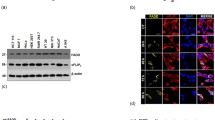
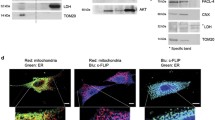
Abstract
Fas-induced apoptosis is initiated through the recruitment of FADD and procaspase 8 to form the death-inducing signaling complex (DISC). In some cells (type I cells) the initiator caspase 8 directly activates effector caspases such as procaspase 3, whereas in others (type II cells) the death signal is amplified through mitochondria. In epithelial cells, Fas-induced hierarchic caspase activation is also linked with DEDD, a member of the DED family that binds to keratin (K) intermediate filaments (IFs). Hepatocytes are type II cells and their IFs are made exclusively of K8/K18. We have shown previously that K8-null mouse hepatocytes, lacking K8/K18 IFs, are more sensitive than their wild-type counterparts to Fas-induced apoptosis. Here, by examining the cell-death kinetics and death-signaling ordering, we found that K8-null hepatocytes exhibited prominent DISC formation, higher procaspase 8 activation and direct procaspase 3 activation as reported for type I cells; however they experienced a reduced Bid cleavage and a stronger procaspase 9 activation. In addition, the K8/K18 loss altered the DEDD ubiquitination status and nuclear/cytoplasmic distribution. Together, the results suggest that the K8/K18 loss induces a switch in Fas-induced death signaling, likely through a DEDD involvement.








Similar content being viewed by others
References
Kumar S (1999) Mechanisms mediating caspase activation in cell death. Cell Death Differ 6:1060–1066. doi:10.1038/sj.cdd.4400600
Zheng TS, Flavell RA (1999) Apoptosis. All’s well that ends dead. Nature 400:410–411. doi:10.1038/22659
Peter ME, Krammer PH (2003) The CD95(APO-1/Fas) DISC and beyond. Cell Death Differ 10:26–35. doi:10.1038/sj.cdd.4401186
Barnhart BC, Alappat EC, Peter ME (2003) The CD95 type I/type II model. Semin Immunol 15:185–193. doi:10.1016/S1044-5323(03)00031-9
Park SM, Peter ME (2008) microRNAs and death receptors. Cytokine Growth Factor Rev 19:303–311. doi:10.1016/j.cytogfr.2008.04.011
Wang X (2001) The expanding role of mitochondria in apoptosis. Genes Dev 15:2922–2933
Barnhart BC, Lee JC, Alappat EC, Peter ME (2003) The death effector domain protein family. Oncogene 22:8634–8644. doi:10.1038/sj.onc.1207103
Fulda S, Meyer E, Debatin KM (2000) Metabolic inhibitors sensitize for CD95 (APO-1/Fas)-induced apoptosis by down-regulating Fas-associated death domain-like interleukin 1-converting enzyme inhibitory protein expression. Cancer Res 60:3947–3956
Dinsdale D, Lee JC, Dewson G, Cohen GM, Peter ME (2004) Intermediate filaments control the intracellular distribution of caspases during apoptosis. Am J Pathol 164:395–407
Lee JC, Schickling O, Stegh AH et al (2002) DEDD regulates degradation of intermediate filaments during apoptosis. J Cell Biol 158:1051–1066. doi:10.1083/jcb.200112124
Schutte B, Henfling M, Ramaekers FC (2006) DEDD association with cytokeratin filaments correlates with sensitivity to apoptosis. Apoptosis 11:1561–1572. doi:10.1007/s10495-006-9113-0
Moll R, Franke WW, Schiller DL (1982) The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell 31:11–24. doi:10.1016/0092-8674(82)90400-7
Schweizer J, Bowden PE, Coulombe PA et al (2006) New consensus nomenclature for mammalian keratins. J Cell Biol 174:169–174. doi:10.1083/jcb.200603161
Coulombe PA, Wong P (2004) Cytoplasmic intermediate filaments revealed as dynamic and multipurpose scaffolds. Nat Cell Biol 6:699–706. doi:10.1038/ncb0804-699
Lane EB, Hogan BL, Kurkinen M, Garrels JI (1983) Co-expression of vimentin and cytokeratins in parietal endoderm cells of early mouse embryo. Nature 303:701–704. doi:10.1038/303701a0
Lu H, Hesse M, Peters B, Magin TM (2005) Type II keratins precede type I keratins during early embryonic development. Eur J Cell Biol 84:709–718. doi:10.1016/j.ejcb.2005.04.001
Ku NO, Omary MB (2000) Keratins turn over by ubiquitination in a phosphorylation-modulated fashion. J Cell Biol 149:547–552. doi:10.1083/jcb.149.3.547
Oshima RG, Baribault H, Caulin C (1996) Oncogenic regulation and function of keratins 8 and 18. Cancer Metastasis Rev 15:445–471. doi:10.1007/BF00054012
Ku NO, Soetikno RM, Omary MB (2003) Keratin mutation in transgenic mice predisposes to Fas but not TNF-induced apoptosis and massive liver injury. Hepatology 37:1006–1014. doi:10.1053/jhep.2003.50181
Ku NO, Strnad P, Zhong BH, Tao GZ, Omary MB (2007) Keratins let liver live: mutations predispose to liver disease and crosslinking generates Mallory-Denk bodies. Hepatology 46:1639–1649. doi:10.1002/hep.21976
Marceau N, Loranger A, Gilbert S, Daigle N, Champetier S (2001) Keratin-mediated resistance to stress and apoptosis in simple epithelial cells in relation to health and disease. Biochem Cell Biol 79:543–555. doi:10.1139/bcb-79-5-543
Marceau N, Schutte B, Gilbert S et al (2007) Dual roles of intermediate filaments in apoptosis. Exp Cell Res 313:2265–2281. doi:10.1016/j.yexcr.2007.03.038
Oshima RG (2002) Apoptosis and keratin intermediate filaments. Cell Death Differ 9:486–492. doi:10.1038/sj.cdd.4400988
Nagata S (1999) Fas ligand-induced apoptosis. Annu Rev Genet 33:29–55. doi:10.1146/annurev.genet.33.1.29
Lacronique V, Mignon A, Fabre M et al (1996) Bcl-2 protects from lethal hepatic apoptosis induced by an anti-Fas antibody in mice. Nat Med 2:80–86. doi:10.1038/nm0196-80
de la Coste A, Fabre M, McDonell N et al (1999) Differential protective effects of Bcl-xL and Bcl-2 on apoptotic liver injury in transgenic mice. Am J Physiol 277:G702–G708
Yin XM, Wang K, Gross A et al (1999) Bid-deficient mice are resistant to Fas-induced hepatocellular apoptosis. Nature 400:886–891. doi:10.1038/23730
Gilbert S, Loranger A, Daigle N, Marceau N (2001) Simple epithelium keratins 8 and 18 provide resistance to Fas-mediated apoptosis. The protection occurs through a receptor-targeting modulation. J Cell Biol 154:763–773. doi:10.1083/jcb.200102130
Gilbert S, Loranger A, Marceau N (2004) Keratins modulate c-Flip/extracellular signal-regulated kinase 1 and 2 antiapoptotic signaling in simple epithelial cells. Mol Cell Biol 24:7072–7081. doi:10.1128/MCB.24.16.7072-7081.2004
Huang DC, Hahne M, Schroeter M et al (1999) Activation of Fas by FasL induces apoptosis by a mechanism that cannot be blocked by Bcl-2 or Bcl-x(L). Proc Natl Acad Sci USA 96:14871–14876. doi:10.1073/pnas.96.26.14871
Baribault H, Penner J, Iozzo RV, Wilson-Heiner M (1994) Colorectal hyperplasia and inflammation in keratin 8-deficient FVB/N mice. Genes Dev 8:2964–2973. doi:10.1101/gad.8.24.2964
Loranger A, Duclos S, Grenier A et al (1997) Simple epithelium keratins are required for maintenance of hepatocyte integrity. Am J Pathol 151:1673–1683
Marceau N, Gilbert S, Loranger A (2004) Uncovering the roles of intermediate filaments in apoptosis. Methods Cell Biol 78:95–129. doi:10.1016/S0091-679X(04)78005-X
Zhao Y, Ding WX, Qian T, Watkins S, Lemasters JJ, Yin XM (2003) Bid activates multiple mitochondrial apoptotic mechanisms in primary hepatocytes after death receptor engagement. Gastroenterology 125:854–867. doi:10.1016/S0016-5085(03)01066-7
Nadakavukaren KK, Nadakavukaren JJ, Chen LB (1985) Increased rhodamine 123 uptake by carcinoma cells. Cancer Res 45:6093–6099
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685. doi:10.1038/227680a0
Scaffidi C, Fulda S, Srinivasan A et al (1998) Two CD95 (APO-1/Fas) signaling pathways. EMBO J 17:1675–1687. doi:10.1093/emboj/17.6.1675
Caulin C, Salvesen GS, Oshima RG (1997) Caspase cleavage of keratin 18 and reorganization of intermediate filaments during epithelial cell apoptosis. J Cell Biol 138:1379–1394. doi:10.1083/jcb.138.6.1379
Ni R, Tomita Y, Matsuda K et al (1994) Fas-mediated apoptosis in primary cultured mouse hepatocytes. Exp Cell Res 215:332–337. doi:10.1006/excr.1994.1349
Gyrd-Hansen M, Farkas T, Fehrenbacher N et al (2006) Apoptosome-independent activation of the lysosomal cell death pathway by caspase-9. Mol Cell Biol 26:7880–7891. doi:10.1128/MCB.00716-06
McDonnell MA, Wang D, Khan SM, Vander Heiden MG, Kelekar A (2003) Caspase-9 is activated in a cytochrome c-independent manner early during TNFalpha-induced apoptosis in murine cells. Cell Death Differ 10:1005–1015. doi:10.1038/sj.cdd.4401271
Berger AB, Sexton KB, Bogyo M (2006) Commonly used caspase inhibitors designed based on substrate specificity profiles lack selectivity. Cell Res 16:961–963. doi:10.1038/sj.cr.7310112
Date T, Mochizuki S, Belanger AJ et al (2003) Differential effects of membrane and soluble Fas ligand on cardiomyocytes: role in ischemia/reperfusion injury. J Mol Cell Cardiol 35:811–821. doi:10.1016/S0022-2828(03)00139-1
Zacks DN, Zheng QD, Han Y, Bakhru R, Miller JW (2004) FAS-mediated apoptosis and its relation to intrinsic pathway activation in an experimental model of retinal detachment. Invest Ophthalmol Vis Sci 45:4563–4569. doi:10.1167/iovs.04-0598
Lee JC, Wang GX, Schickling O, Peter ME (2005) Fusing DEDD with ubiquitin changes its intracellular localization and apoptotic potential. Apoptosis 10:1483–1495. doi:10.1007/s10495-005-1833-z
Schmitz I, Weyd H, Krueger A et al (2004) Resistance of short term activated T cells to CD95-mediated apoptosis correlates with de novo protein synthesis of c-FLIPshort. J Immunol 172:2194–2200
Brumatti G, Yon M, Castro FA et al (2008) Conversion of CD95 (Fas) Type II into Type I signaling by sub-lethal doses of cycloheximide. Exp Cell Res 314:554–563. doi:10.1016/j.yexcr.2007.11.003
Scaffidi C, Schmitz I, Zha J, Korsmeyer SJ, Krammer PH, Peter ME (1999) Differential modulation of apoptosis sensitivity in CD95 type I and type II cells. J Biol Chem 274:22532–22538. doi:10.1074/jbc.274.32.22532
Galarneau L, Loranger A, Gilbert S, Marceau N (2007) Keratins modulate hepatic cell adhesion, size and G1/S transition. Exp Cell Res 313:179–194. doi:10.1016/j.yexcr.2006.10.007
Mathew J, Galarneau L, Loranger A, Gilbert S, Marceau N (2008) Keratin-protein kinase C interaction in reactive oxygen species-induced hepatic cell death through mitochondrial signaling. Free Radic Biol Med 45:413–424. doi:10.1016/j.freeradbiomed.2008.04.031
Werneburg NW, Guicciardi ME, Bronk SF, Gores GJ (2002) Tumor necrosis factor-alpha-associated lysosomal permeabilization is cathepsin B dependent. Am J Physiol Gastrointest Liver Physiol 283:G947–G956
Earnshaw WC, Martins LM, Kaufmann SH (1999) Mammalian caspases: structure, activation, substrates, and functions during apoptosis. Annu Rev Biochem 68:383–424. doi:10.1146/annurev.biochem.68.1.383
Srinivasula SM, Ahmad M, Fernandes-Alnemri T, Alnemri ES (1998) Autoactivation of procaspase-9 by Apaf-1-mediated oligomerization. Mol Cell 1:949–957. doi:10.1016/S1097-2765(00)80095-7
Chaigne-Delalande B, Moreau JF, Legembre P (2008) Rewinding the DISC. Arch Immunol Ther Exp (Warsz) 56:9–14. doi:10.1007/s00005-008-0002-9
Acknowledgements
We thank H. Baribault for the gift of the K8-null mice, R. Kemler for the TROMA-1 hybridoma, and M. E. Peter for the anti-DEDD polyclonal antibody. We also thank S. Champetier for producing the K8-containing retrovirus and D. Poirier for providing the cyt c immunofluorescence protocol. We are grateful to J. Lavoie and L. Galarneau for helpful discussions and critical reading of the manuscript. This work was supported by a grant from Canadian Institutes of Health Research.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gilbert, S., Ruel, A., Loranger, A. et al. Switch in Fas-activated death signaling pathway as result of keratin 8/18-intermediate filament loss. Apoptosis 13, 1479–1493 (2008). https://doi.org/10.1007/s10495-008-0274-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-008-0274-x