.
Author information
Authors and Affiliations
Contributions
MVA and LMG performed experiments, analyzed data and designed figures. MVA also contributed to the writing of the manuscript and conceived experiments. MJC, MGG, and JB performed experiments; MGG contributed with reagents and analyzed data. MB conceived research and experiments, analyzed data and wrote manuscript. Authors discussed and commented the results on the manuscript.
Corresponding author
Ethics declarations
Competing interests
All authors declare no competing financial interests.
Research involving animals right
The animal handling procedures in this study were conducted in accordance with the guidelines and regulations established by the Institutional Animal Care and Use Committee (IACUC) under the protocol number IACUC IMTIB 0001/19. Furthermore, all procedures complied with relevant national and international standards for the ethical use of animals in scientific research. Study design and execution followed the principles outlined in the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Valenzuela Alvarez, M.J.P., Gutierrez, L.M., Bayo, J.M. et al. Osteosarcoma cells exhibit functional interactions with stromal cells, fostering a lung microenvironment conducive to the establishment of metastatic tumor cells. Mol Biol Rep 51, 467 (2024). https://doi.org/10.1007/s11033-024-09315-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11033-024-09315-w