Abstract
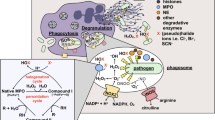
Apoptosis and phagocytosis are crucial processes required for developmental morphogenesis, pathogen deterrence and immunomodulation in metazoans. We present data showing that amebocytes of the chelicerate, Limulus polyphemus, undergo phagocytosis-induced cell death after ingesting spores of the fungus, Beauveria bassiana, in vitro. The observed biochemical and morphological modifications associated with dying amebocytes are congruent with the hallmarks of apoptosis, including: extracellularisation of phosphatidylserine, intranucleosomal DNA fragmentation and an increase in caspase 3/7-like activities. Previous studies have demonstrated that phosphatidylserine is a putative endogenous activator of hemocyanin-derived phenoloxidase, inducing conformational changes that permit phenolic substrate access to the active site. Here, we observed extracellular hemocyanin-derived phenoloxidase activity levels increase in the presence of apoptotic amebocytes. Enzyme activity induced by phosphatidylserine or apoptotic amebocytes was reduced completely upon incubation with the phosphatidylserine binding protein, annexin V. We propose that phosphatidylserine redistributed to the outer plasma membrane of amebocytes undergoing phagocytosis-induced apoptosis could interact with hemocyanin, thus facilitating its conversion into a phenoloxidase-like enzyme, during immune challenge.








Similar content being viewed by others
Notes
All buffers marked were purchased prepared from their respective manufacturers.
References
Brinkmann V, Reichard U, Goosmann C et al (2004) Neutrophil extracellular traps kill bacteria. Science 303:1532–1535
Wang XW, Tan NS, Ho B, Ding JL et al (2006) Evidence for the ancient origin of the NF-kappaB/IkappaB cascade: its archaic role in pathogen infection and immunity. Proc Natl Acad Sci USA 103:4204–4209
Kroemer G, Galluzzi L, Vandenabeele P et al (2009) Classification of cell death: recommendations of the nomenclature committee on cell death 2009. Cell Death Differ 16:3–11
Brown GC, Neher JJ (2012) Eaten alive! Cell death by primary phagocytosis: ‘phagoptosis’. Trends Biochem Sci 37:325–332
Elmore S (2007) Apoptosis: a review of programmed cell death. Toxicol Pathol 35:495–516
Cooper DM, Mitchell-Foster K (2011) Death for survival: What do we know about innate immunity and cell death in insects? ISJ 8:162–172
Menze MA, Fortner G, Nag S, Hand SC et al (2010) Mechanisms of apoptosis in crustacea: What conditions induce versus suppress cell death? Apoptosis 15:293–312
Kiss T (2010) Apoptosis and its functional significance in molluscs. Apoptosis 15:313–321
DeLeo FR (2004) Modulation of phagocyte apoptosis by bacterial pathogens. Apoptosis 9:399–413
Thi EP, Lambertz U, Reiner NE (2012) Sleeping with the enemy: how intracellular pathogens cope with macrophage lifestyle. PLoS Pathog 8:e1002551
Frankenberg T, Kirschnek S, Hacker H, Hacker G (2008) Phagocytosis-induced apoptosis of macrophages is linked to uptake, killing and degradation of bacteria. Eur J Immunol 38:204–215
Kirschnek S, Ying S, Fischer SF et al (2005) Phagocytosis-induced apoptosis is mediated by up-regulation and activation of the Bcl-2 homology domain 3-only protein Bim. J Immunol 174:671–679
Kennedy AD, DeLeo FR (2009) Neutrophil apoptosis and the resolution of infection. Immunol Res 43:25–61
Decker H, Jaenicke E (2004) Recent findings on phenoloxidase activity and antimicrobial activity of hemocyanins. Devel Comp Immunol 28:673–687
Jiang N, Tan NS, Ho B, Ding JL (2007) Respiratory protein generated reactive oxygen species as an antimicrobial strategy. Nature Immunol 8:1114–1122
Zhang Y, Yan F, Hu Z, Zhao X et al (2009) Hemocyanin from shrimp Litopenaeus vannamei shows hemolytic activity. Fish Shellfish Immunol 27:33–335
Coates CJ, Kelly SM, Nairn J (2011) Possible role of phosphatidylserine-hemocyanin interaction in the innate immune response of Limulus polyphemus. Dev Comp Immunol 35:155–163
Zhao X, Guo L, Zhang Y, Liu Y et al (2012) SNPs of hemocyanin C-terminal fragment in shrimp Litopenaeus vannamei. FEBS Lett 586:403–410
Coates CJ, Whalley T, Nairn J (2012) Phagocytic activity of Limulus polyphemus amebocytes in vitro. J Invert Pathol 111:205–210
Altman SA, Randers L, Rao G (1993) Comparison of trypan blue dye exclusion and fluorometric assays for mammalian cell viability determinants. Biotech Prog 9:671–674
Logue SE, Elgendy M, Martin SJ (2009) Expression, purification and use of recombinant annexin V for the detection of apoptotic cells. Nat Protoc 4:1383–1395
Evans TC Jr, Nelsestuen GL (1994) Calcium and membrane-binding of monomeric and multimeric annexin II. Biochemistry 33:13231–13238
Ding JL, Thangamani S, Kusuma N, Seow WK et al (2005) Spatial and temporal coordination of expression of immune genes during Pseudomonas infection of horseshoe crab, Carcinoscorpius rotundicauda. Genes Immun 6:557–574
Coates CJ, Bradford EL, Krome CA, Nairn J (2012) Effect of temperature on biochemical and cellular properties of Limulus polyphemus. Aquaculture 334–337:30–38
Armstrong PB (1980) Adhesion and spreading of Limulus blood cells on artificial surfaces. J Cell Sci 44:243–262
Conrad ML, Pardy RL, Wainwright N, Child A, Armstrong PB (2006) Response of the blood clotting system of the American horseshoe crab, Limulus polyphemus, to a novel form of lipopolysaccharide from a green alga. Comp Biochem Physiol Part A 144:423–428
Li H, Zhu H, Xu C-J, Yuan J (1998) Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 94:491–501
Marchetti P, Castedo M, Susin SA, Zamzami N et al (1996) Mitochondrial permeability transition is a central coordinating event of apoptosis. J Exp Med 184:1155–1160
Jagasia R, Grote P, Westermann B, Condradt B (2005) DRP-1-mediated mitochondrial fragmentation during EGL-1-induced cell death in C. elegans. Nature 433:754–760
Henry CM, Hollville E, Martine SJ (2013) Measuring apoptosis by microscopy and flow cytometry. Methods 61:90–97
Green DR, Oberst A, Dillon CP, Weinlich R, Salvesen GS (2011) RIPK-dependent necrosis and its regulation by caspases: a mystery in five acts. Mol Cell 44:9–16
Baird S, Kelly SM, Price NC, Jaenicke E et al (2007) Hemocyanin conformational changes associated with SDS-induced phenol oxidase activation. Biochim Biophys Acta 1774:1380–1394
Watson RW, Redmond HP, Wang JH, Condron C, Bouchier-Hayes D (1996) Neutrophils undergo apoptosis following ingestion of Escherichia coli. J Immunol 156:3986–3992
Colamussi ML, White MR, Crouch E, Hartshorn KL (1999) Influenza A virus accelerates apoptosis and markedly potentiates apoptotic effects of bacteria. Blood 93:2395–2403
Rotstein D, Parodo J, Taneja R, Marshall JC (2000) Phagocytosis of Candida albicans induces apoptosis of human neutrophils. SHOCK 14:278–283
Zhang B, Hirahashi J, Cullere X, Mayadas TN (2003) Elucidation of the molecular events leading to neutrophil apoptosis following phagocytosis. J Biol Chem 278:28443–28454
Krzyzowska M, Schollenberger A, Skierski J, Niemialtowski M (2002) Apoptosis during ectromelia orthopoxvirus infection is DEVDase dependent: in vitro and in vivo studies. Microb Infect 4:599–611
Phongdara A, Wanna W, Chotigeat W (2006) Molecular cloning and expression of caspase from white shrimp Penaeus merguiensis. Aquaculture 252:114–120
Song Z, McCall K, Steller H (1997) DCP-1, a Drosophila cell death protease essential for development. Science 275:536–540
Albee L, Shi B, Perlman H (2007) Aspartic protease and caspase 3/7 activation are central to macrophage apoptosis following infection with Escherichia coli. J Leuk Biol 81:229–237
Gille Ch, Leiber A, Mundle I, Spring B et al (2009) Phagocytosis and postphagocytic reaction of cord blood and adult blood monocyte after infection with green fluorescent protein-labelled Escherichia coli and group B Streptococci. Cytom Part B 76B:271–284
Dreschers S, Gille C, Haas M, Grosse-Ophoff J et al (2013) Infection-induced bystander apoptosis of monocytes is TNF-alpha-mediated. PLoS ONE 8:e53589
Smith VJ (2010) Immunology of invertebrates: cellular. Encyclopedia of life sciences (ELS). Wiley, Chicester. doi:10.1002/9780470015902.a0002344.pub2
Franc NC (2002) Phagocytosis of apoptotic cells in mammals, Caenorhabditis elegans and Drosophila melanogaster: molecular mechanisms and physiological consequences. Front Biosci 7:1298–1313
Hillman MR (2004) Strategies and mechanisms for host and pathogen survival in acute and persistent viral infections. Proc Nat Acad Sci, USA 101:14560–14566
McLean JE, Ruck A, Shirazian S, Pooyaei-Mehr F, Zakeri ZF (2008) Viral manipulation of cell death. Cur Pharm Des 14:198–220
Watthanasurorot A, Jiravanichpaisal P, Soderhall K, Soderhall I (2013) A calreticulin/gC1qR complex prevents cells from dying: a conserved mechanism from arthropods to humans. J Mol Cell Biol 5:120–131
Hsu J-P, Huang C, Liao C-M, Hsuan S-L, Hung H–H, Chien M-S (2005) Engulfed pathogen-induced apoptosis in haemocytes of giant freshwater prawn, Macrobrachium rosenbergii. J Fish Dis 28:729–735
Lavrov DV, Boore JL, Brown WM (2000) The complete mitochondrial DNA sequence of the horseshoe crab Limulus polyphemus. Mol Biol Evol 17:813–824
Wu Y, Tibrewal N, Birge RB (2006) Phosphatidylserine recognition by phagocytes: a view to a kill. Trends Cell Biol 16:189–197
Clark MR (2011) Flippin’ lipids. Nat Immunol 12:373–375
Lee SH, Meng XW, Flatten KS, Loegering DA, Kaufmann SH (2013) Phosphatidylserine exposure during apoptosis reflects bidirectional trafficking between plasma membrane and cytoplasm. Cell Death Differ 20:64–76
Levin J, Bang FB (1968) Clottable protein in Limulus: its localization and kinetics of its coagulation by endotoxin. Thrombosis et Diathesis Haemorrhagica 19:186–197
Nagai T, Kawabata S (2000) A link between blood coagulation and prophenoloxidase activation in the arthropod host defence. J Biol Chem 275:29264–29267
Nellaiappan K, Sugumaran M (1996) On the presence of prophenoloxidase in the hemolymph of the horseshoe crab, Limulus. Comp Biochem Phys Part B 113:163–168
Nagai T, Osaki T, Kawabata S (2001) Functional conversion of hemocyanin to phenoloxidase by horseshoe crab antimicrobial peptides. J Biol Chem 276:27166–27170
Meers P, Mealy T (1993) Calcium-dependent annexin V binding to phospholipids: stoichiometry, specificity, and the role of negative charge. Biochemistry 32:11711–11721
Pigault C, Follenius-Wund A, Schmutz M, Freyssinet J-M, Brisson A (1994) Formation of two-dimensional arrays of annexin V on phosphatidylserine-containing liposomes. J Mol Biol 236:199–208
Wright J, McCaskill-Clark W, Cain JA, Patterson A, Coates CJ, Nairn J (2012) Effects of known phenoloxidase inhibitors on hemocyanin-derived phenoloxidase from Limulus polyphemus. Comp Biochem Physiol Part B 163:303–308
Martin CJ, Booty MG, Rosebrock TR (2012) Efferocytosis is an innate antibacterial mechanism. Cell Host Microbe 12:289–300
Zwaal RFA, Comfurius P, Bevers EM (1998) Lipid-protein interactions in blood coagulation. Biochim Biophys Acta 1376:433–453
Stace CL, Ktistakis NT (2006) Phosphatidic acid-and phosphatidylserine-binding proteins. Biochim Biophys Acta 1761:913–926
Nakamura T, Tokunaga F, Morita T et al (1988) Intracellular serine-protease zymogen, factor C, from horseshoe crab hemocytes. Eur J Biochem 176:89–94
Bidla A, Hauling T, Dushay MS, Theopold U (2009) Activation of insect phenoloxidase after injury: endogenous versus foreign elicitors. J Innat Immun 1:301–308
Vance JE, Steenbergen R (2005) Metabolism and functions of phosphatidylserine. Prog Lipid Res 44:207–234
Zaini NAM, Osman A, Hamid AA, Ebrahimpour A, Saari N (2013) Purification and characterisation of membrane-bound Polyphenoloxidase (mPPO) from snake fruit [Salacca zalacca (Gaertn.) Voss]. Food Chem 136:407–414
Sokolova IM (2009) Apoptosis in molluscan immune defense. ISJ 6:49–58
Bidla A, Dushay MS, Theopold U (2007) Crystal rupture after injury in Drosophila requires JNK pathway, small GTPases and the TNF homolog Eiger. J Cell Sci 120:1209–1215
Jiravanichpaisal P, Lee BL, Soderhall K (2006) Cell-mediated immunity in arthropods: Hemoatopoiesis, coagulation, melanisation and opsonisation. Immunbiol 211:213–236
Kawabata S, Koshiba T, Shibata T (2009) The lipopolysaccharide-activated innate immune response network of the horseshoe crab. ISJ 6:59–77
MacPherson JC, Pavlovich JG, Jacobs RS (1998) Phospholipid composition of the granular amebocyte from the horseshoe crab, Limulus polyphemus. Lipids 33:931–940
Armstrong P, Levin J (1979) In vitro phagocytosis by Limulus blood cells. J Invert Pathol 34:145–151
Acknowledgments
This work was supported by the University of Stirling. With thanks to Alex Mühlhölzl, C.T.O., Marine Biotech Limited, for providing access to L. polyphemus. We are grateful to Prof. Seamus J. Martin and Conor M. Henry (Molecular Cell Biology Laboratory, Trinity College Dublin) for kindly providing the pProEx.Htb.annexin V plasmid.
Conflict of interest
The authors declare no financial or other potential conflicts of interest.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Coates, C.J., Whalley, T., Wyman, M. et al. A putative link between phagocytosis-induced apoptosis and hemocyanin-derived phenoloxidase activation. Apoptosis 18, 1319–1331 (2013). https://doi.org/10.1007/s10495-013-0891-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-013-0891-x