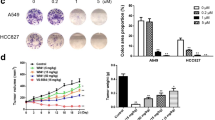
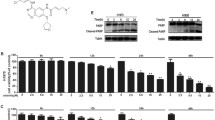
Abstract
Paclitaxel is one of the most commonly used drugs for the treatment of nonsmall cell lung cancer (NSCLC). However acquired resistance to paclitaxel, epithelial to mesenchymal transition and cancer stem cell formation are the major obstacles for successful chemotherapy with this drug. Some of the major reasons behind chemoresistance development include increased ability of the cancer cells to survive under stress conditions by autophagy, increased expression of drug efflux pumps, tubulin mutations etc. In this study we found that inhibition of autophagy with chloroquine prevented development of paclitaxel resistance in A549 cells with time and potentiated the effect of paclitaxel by increased accumulation of superoxide-producing damaged mitochondria, with elevated ROS generation, it also increased the apoptotic rate and sub G0/ G1 phase arrest with time in A549 cells treated with paclitaxel and attenuated the metastatic potential and cancer stem cell population of the paclitaxel-resistant cells by ROS mediated modulation of the Wnt/β-catenin signaling pathway, thereby increasing paclitaxel sensitivity. ROS here played a crucial role in modulating Akt activity when autophagy process was hindered by chloroquine, excessive ROS accumulation in the cell inhibited Akt activity. In addition, chloroquine pre-treatment followed by taxol (10 nM) treatment did not show significant toxicity towards non-carcinomas WI38 cells (lung fibroblast cells). Thus autophagy inhibition by CQ pre-treatment can be used as a fruitful strategy to combat the phenomenon of paclitaxel resistance development as well as metastasis in lung cancer.











Similar content being viewed by others
Change history
28 March 2019
The original version of this article unfortunately contained an error in acknowledgment text. The authors would like to include a statement: “Moumita Dasgupta is supported by Junior Research Fellowship from University Grant Commission, India.” in acknowledgment section.
Abbreviations
- NSCLC:
-
Non small cell lung cancer
- ROS:
-
Reactive oxygen species
- Tx:
-
Paclitaxel/taxol
- MDR1:
-
Multidrug resistant protein 1
- MRP1:
-
Multidrug resistance-associated protein 1
- Pgp:
-
P-glycoprotein
- EMT:
-
Epithelial to mesenchymal transition
- ROS:
-
Reactive oxygen species
- CQ:
-
Chloroquine
- DMEM:
-
Dulbecco’s modified eagle’s Media
- DMSO:
-
Dimethyl sulfoxide
- MDC:
-
Monodancyl cadaverine
- MSR:
-
Mitosox red
- OCR:
-
Oxygen consumption rate
- ECAR:
-
Extracellular acidification rate
- PE:
-
Phycoerythrin
- CSC:
-
Cancer stem cell
- NAC:
-
N-acetyl-cysteine
References
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A (2015) Global cancer statistics, 2012. CA Cancer J Clin 65:87–108
McGuire A, Martin M, Lenz C, Sollano JA (2015) Treatment cost of non-small cell lung cancer in three European countries: comparisons across France, Germany and England using administrative databases. J Med Econ 18(7):525–532
Rowinsky EK, Donehower RC (1995) Paclitaxel (taxol). N Engl J Med 332:1004–1014
Bharadwaj R, Yu H (2004) The spindle checkpoint, aneuploidy, and cancer. Oncogene 23:2016–2027
Brito DA, Yang Z, Rieder CL (2008) Microtubules do not promote mitotic slippage when the spindle assembly checkpoint cannot be satisfied. J Cell Biol 182:623–629
Gottesman MM, Pastan I (1993) Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu Rev Biochem 62:385–427
Gottesman MM, Fojo T, Bates SE (2002) Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer 2:48–58
Kavallaris M, Kuo DY, Burkhart CA et al (1997) Taxol-resistant epithelial ovarian tumors are associated with altered expression of specific beta-tubulin isotypes. J Clin Investig 100:1282–1293
Singh A, Settleman J (2010) EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene 29:4741–4751
Kriegenburg F, Ungermann C, Reggiori F (2018) Coordination of autophagosome-lysosome fusion by Atg8 family members. Curr Biol 28:R512–R518
Mizushima N, Komatsu M (2011) Autophagy: renovation of cells and tissues. Cell 147:728–741
Eskelinen EL (2011) The dual role of autophagy in cancer. Curr Opin Pharmacol 11:294–300
Sui X, Chen R, Wang Z et al (2013) Autophagy and chemotherapy resistance: a promising therapeutic target for cancer treatment. Cell Death Dis 4:e838
Yoon JH, Ahn SG, Lee BH, Jung SH, Oh SH (2012) Role of autophagy in chemoresistance: regulation of the ATM-mediated DNA-damage signaling pathway through activation of DNA-PKcs and PARP-1. Biochem Pharmacol 83:747–757
Zhou Y, Sun K, Ma Y et al (2014) Autophagy inhibits chemotherapy-induced apoptosis through downregulating Bad and Bim in hepatocellular carcinoma cells. Sci Rep 4:5382
Datta S, Choudhury D, Das A et al (2017) Paclitaxel resistance development is associated with biphasic changes in reactive oxygen species, mitochondrial membrane potential and autophagy with elevated energy production capacity in lung cancer cells: a chronological study. Tumour Biol 39:1010428317694314
Li J, Hou N, Faried A, Tsutsumi S, Takeuchi T, Kuwano H (2009) Inhibition of autophagy by 3-MA enhances the effect of 5-FU-induced apoptosis in colon cancer cells. Ann Surg Oncol 16:761–771
Sasaki K, Tsuno NH, Sunami E et al (2010) Chloroquine potentiates the anti-cancer effect of 5-fluorouracil on colon cancer cells. BMC Cancer 10:370
Yang YP, Hu LF, Zheng HF et al (2013) Application and interpretation of current autophagy inhibitors and activators. Acta pharmacol Sin 34:625–635
Shingu T, Fujiwara K, Bogler O et al (2009) Inhibition of autophagy at a late stage enhances imatinib-induced cytotoxicity in human malignant glioma cells. Int J Cancer 124:1060–1071
Kanzawa T, Germano IM, Komata T, Ito H, Kondo Y, Kondo S (2004) Role of autophagy in temozolomide-induced cytotoxicity for malignant glioma cells. Cell Death Differ 11:448–457
Solomon VR, Lee H (2009) Chloroquine and its analogs: a new promise of an old drug for effective and safe cancer therapies. Eur J Pharmacol 625:220–233
Ganguli A, Choudhury D, Datta S, Bhattacharya S, Chakrabarti G (2014) Inhibition of autophagy by chloroquine potentiates synergistically anti-cancer property of artemisinin by promoting ROS dependent apoptosis. Biochimie 107:338–349
Zheng Y, Zhao YL, Deng X et al (2009) Chloroquine inhibits colon cancer cell growth in vitro and tumor growth in vivo via induction of apoptosis. Cancer Investig 27:286–292
Fan C, Wang W, Zhao B, Zhang S, Miao J (2006) Chloroquine inhibits cell growth and induces cell death in A549 lung cancer cells. Bioorgan Med Chem 14:3218–3222
Qu X, Sheng J, Shen L et al (2017) Autophagy inhibitor chloroquine increases sensitivity to cisplatin in QBC939 cholangiocarcinoma cells by mitochondrial ROS. PloS ONE 12:e0173712
Wang Y, Peng RQ, Li DD et al (2011) Chloroquine enhances the cytotoxicity of topotecan by inhibiting autophagy in lung cancer cells. Chin J Cancer 30:690–700
Yang J, Zhang K, Wu J et al (2016) Wnt5a increases properties of lung cancer stem cells and resistance to Cisplatin through activation of Wnt5a/PKC signaling pathway. Stem Cells Int 2016:1690896
Deitrick J, Pruitt WM (2016) Wnt/beta Catenin-mediated signaling commonly altered in colorectal cancer. Prog Mol Biol Trans Sci 144:49–68
MacDonald BT, Tamai K, He X (2009) Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell 17:9–26
Anderson EC, Wong MH (2010) Caught in the Akt: regulation of Wnt signaling in the intestine. Gastroenterology 139:718–722
Hart JR, Vogt PK (2011) Phosphorylation of AKT: a mutational analysis. Oncotarget 2:467–476
Cao J, Xu D, Wang D et al (2009) ROS-driven Akt dephosphorylation at Ser-473 is involved in 4-HPR-mediated apoptosis in NB4 cells. Free Radic Biol Med 47:536–547
Juan O, Albert A, Ordono F et al (2002) Low-dose weekly paclitaxel as second-line treatment for advanced non-small cell lung cancer: a phase II study. Jpn J Clin Oncol 32:449–454
Strober W (2001) Trypan blue exclusion test of cell viability. Curr Protoc Immunol. https://doi.org/10.1002/0471142735.ima03bs21 (Appendix 3:Appendix 3B)
Choudhury D, Ganguli A, Dastidar DG, Acharya BR, Das A, Chakrabarti G (2013) Apigenin shows synergistic anticancer activity with curcumin by binding at different sites of tubulin. Biochimie 95:1297–1309
Choudhury D, Das A, Bhattacharya A, Chakrabarti G (2010) Aqueous extract of ginger shows antiproliferative activity through disruption of microtubule network of cancer cells. Food Chem Toxicol 48:2872–2880
Acharya BR, Bhattacharyya S, Choudhury D, Chakrabarti G (2011) The microtubule depolymerizing agent naphthazarin induces both apoptosis and autophagy in A549 lung cancer cells. Apoptosis 16:924–939
Das A, Chakrabarty S, Choudhury D, Chakrabarti G (2010) 1,4-Benzoquinone (PBQ) induced toxicity in lung epithelial cells is mediated by the disruption of the microtubule network and activation of caspase-3. Chem Res Toxicol 23:1054–1066
Xia XY, Wu YM, Hou BS et al (2008) Evaluation of sperm mitochondrial membrane potential by JC-1 fluorescent staining and flow cytometry. Zhonghua nan ke xue 14:135–138
Biederbick A, Kern HF, Elsasser HP (1995) Monodansylcadaverine (MDC) is a specific in vivo marker for autophagic vacuoles. Eur J Cell Biol 66:3–14
Jiang RD, Shen H, Piao YJ (2010) The morphometrical analysis on the ultrastructure of A549 cells. Rom J Morphol Embryol 51:663–667
Das Mukherjee D, Kumar NM, Tantak MP et al (2016) Development of novel Bis(indolyl)-hydrazide-hydrazone derivatives as potent microtubule-targeting cytotoxic agents against A549 lung cancer Cells. Biochemistry 55:3020–3035
Yin JX, Yang RF, Li S et al (2010) Mitochondria-produced superoxide mediates angiotensin II-induced inhibition of neuronal potassium current. Am J Physiol Cell Physiol 298:C857–C865
Zimmerman MC, Oberley LW, Flanagan SW (2007) Mutant SOD1-induced neuronal toxicity is mediated by increased mitochondrial superoxide levels. J Neurochem 102:609–618
Dai T, Hu Y, Zheng H (2017) Hypoxia increases expression of CXC chemokine receptor 4 via activation of PI3K/Akt leading to enhanced migration of endothelial progenitor cells. Eur Rev Med Pharmacol Sci 21:1820–1827
Bertolini G, Roz L, Perego P et al (2009) Highly tumorigenic lung cancer CD133 + cells display stem-like features and are spared by cisplatin treatment. Proc Natl Acad Sci USA 106:16281–16286
Wang P, Gao Q, Suo Z et al (2013) Identification and characterization of cells with cancer stem cell properties in human primary lung cancer cell lines. PloS ONE 8:e57020
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Green K, Brand MD, Murphy MP (2004) Prevention of mitochondrial oxidative damage as a therapeutic strategy in diabetes. Diabetes 53(Suppl 1):S110–S118
Kirkinezos IG, Moraes CT (2001) Reactive oxygen species and mitochondrial diseases. Semin Cell Dev Biol 12:449–457
Verheyen EM, Gottardi CJ (2010) Regulation of Wnt/beta-catenin signaling by protein kinases. Dev Dyn 239:34–44
Hu Y, Qiao L, Wang S et al (2000) 3-(Hydroxymethyl)-bearing phosphatidylinositol ether lipid analogues and carbonate surrogates block PI3-K, Akt, and cancer cell growth. J Med Chem 43:3045–3051
Liou GY, Storz P (2010) Reactive oxygen species in cancer. Free Radic Res 44:479–496
Yusuf RZ, Duan Z, Lamendola DE, Penson RT, Seiden MV (2003) Paclitaxel resistance: molecular mechanisms and pharmacologic manipulation. Curr Cancer Drug Targets 3:1–19
Fan QW, Cheng C, Hackett C et al (2010) Akt and autophagy cooperate to promote survival of drug-resistant glioma. Sci Signal 3:ra81
Liang X, Tang J, Liang Y, Jin R, Cai X (2014) Suppression of autophagy by chloroquine sensitizes 5-fluorouracil-mediated cell death in gallbladder carcinoma cells. Cell Biosci 4:10
Verschooten L, Barrette K, Van Kelst S et al (2012) Autophagy inhibitor chloroquine enhanced the cell death inducing effect of the flavonoid luteolin in metastatic squamous cell carcinoma cells. PLoS ONE 7:e48264
Peng X, Gong F, Chen Y et al (2014) Autophagy promotes paclitaxel resistance of cervical cancer cells: involvement of Warburg effect activated hypoxia-induced factor 1-alpha-mediated signaling. Cell Death Dis 5:e1367
Korolchuk VI, Mansilla A, Menzies FM, Rubinsztein DC (2009) Autophagy inhibition compromises degradation of ubiquitin-proteasome pathway substrates. Mol Cell 33:517–527
Suski JM, Lebiedzinska M, Bonora M, Pinton P, Duszynski J, Wieckowski MR (2012) Relation between mitochondrial membrane potential and ROS formation. Methods Mol Biol 810:183–205
Andre N, Braguer D, Brasseur G et al (2000) Paclitaxel induces release of cytochrome c from mitochondria isolated from human neuroblastoma cells’. Cancer Res 60:5349–5353
Redmann M, Benavides GA, Berryhill TF et al (2017) Inhibition of autophagy with bafilomycin and chloroquine decreases mitochondrial quality and bioenergetic function in primary neurons. Redox Biol 11:73–81
Mullarky E, Cantley LC (2015) Diverting Glycolysis to Combat Oxidative Stress. In: Nakao K, Minato N, Uemoto S (eds) Innovative Medicine, Basic Research and Development, Tokyo, pp 3–23
Wang J, Wei Q, Wang X et al (2016) Transition to resistance: an unexpected role of the EMT in cancer chemoresistance. Genes Dis 3:3–6
Leslie NR, Bennett D, Lindsay YE, Stewart H, Gray A, Downes CP (2003) Redox regulation of PI 3-kinase signalling via inactivation of PTEN. EMBO J 22:5501–5510
Liu C, Gong K, Mao X, Li W (2011) Tetrandrine induces apoptosis by activating reactive oxygen species and repressing Akt activity in human hepatocellular carcinoma. Int J Cancer 129:1519–1531
Acknowledgements
The authors wish to thank Dr. Sib Sankar Roy, CSIR – Indian Institute of Chemical Biology, Kolkata, India for helping the use of Seahorse XFe24 Extracellular Flux Analyser facility. The work was supported by grants from Department of Science and Technology, Govt. of India (No. SR/SO/BB-14/2008) and Department of Biotechnology, Government of India (No. BT/ PR12889/AGR/36/624/2009) to G.Chakbarati. FACS and fluorescence microscope instruments facility were developed by grants from National Common Minimum Project, Government. of India. Confocal microscope instrument facility was developed by grants from DBT-IPLS facility. S Datta and D Das Mukherjee were supported by Senior Research fellowship from Council of Scientific and industrial research (CSIR), Government. of India. S. Bandopadhyay is supported by Junior research fellowship from Council of Scientific and industrial research (CSIR), India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflicts of interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Datta, S., Choudhury, D., Das, A. et al. Autophagy inhibition with chloroquine reverts paclitaxel resistance and attenuates metastatic potential in human nonsmall lung adenocarcinoma A549 cells via ROS mediated modulation of β-catenin pathway. Apoptosis 24, 414–433 (2019). https://doi.org/10.1007/s10495-019-01526-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-019-01526-y